The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence
Abstract
1. Introduction
2. Results
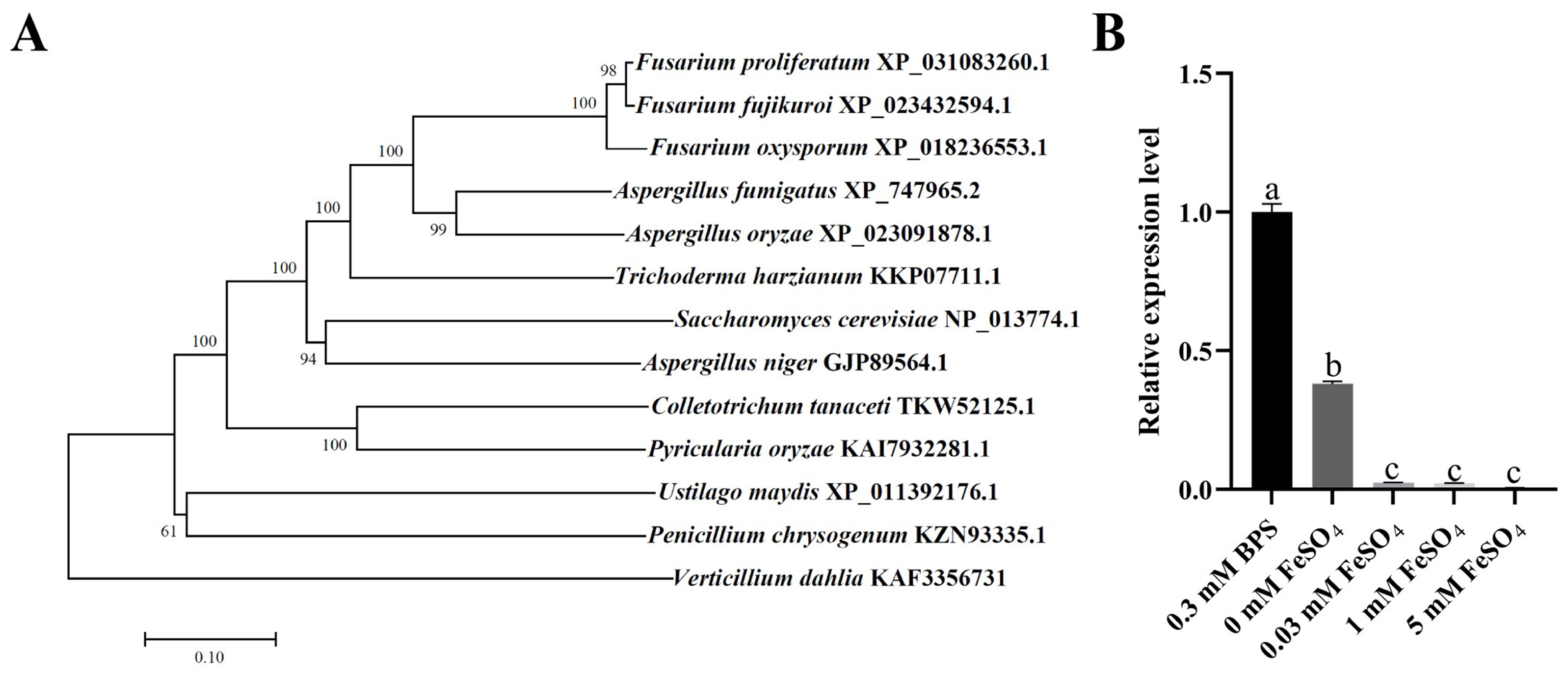
2.1. Identification and Expression of FpfetC in F. proliferatum
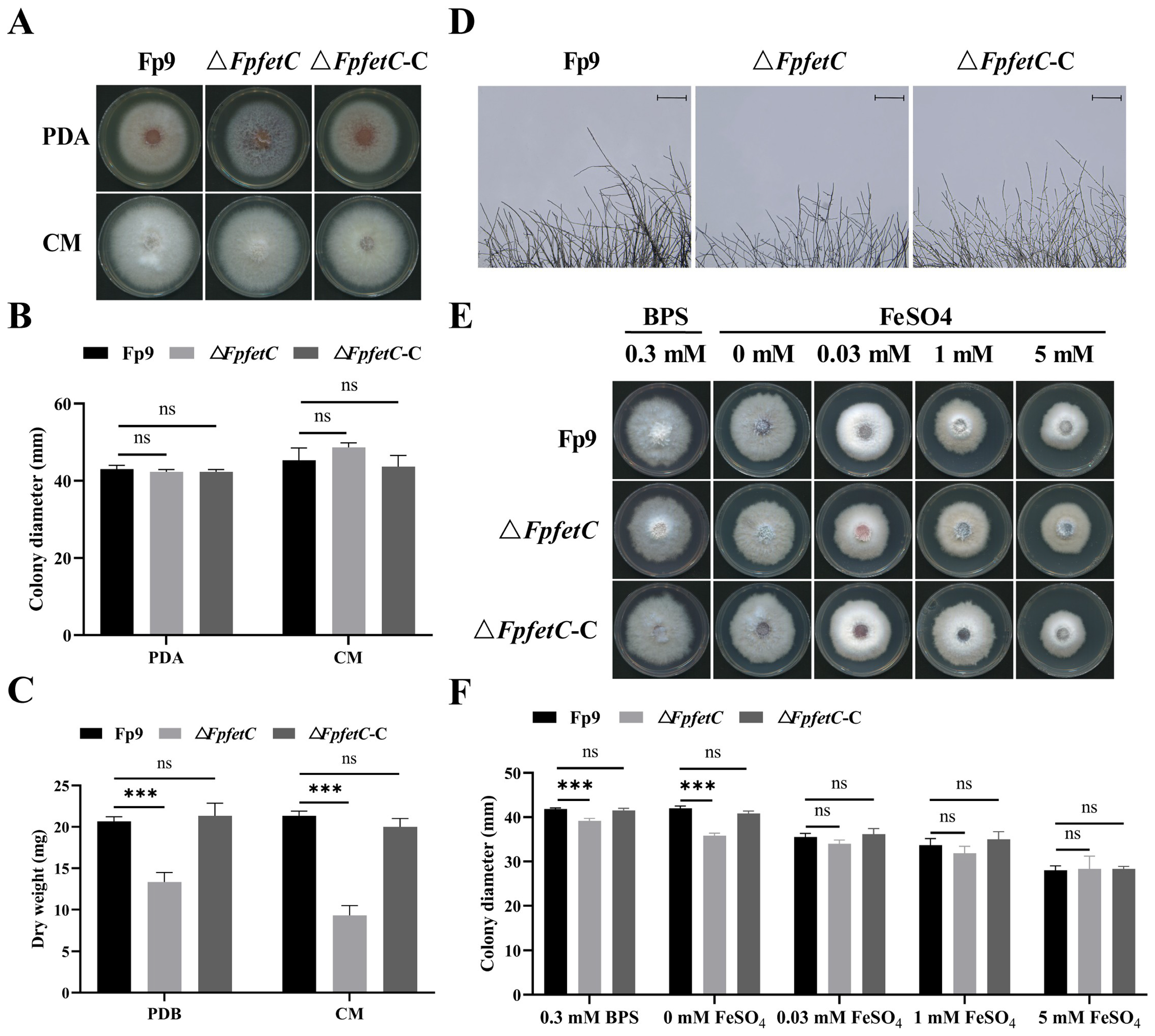
2.2. Loss of FpfetC Decreased Growth Under Iron-Limited Conditions
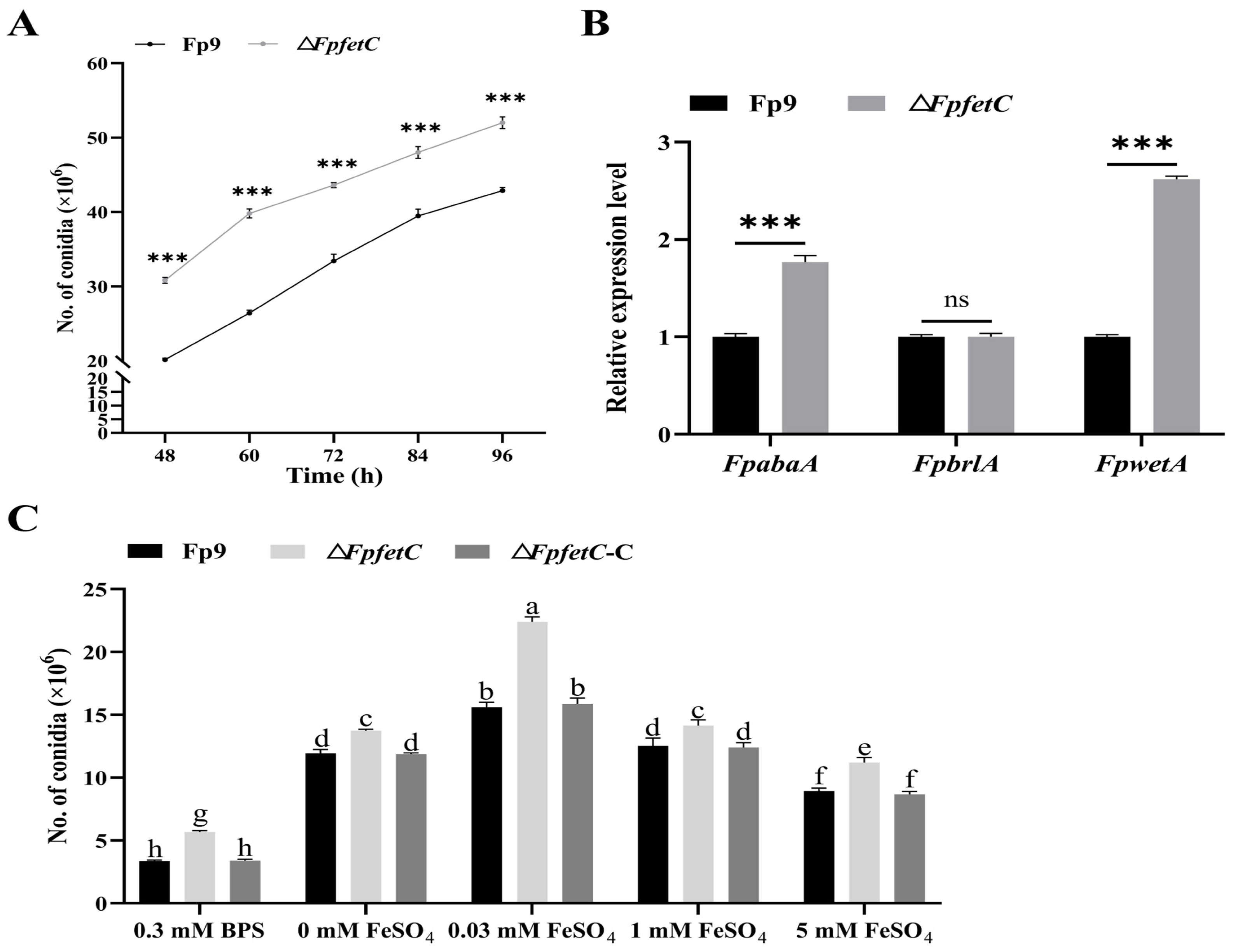
2.3. Deletion of FpfetC Stimulated Formation of Asexual Conidia
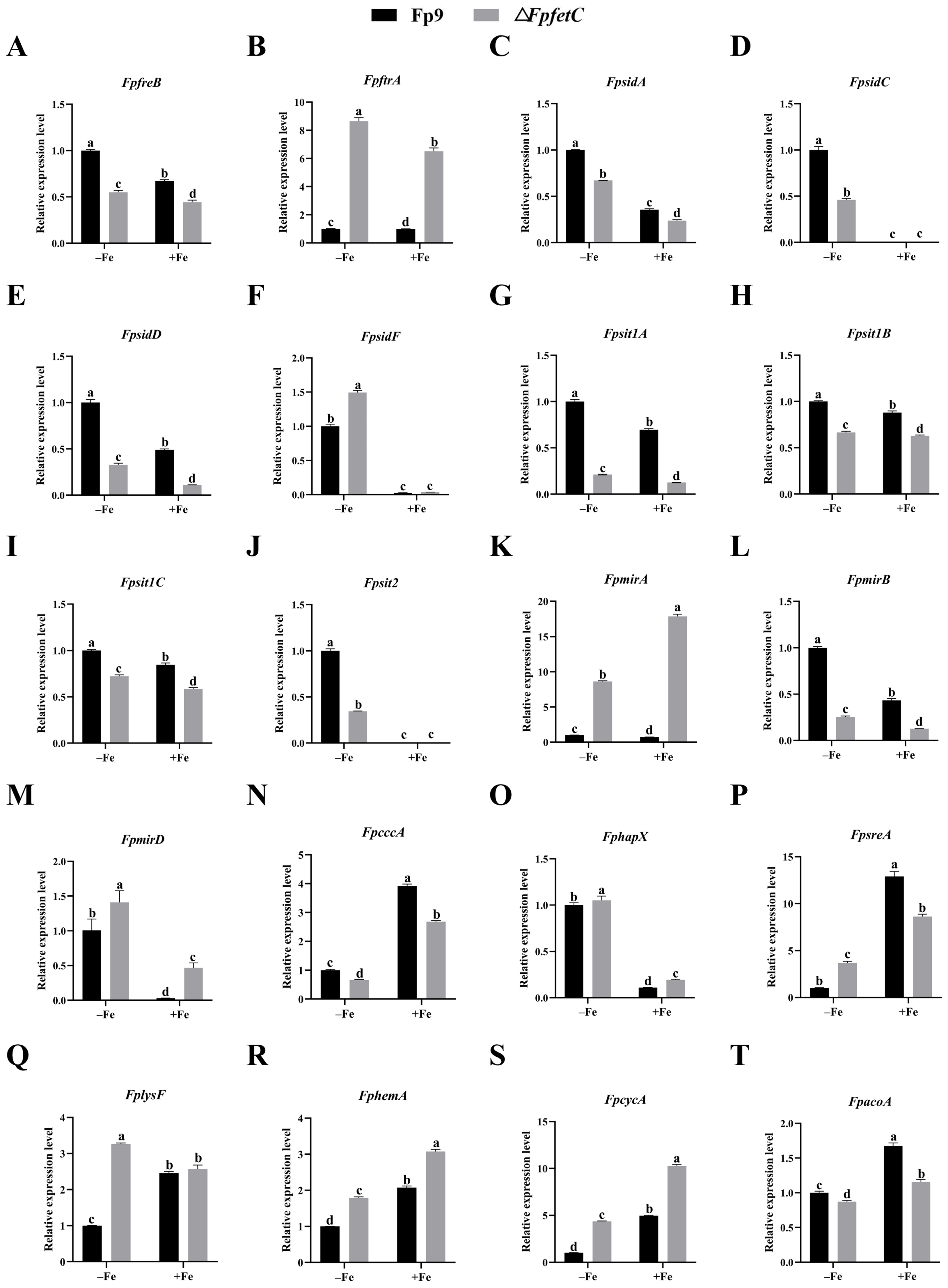
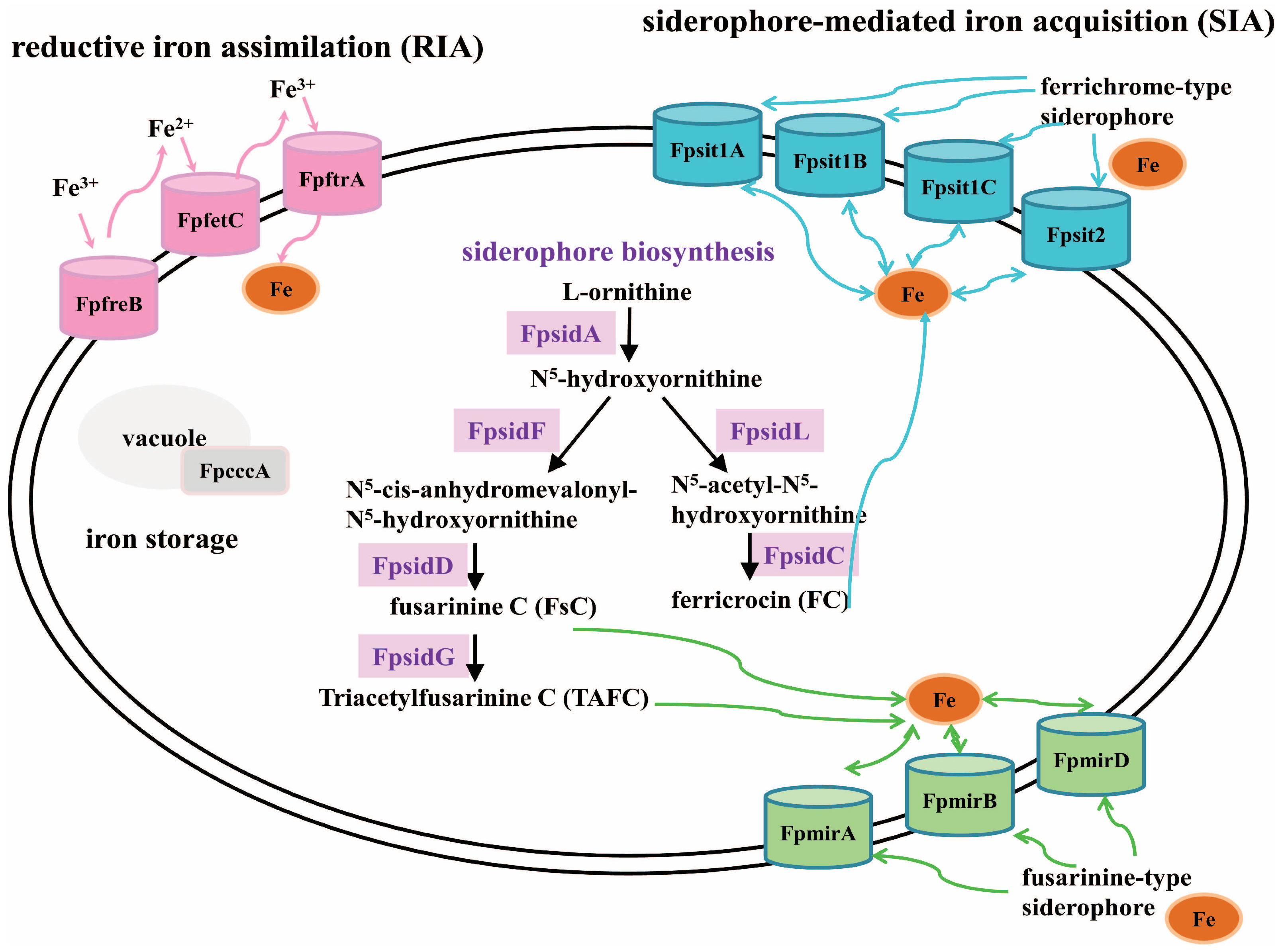
2.4. Deletion of FpfetC Altered Transcription Pattern of Iron-Associated Genes
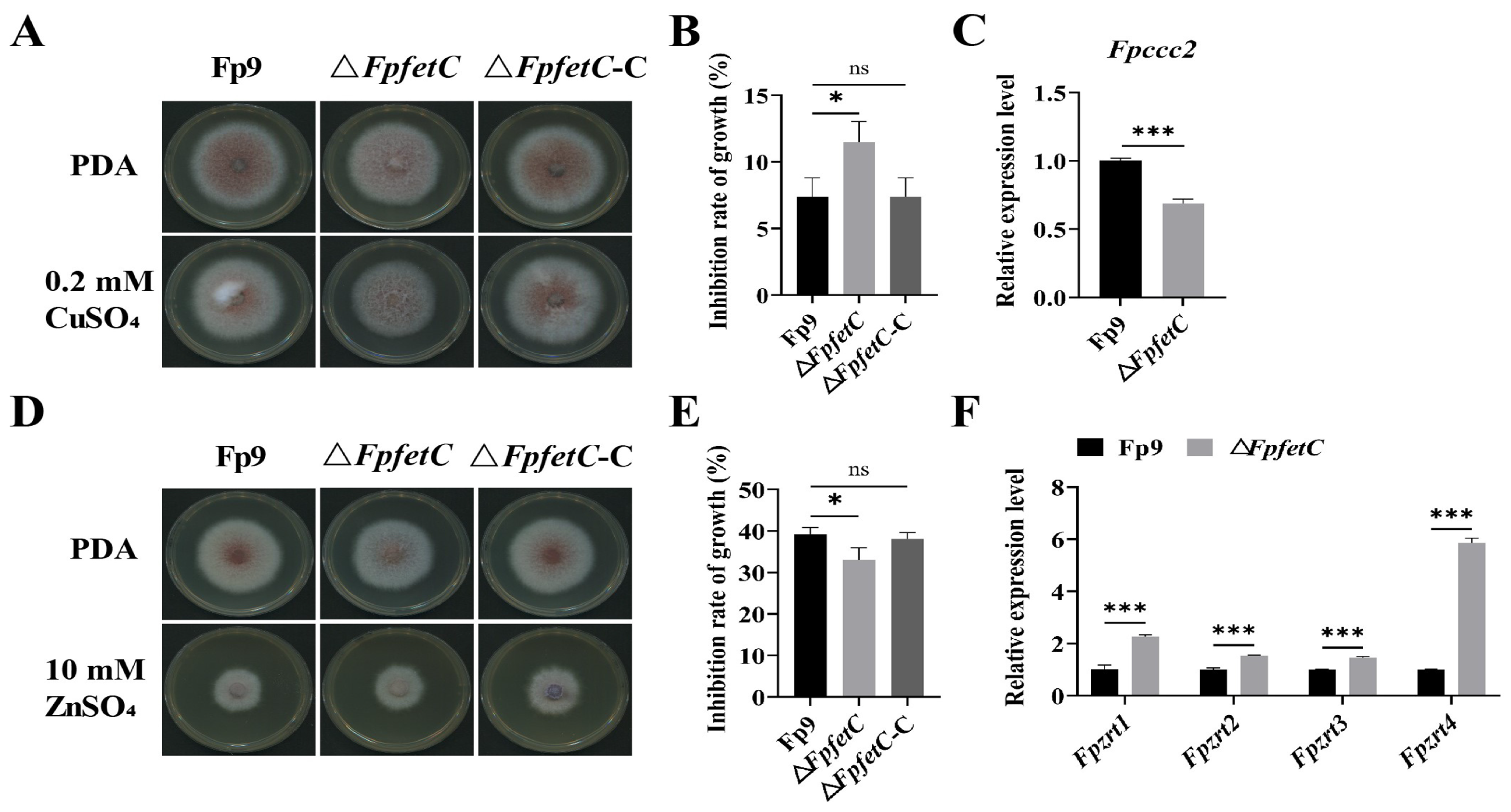
2.5. Loss of FpfetC Affected the Response to Metal Ion Stresses
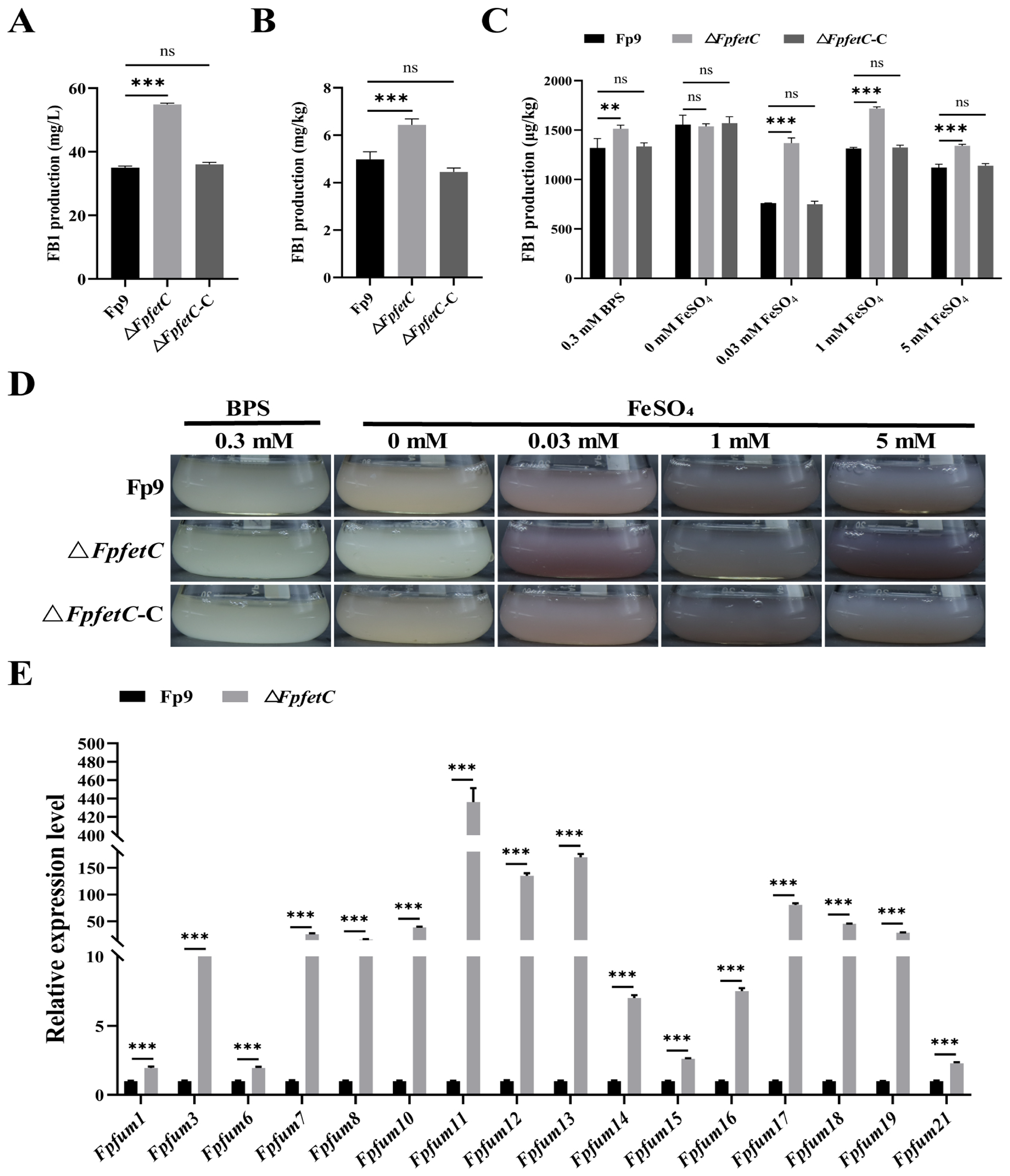
2.6. The Absence of FpfetC Led to Elevated Production of Fumonisin FB1
2.7. Loss of FpfetC Enhanced the Ability of Fungal Infection and Colonization
3. Discussion
3.1. FpfetC Regulated Vegetative Growth in an Iron-Dependent Pattern in F. proliferatum
3.2. FpfetC Was Essential for Transcription Regulation of Iron Uptake in F. proliferatum
3.3. FpfetC Was Involved in Response to Copper and Zinc Stresses in F. proliferatum
3.4. FpfetC Negatively Regulated Fumonisin FB1 Production in F. proliferatum
3.5. FpfetC Was Crucial for the Pathogenicity in F. proliferatum
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Phylogenetic Analysis
4.3. Gene Deletion and Complementation
4.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.5. Determination of FB1 Production
4.6. Plant Infection Assay
4.7. Scanning Electron Microscopy (SEM)
4.8. Transmission Electron Microscopy (TEM)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of iron homeostasis: New players in metabolism, cell death, and disease. Trends Biochem. Sci. 2016, 41, 274–286. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta – Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef]
- Misslinger, M.; Hortschansky, P.; Brakhage, A.A.; Haas, H. Fungal iron homeostasis with a focus on Aspergillus fumigatus. Biochim. Biophys. Acta – Mol. Cell Res. 2021, 1868, 118885. [Google Scholar] [CrossRef]
- Hu, G.; Chen, S.H.; Qiu, J.; Bennett, J.E.; Myers, T.G.; Williamson, P.R. Microevolution during serial mouse passage demonstrates FRE3 as a virulence adaptation gene in Cryptococcus neoformans. mBio 2014, 5, e00941-14. [Google Scholar] [CrossRef]
- Albarouki, E.; Deising, H.B. Infection structure–specific reductive iron assimilation is required for cell wall integrity and full virulence of the maize pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 2013, 26, 695–708. [Google Scholar] [CrossRef]
- Ding, J.L.; Feng, M.G.; Ying, S.H. Two ferrous iron transporter-like proteins independently participate in asexual development under iron limitation and virulence in Beauveria bassiana. Fungal Genet. Biol. 2024, 173, 103908. [Google Scholar] [CrossRef]
- Ding, J.L.; Lu, M.; Liu, X.L.; Feng, M.G.; Ying, S.H. Essential roles of ferric reductase-like proteins in growth, development, stress response, and virulence of the filamentous entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2024, 282, 127661. [Google Scholar] [CrossRef]
- Saikia, S.; Oliveira, D.; Hu, G.; Kronstad, J. Role of ferric reductases in iron acquisition and virulence in the fungal pathogen Cryptococcus neoformans. Infect. Immun. 2014, 82, 839–850. [Google Scholar] [CrossRef]
- Xu, N.; Qian, K.; Dong, Y.; Chen, Y.; Yu, Q.; Zhang, B.; Xing, L.; Li, M. Novel role of the Candida albicans ferric reductase gene CFL1 in iron acquisition, oxidative stress tolerance, morphogenesis and virulence. Res. Microbiol. 2014, 165, 252–261. [Google Scholar] [CrossRef]
- Xie, N.; Ruprich-Robert, G.; Silar, P.; Herbert, E.; Ferrari, R.; Chapeland-Leclerc, F. Characterization of three multicopper oxidases in the filamentous fungus Podospora anserina: A new role of an ABR1–like protein in fungal development? Fungal Genet. Biol. 2018, 116, 1–13. [Google Scholar] [PubMed]
- Yu, H.; Hwang, S.F.; Strelkov, S.E. The host range of Fusarium proliferatum in western Canada. Pathogens 2024, 13, 407. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Ge, S.L.; Liang, W.H.; Liao, W.Y.; Li, W.; Jiao, G.; Wei, X.J.; Shao, G.N.; Xie, L.H.; et al. Genomic footprints related with adaptation and fumonisins production in Fusarium proliferatum. Front. Microbiol. 2022, 13, 1004454. [Google Scholar]
- Wang, L.; Ge, S.; Liang, W.; Liao, W.; Li, W.; Jiao, G.; Wei, X.; Shao, G.; Xie, L.; Sheng, Z.; et al. Genome-wide characterization reveals variation potentially involved in pathogenicity and mycotoxins biosynthesis of Fusarium proliferatum causing spikelet rot disease in rice. Toxins 2022, 14, 568. [Google Scholar] [CrossRef]
- Braun, M.S.; Wink, M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 769–791. [Google Scholar]
- Li, T.; Su, X.; Qu, H.; Duan, X.; Jiang, Y. Biosynthesis, regulation, and biological significance of fumonisins in fungi: Current status and prospects. Crit. Rev. Microbiol. 2022, 48, 450–462. [Google Scholar]
- Ponce-García, N.; Serna-Saldivar, S.O.; Garcia-Lara, S. Fumonisins and their analogues in contaminated corn and its processed foods – a review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2018, 35, 2183–2203. [Google Scholar]
- International Agency for Research on Cancer (IARC). Fumonisin B1. IARC monographs on the evaluation of carcinogenic risks to humans: Some traditional medicines, some mycotoxins, naphthalene and styrene. IARC 2002, 82, 301–366. [Google Scholar]
- Yang, D.; Ye, Y.; Sun, J.; Wang, J.S.; Huang, C.; Sun, X. Occurrence, transformation, and toxicity of fumonisins and their covert products during food processing. Crit. Rev. Food Sci. Nutr. 2024, 64, 3660–3673. [Google Scholar]
- Kaur, B.; Ranawana, V.; Henry, J. The glycemic index of rice and rice products: A review, and table of GI values. Crit. Rev. Food Sci. Nutr. 2016, 56, 215–236. [Google Scholar]
- Song, Y.; Wang, C.; Linderholm, H.W.; Fu, Y.; Cai, W.; Xu, J.; Zhuang, L.; Wu, M.; Shi, Y.; Wang, G.; et al. The negative impact of increasing temperatures on rice yields in southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar]
- Huang, S.W.; Wang, L.; Liu, L.M.; Tang, S.Q.; Zhu, D.F.; Savary, S. Rice spikelet rot disease in China–1. Characterization of fungi associated with the disease. Crop Prot. 2011, 30, 1–9. [Google Scholar]
- Philpott, C.C. Iron uptake in fungi: A system for every source. Biochim. Biophys. Acta. 2006, 1763, 636–645. [Google Scholar]
- Chakraborty, T.; Tóth, Z.; Tóth, R.; Vágvölgyi, C.; Gácser, A. Iron metabolism, pseudohypha production, and biofilm formation through a multicopper oxidase in the human-pathogenic fungus Candida parapsilosis. mSphere 2020, 5, e00227-20. [Google Scholar]
- Forester, N.T.; Lane, G.A.; Steringa, M.; Lamont, I.L.; Johnson, L.J. Contrasting roles of fungal siderophores in maintaining iron homeostasis in Epichloë festucae. Fungal Genet. Biol. 2018, 111, 60–72. [Google Scholar]
- Zhang, W.; Forester, N.T.; Applegate, E.R.; Liu, X.; Johnson, L.J. High-affinity iron uptake is required for optimal Epichloë festucae colonization of Lolium perenne and seed transmission. Mol. Plant Pathol. 2023, 24, 1430–1442. [Google Scholar]
- Zheng, M.T.; Ding, H.; Huang, L.; Wang, Y.H.; Yu, M.N.; Zheng, R.; Yu, J.J.; Liu, Y.F. Low-affinity iron transport protein Uvt3277 is important for pathogenesis in the rice false smut fungus Ustilaginoidea virens. Curr. Genet. 2017, 63, 131–144. [Google Scholar]
- Hissen, A.H.; Wan, A.N.; Warwas, M.L.; Pinto, L.J.; Moore, M.M. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect. Immun. 2005, 73, 5493–5503. [Google Scholar]
- Greenshields, D.L.; Liu, G.; Feng, J.; Selvaraj, G.; Wei, Y. The siderophore biosynthetic gene SID1, but not the ferroxidase gene FET3, is required for full Fusarium graminearum virulence. Mol. Plant Pathol. 2007, 8, 411–421. [Google Scholar]
- Condon, B.J.; Oide, S.; Gibson, D.M.; Krasnoff, S.B.; Turgeon, B.G. Reductive iron assimilation and intracellular siderophores assist extracellular siderophore-driven iron homeostasis and virulence. Mol. Plant Microbe In. 2014, 27, 793–808. [Google Scholar]
- Li, Y.; Wang, Z.; Liu, X.; Song, Z.; Li, R.; Shao, C.; Yin, Y. Siderophore biosynthesis but not reductive iron assimilation is essential for the dimorphic fungus Nomuraea rileyi conidiation, dimorphism transition, resistance to oxidative stress, pigmented microsclerotium formation, and virulence. Front. Microbiol. 2016, 7, 931. [Google Scholar]
- Singh, A.; Severance, S.; Kaur, N.; Wiltsie, W.; Kosman, D.J. Assembly, activation, and trafficking of the Fet3p.Ftr1p high affinity iron permease complex in Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 13355–13364. [Google Scholar]
- Li, L.; Ward, D.M. Iron toxicity in yeast: Transcriptional regulation of the vacuolar iron importer Ccc1. Curr. Genet. 2018, 64, 413–416. [Google Scholar]
- Vest, K.E.; Wang, J.; Gammon, M.G.; Maynard, M.K.; White, O.L.; Cobine, J.A.; Mahone, W.K.; Cobine, P.A. Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae. Open Biol. 2016, 6, 150223. [Google Scholar]
- Yuan, D.S.; Stearman, R.; Dancis, A.; Dunn, T.; Beeler, T.; Klausner, R.D. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc. Natl. Acad. Sci. USA. 1995, 92, 2632–2636. [Google Scholar]
- Antsotegi-Uskola, M.; Markina-Inarrairaegui, A.; Ugalde, U. Copper resistance in Aspergillus nidulans relies on the PI-type ATPase CrpA, regulated by the transcription factor AceA. Front. Microbiol. 2017, 8, 912. [Google Scholar]
- Yang, K.; Shadkchan, Y.; Tannous, J.; Landero Figueroa, J.A.; Wiemann, P.; Osherov, N.; Wang, S.; Keller, N.P. Contribution of ATPase copper transporters in animal but not plant virulence of the crossover pathogen Aspergillus flavus. Virulence 2018, 9, 1273–1286. [Google Scholar] [CrossRef]
- Wu, C.Y.; Bird, A.J.; Chung, L.M.; Newton, M.A.; Winge, D.R.; Eide, D.J. Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genomics 2008, 9, 370. [Google Scholar]
- Bailão, E.F.; Lima Pde, S.; Silva-Bailão, M.G.; Bailão, A.M.; Fernandes Gda, R.; Kosman, D.J.; Soares, C.M. Paracoccidioides spp. ferrous and ferric iron assimilation pathways. Front. Microbiol. 2015, 6, 821. [Google Scholar]
- Yasmin, S.; Abt, B.; Schrettl, M.; Moussa, T.A.; Werner, E.R.; Haas, H. The interplay between iron and zinc metabolism in Aspergillus fumigatus. Fungal Genet. Biol. 2009, 46, 707–713. [Google Scholar]
- Vicentefranqueira, R.; Amich, J.; Marín, L.; Sánchez, C.I.; Leal, F.; Calera, J.A. The transcription factor ZafA regulates the homeostatic and adaptive response to zinc starvation in Aspergillus fumigatus. Genes 2018, 9, 318. [Google Scholar] [CrossRef]
- Vicentefranqueira, R.; Leal, F.; Marín, L.; Sánchez, C.I.; Calera, J.A. The interplay between zinc and iron homeostasis in Aspergillus fumigatus under zinc-replete conditions relies on the iron-mediated regulation of alternative transcription units of zafA and the basal amount of the ZafA zinc-responsiveness transcription factor. Environ. Microbiol. 2019, 21, 2787–2808. [Google Scholar]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Manfiolli, A.O.; de Castro, P.A.; Dos Reis, T.F.; Dolan, S.; Doyle, S.; Jones, G.; Riaño Pachón, D.M.; Ulaş, M.; Noble, L.M.; Mattern, D.J.; et al. Aspergillus fumigatus protein phosphatase PpzA is involved in iron assimilation, secondary metabolite production, and virulence. Cell. Microbiol. 2017, 19, e12770. [Google Scholar] [CrossRef]
- Donzelli, B.G.G.; Turgeon, B.G.; Gibson, D.M.; Krasnoff, S.B. Disruptions of the genes involved in lysine biosynthesis, iron acquisition, and secondary metabolisms affect virulence and fitness in Metarhizium robertsii. Fungal Genet. Biol. 2017, 98, 23–34. [Google Scholar] [CrossRef][Green Version]
- Dai, B.; Xu, Y.; Wu, H.; Chen, J. Rim101-upregulated Fets contribute to dark pigment formation in gray cells of Candida albicans. Acta Biochim. Biophys. Sin. 2021, 53, 1723–1730. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, D.; Wu, H.L.; Ling, H.Q. Iron in plant-pathogen interactions. J. Exp. Bot. 2021, 72, 2114–2124. [Google Scholar] [CrossRef]
- Haas, H. Iron–a key nexus in the virulence of Aspergillus fumigatus. Front. Microbiol. 2012, 3, 28. [Google Scholar] [CrossRef]
- Balhara, M.; Chaudhary, R.; Ruhil, S.; Singh, B.; Dahiya, N.; Parmar, V.S.; Jaiwal, P.K.; Chhillar, A.K. Siderophores; iron scavengers: The novel & promising targets for pathogen specific antifungal therapy. Expert Opin. Ther. Tar. 2016, 20, 1477–1489. [Google Scholar]
- Zarember, K.A.; Cruz, A.R.; Huang, C.Y.; Gallin, J.I. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigatus. Antimicrob. Agents Ch. 2009, 53, 2654–2656. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Liu, L.M.; Hou, Y.X.; Xu, Y.H.; Liang, M.Q.; Gao, J.; Li, Q.Q.; Huang, S.W. Infection and colonization of pathogenic fungus Fusarium proliferatum in rice spikelet rot disease. Rice Sci. 2019, 26, 60–68. [Google Scholar]
- Vasquez-Montaño, E.; Hoppe, G.; Vega, A.; Olivares-Yañez, C.; Canessa, P. Defects in the ferroxidase that participates in the reductive iron assimilation system results in hypervirulence in Botrytis Cinerea. mBio 2020, 11, e01379-20. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, N.; Yu, Q.; Ding, X.; Qian, K.; Zhao, Q.; Wang, Y.; Zhang, B.; Xing, L.; Li, M. Novel insight into the expression and function of the multicopper oxidases in Candida albicans. Microbiology 2013, 159, 1044–1055. [Google Scholar] [CrossRef][Green Version]
- Pontes, J.G.M.; Fernandes, L.S.; Dos Santos, R.V.; Tasic, L.; Fill, T.P. Virulence factors in the phytopathogen-host interactions: An overview. J. Agric. Food Chem. 2020, 68, 7555–7570. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A. Aspergillus species and their associated mycotoxins. Methods Mol. Biol. 2017, 1542, 33–49. [Google Scholar] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska., J.; Stępień., Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Meena, M.; Gupta., S.K.; Swapnil., P.; Zehra., A.; Dubey., M.K.; Upadhyay., R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017, 8, 1451. [Google Scholar]
- Beccari, G.; Tini, F.; Foroud, N.A.; Ederli, L.; Gardiner, D.M.; Benfield, A.H.; Harris, L.J.; Sulyok, M.; Romani, R.; Bellezza, I.; et al. A comparison between the role of enniatins and deoxynivalenol in Fusarium virulence on different tissues of common wheat. BMC Plant Biol. 2024, 24, 463. [Google Scholar]
- Gunupuru, L.R.; Perochon, A.; Doohan, P.R. Deoxynivalenol resistance as a component of FHB resistance. Trop. Plant Pathol. 2017, 42, 175–183. [Google Scholar]
- Iqbal, N.; Czékus, Z.; Poór, P.; Ördög, A. Plant defence mechanisms against mycotoxin Fumonisin B1. Chem. Biol. Interact. 2021, 343, 109494. [Google Scholar]
- Gwinn, K.D.; Hansen, Z.; Kelly, H.; Ownley, B.H. Diseases of Cannabis sativa caused by diverse Fusarium species. Front. Agron. 2022, 3, 796062. [Google Scholar] [CrossRef]
- Conti Taguali, S.; Riolo, M.; Dopazo, V.; Meca, G.; Cacciola, S.O. Characterization of mycotoxins produced by two Fusarium species responsible for postharvest rot of banana fruit. J. Plant Pathol. 2024, 106, 1785–1800. [Google Scholar] [CrossRef]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.M.; Hou, Y.X.; Wang, L.; Huang, S.W. Effects of disruption of five FUM genes on fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef]
- Xie, L.; Wu, Y.; Wang, Y.; Jiang, Y.; Yang, B.; Duan, X.; Li, T. Fumonisin B1 induced aggressiveness and infection mechanism of Fusarium proliferatum on banana fruit. Environ. Pollut. 2021, 288, 117793. [Google Scholar]
- Vismer, H.F.; Shephard, G.S.; van der Westhuizen, L.; Mngqawa, P.; Bushula-Njah, V.; Leslie, J.F. Mycotoxins produced by Fusarium proliferatum and F. pseudonygamai on maize, sorghum and pearl millet grains in vitro. Int. J. Food Microbiol. 2019, 296, 31–36. [Google Scholar]
- Aguiar, M.; Orasch, T.; Shadkchan, Y.; Caballero, P.; Pfister, J.; Sastré-Velásquez, L.E.; Gsaller, F.; Decristoforo, C.; Osherov, N.; Haas, H. Uptake of the siderophore triacetylfusarinine C, but not fusarinine C, is crucial for virulence of Aspergillus fumigatus. mBio 2022, 13, e02192-22. [Google Scholar] [CrossRef]
- Voß, B.; Kirschhöfer, F.; Brenner-Weiß, G.; Fischer, R. Alternaria alternata uses two siderophore systems for iron acquisition. Sci. Rep. 2020, 10, 3587. [Google Scholar]
- Albarouki, E.; Schafferer, L.; Ye, F.; von Wirén, N.; Haas, H.; Deising, H.B. Biotrophy-specific downregulation of siderophore biosynthesis in Colletotrichum graminicola is required for modulation of immune responses of maize. Mol. Microbiol. 2014, 92, 338–355. [Google Scholar] [CrossRef]
- Donzelli, B.G.G.; Gibson, D.M.; Krasnoff, S.B. Intracellular siderophore but not extracellular siderophore is required for full virulence in Metarhizium robertsii. Fungal Genet. Biol. 2015, 82, 56–68. [Google Scholar]
- Wang, Y.; Deng, C.; Tian, L.; Xiong, D.; Tian, C.; Klosterman, S.J. The transcription factor VdHapX controls iron homeostasis and is crucial for virulence in the vascular pathogen Verticillium dahliae. mSphere 2018, 3, e00400-18. [Google Scholar]
- Hong, S.; Sun, Y.; Chen, H.; Wang, C. Suppression of the insect cuticular microbiomes by a fungal defensin to facilitate parasite infection. ISME J. 2023, 17, 1–11. [Google Scholar]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [PubMed]
- Orasch, T.; Dietl, A.M.; Shadkchan, Y.; Binder, U.; Bauer, I.; Lass-Flörl, C.; Osherov, N.; Haas, H. The leucine biosynthetic pathway is crucial for adaptation to iron starvation and virulence in Aspergillus fumigatus. Virulence 2019, 10, 925–934. [Google Scholar] [PubMed]
- Herrera, M.L.; Vallor, A.C.; Gelfond, J.A.; Patterson, T.F.; Wickes, B.L. Strain-dependent variation in 18S ribosomal DNA copy numbers in Aspergillus fumigatus. J. Clin. Microbiol. 2009, 47, 1325–1332. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Ediage, E.N.; Van Poucke, C.; De Saeger, S. A multi-analyte LC–MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 177, 397–404. [Google Scholar]
- Tebele, S.M.; Gbashi, S.; Adebo, O.; Changwa, R.; Naidu, K.; Njobeh, P.B. Quantification of multi-mycotoxin in cereals (maize, maize porridge, sorghum and wheat) from Limpopo province of South Africa. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1922–1938. [Google Scholar]
- Huang, S.W.; Wang, L.; Liu, L.M.; Tang, S.Q.; Zhu, D.F.; Savary, S. Rice spikelet rot disease in China–2. Pathogenicity tests, assessment of the importance of the disease, and preliminary evaluation of control options. Crop Prot. 2011, 30, 10–17. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Liu, L.; Hou, Y.; Li, Q.; Huang, S. Screening for strains of rice spikelet rot disease pathogenic fungus with high fumonisin production and strong pathogenicity. Chin. J. Rice Sci. 2018, 32, 610–616. [Google Scholar]
- Xiang, X.; Zhang, P.; Yu, P.; Zhang, Y.; Yang, Z.; Sun, L.; Wu, W.; Khan, R.M.; Abbas, A.; Cheng, S.; et al. LSSR1 facilitates seed setting rate by promoting fertilization in rice. Rice 2019, 12, 31. [Google Scholar] [PubMed]
- Saddique, M.A.B.; Ali, Z.; Khan, A.S.; Rana, I.A.; Shamsi, I.H. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice 2018, 11, 34. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, W.; Ge, S.; Sheng, Z.; Hu, S.; Jiao, G.; Shao, G.; Xie, L.; Tang, S.; Hu, P. The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence. Int. J. Mol. Sci. 2025, 26, 2883. https://doi.org/10.3390/ijms26072883
Wang L, Li W, Ge S, Sheng Z, Hu S, Jiao G, Shao G, Xie L, Tang S, Hu P. The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence. International Journal of Molecular Sciences. 2025; 26(7):2883. https://doi.org/10.3390/ijms26072883
Chicago/Turabian StyleWang, Ling, Wen Li, Shuailing Ge, Zhonghua Sheng, Shikai Hu, Guiai Jiao, Gaoneng Shao, Lihong Xie, Shaoqing Tang, and Peisong Hu. 2025. "The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence" International Journal of Molecular Sciences 26, no. 7: 2883. https://doi.org/10.3390/ijms26072883
APA StyleWang, L., Li, W., Ge, S., Sheng, Z., Hu, S., Jiao, G., Shao, G., Xie, L., Tang, S., & Hu, P. (2025). The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence. International Journal of Molecular Sciences, 26(7), 2883. https://doi.org/10.3390/ijms26072883






