Greek Raw Honey from Pindos Mountain Improves Redox Homeostasis of RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
2.1. Determination of Total Phenolic Content (TPC)
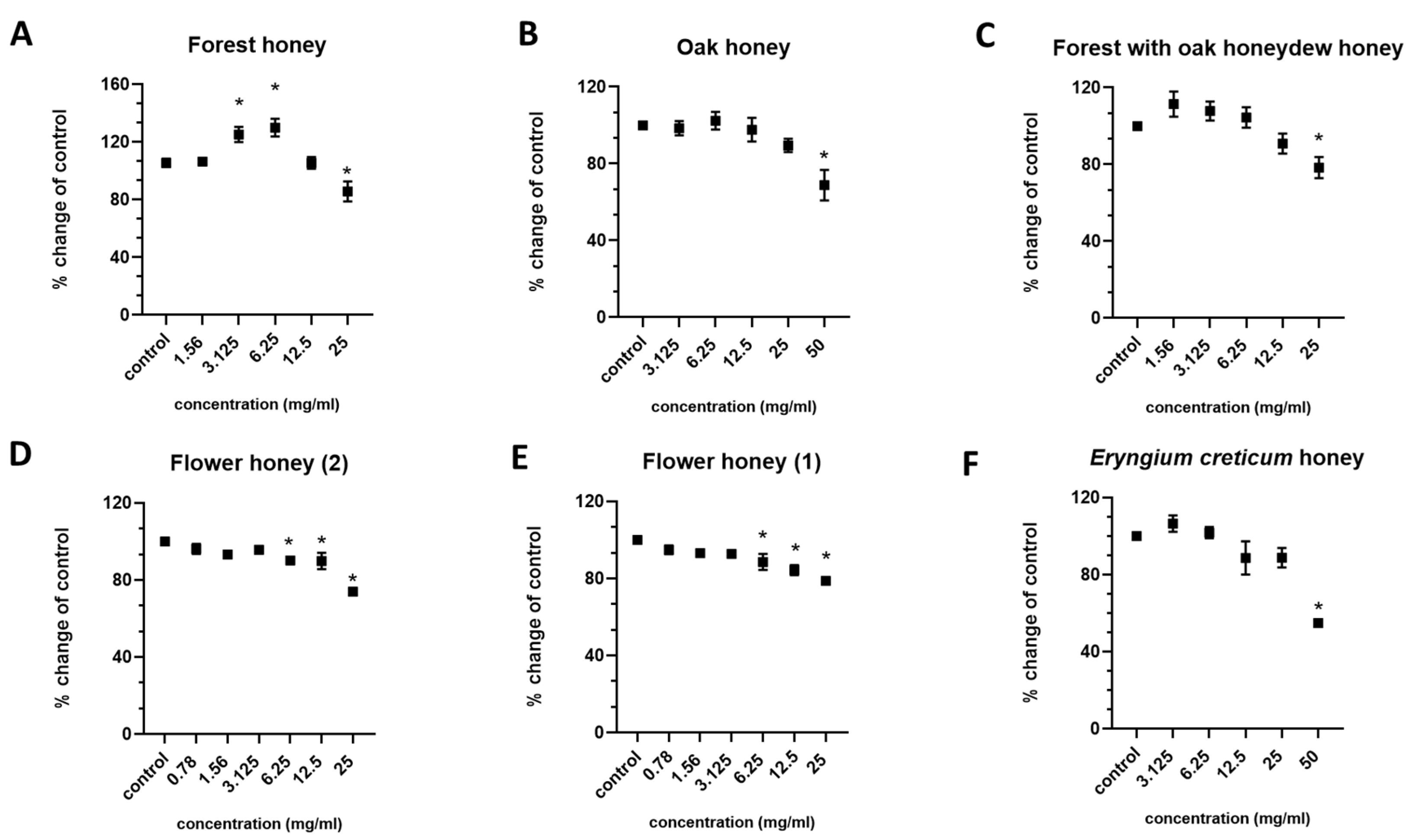
2.2. Determination of the Effects on Cell Viability
2.3. Determination of the Effects on Intracellular Reduced Glutathione (GSH) and Reactive Oxygen Species (ROS) Levels
2.4. Determination of the Effects on Total Antioxidant Capacity (TAC), Thiobarbituric Acid Reactive Substances (TBARS), and Protein Carbonyls (PCARBS) Levels
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Total Phenolic Content (TPC)
4.3. Cell Cultures
4.4. Cell Viability Assay
4.5. Flow Cytometry
4.6. Spectrophotometry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xagoraris, M.; Revelou, P.-K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Greek Honey Authentication: Botanical Approach. Encyclopedia 2021, 1, 1322–1333. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability and Evidence of Protective Effects Against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.L.; Panyoyai, N.; Paramita, V.D.; Mantri, N.; Kasapis, S. Physicochemical and Viscoelastic Properties of Honey from Medicinal Plants. Food Chem. 2018, 241, 143–149. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Sulaiman, G.M.; Albukhaty, S.; Al-Saffar, A.Z.; Elshibani, F.A.; Ragab, E.A. Chrysin, The Flavonoid Molecule of Antioxidant Interest. ChemistrySelect 2023, 8, e202303306. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Molecules Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic Acid Composition, Antiatherogenic and Anticancer Potential of Honeys Derived from Various Regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Ahmed, M.A.; Alsulami, T.; Hakeem, M.J.; Ibraheem, M.A.; Al-Nouri, D.M. Phenolic Compounds, Antioxidant Activity, Ascorbic Acid, and Sugars in Honey from Ingenious Hail Province of Saudi Arabia. Appl. Sci. 2022, 12, 8334. [Google Scholar] [CrossRef]
- Erejuwa, O.O. Effect of Honey in Diabetes Mellitus: Matters Arising. J. Diabetes Metab. Disord. 2014, 13, 23. [Google Scholar] [CrossRef]
- Candiracci, M.; Piatti, E.; Dominguez-Barragán, M.; García-Antrás, D.; Morgado, B.; Ruano, D.; Gutiérrez, J.F.; Parrado, J.; Castaño, A. Anti-Inflammatory Activity of a Honey Flavonoid Extract on Lipopolysaccharide-Activated N13 Microglial Cells. J. Agric. Food Chem. 2012, 60, 12304–12311. [Google Scholar] [CrossRef]
- Chan, C.W.; Deadman, B.J.; Manley-Harris, M.; Wilkins, A.L.; Alber, D.G.; Harry, E. Analysis of the Flavonoid Component of Bioactive New Zealand mānuka (Leptospermum scoparium) Honey and the Isolation, Characterisation and Synthesis of an Unusual Pyrrole. Food Chem. 2013, 141, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of Honeys According to Their Content of Phenolic Compounds Using High Performance Liquid Chromatography/Tandem Mass Spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Georghiou, K.; Delipetrou, P. Patterns and Traits of the Endemic Plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–153. [Google Scholar] [CrossRef]
- Graikou, K.; Andreou, A.; Chinou, I. Chemical Profile of Greek Arbutus Unedo Honey: Biological Properties. J. Apic. Res. 2022, 61, 100–106. [Google Scholar] [CrossRef]
- Xagoraris, M.; Skouria, A.; Revelou, P.K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Response Surface Methodology to Optimize the Isolation of Dominant Volatile Compounds from Monofloral Greek Thyme Honey Using Spme-Gc-Ms. Molecules 2021, 26, 3612. [Google Scholar] [CrossRef]
- Tsavea, E.; Vardaka, F.P.; Savvidaki, E.; Kellil, A.; Kanelis, D.; Bucekova, M.; Grigorakis, S.; Godocikova, J.; Gotsiou, P.; Dimou, M.; et al. Physicochemical Characterization and Biological Properties of Pine Honey Produced across Greece. Foods 2022, 11, 943. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.L.P.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C.; et al. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography–High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Kleisiari, C.; Kleftodimos, G.; Vlontzos, G. Be(e)Ha(i)Viour(e): Assessment of Honey Consumption in Europe. Br. Food J. 2023, 125, 1374–1389. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Introductory Remarks. Oxidative Stress 1985, 5, 1–8. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Allan Butterfield, D.; Koppal, T.; Howard, B.; Subramaniam, R.; Hall, N.; Hensley, K.; Yatin, S.; Allen, K.; Aksenov, M.; Aksenova, M.; et al. Structural and Functional Changes in Proteins Induced by Free Radical-Mediated Oxidative Stress and Protective Action of the Antioxidants N-Tert-Butyl-Phenylnitrone and Vitamin E A. Ann. N. Y. Acad. Sci. 1998, 854, 448–462. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Bhatia, V.; Sharma, S. Role of Mitochondrial Dysfunction, Oxidative Stress and Autophagy in Progression of Alzheimer’s Disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of Oxidative Stress, Cellular Communication and Signaling Pathways in Cancer. Cell Commun. Signal. 2024, 22, 1–16. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Ramani, S.; Pathak, A.; Dalal, V.; Paul, A.; Biswas, S. Oxidative Stress in Autoimmune Diseases: An Under Dealt Malice. Curr. Protein Pept. Sci. 2020, 21, 611–621. [Google Scholar] [CrossRef]
- Dubois-deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H.; Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Dunnick, J.K.; Halley, J.R. Toxicity and Carcinogenicity Studies of Quercetin, a Natural Component of Foods. Toxicol. Sci. 1992, 19, 423–431. [Google Scholar] [CrossRef]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenol-Enriched Extracts of Prunus Spinosa Fruits: Anti-Inflammatory and Antioxidant Effects in Human Immune Cells Ex Vivo in Relation to Phytochemical Profile. Molecules 2022, 27, 1691. [Google Scholar] [CrossRef]
- Ntakoulas, D.D.; Pasias, I.N.; Raptopoulou, K.G.; Proestos, C. Authenticity of Greek Honey Based on Phenolic Compounds and Physicochemical Characteristics. Food Chem. 2025, 476, 143465. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Zaburaeva, H.S.; Daukaev, A.A.; Zaburaev, C.S.; Sedieva, M.B. Biodiversity as the Basis for Sustainable Development of Mountain Areas. In Proceedings of the International Scientific and Practical Conference “Agro-SMART-Smart Solutions for Agriculture” (Agro-SMART 2018), Tyumen, Russia, 16–20 July 2018. [Google Scholar] [CrossRef]
- Clement, H. Le Traite Rustica de La Apiculture; Editions Rustica/FLER: Saint Maur des Fossés, France, 2017; ISBN 978-618-5049-54-6. [Google Scholar]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and Antioxidant Activity of Different Types of Honey Derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Pakosz, P. Comparison of Antioxidant Properties and Color of Selected Polish Honeys and Manuka Honey. Foods 2024, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- Tananaki, C.; Rodopoulou, M.A.; Dimou, M.; Kanelis, D.; Liolios, V. The Total Phenolic Content and Antioxidant Activity of Nine Monofloral Honey Types. Appl. Sci. 2024, 14, 4329. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, P.T.; Bashir, S.M.; Rather, M.A.; Dar, K.B.; Taban, Q.; Sajood, S.; Ali, A.; Rather, Z.A.; Amin, I.; Dar, M.A. Antiproliferative and Apoptotic Activities of Natural Honey. Ther. Appl. Honey Its Phytochem. 2020, 1, 345–360. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and Cancer: A Mechanistic Review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Abd-Elhakim, Y.M.; Ismail, S.A.A. Involvement of the Anti-Inflammatory, Anti-Apoptotic, and Anti-Secretory Activity of Bee Venom in Its Therapeutic Effects on Acetylsalicylic Acid-Induced Gastric Ulceration in Rats. Toxicology 2019, 419, 11–23. [Google Scholar] [CrossRef]
- Othman, N.H. Honey and Cancer: Sustainable Inverse Relationship Particularly for Developing Nations—A Review. Evid. -Based Complement. Altern. Med. 2012, 2012, 410406. [Google Scholar]
- Andre, C.; Larondelle, Y.; Evers, D. Dietary Antioxidants and Oxidative Stress from a Human and Plant Perspective: A Review. Curr. Nutr. Food Sci. 2010, 6, 2–12. [Google Scholar] [CrossRef]
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by Quercetin in Doxorubicin-Treated Rats. Heliyon 2020, 6, e03803. [Google Scholar]
- Abdel-Latif, M.M.M.; Abouzied, M.M. Molecular Mechanisms of Natural Honey Against H. Pylori Infection Via Suppression of NF-ΚB and AP-1 Activation in Gastric Epithelial Cells. Arch. Med. Res. 2016, 47, 340–348. [Google Scholar] [CrossRef]
- Hashim, K.N.; Chin, K.Y.; Ahmad, F. The Mechanism of Honey in Reversing Metabolic Syndrome. Molecules 2021, 26, 808. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Othman, N.H. Honey as a Potential Natural Anticancer Agent: A Review of Its Mechanisms. Evid. -Based Complement. Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef]
- Gasparrini, M.; Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective Effects of Manuka Honey on LPS-Treated RAW 264.7 Macrophages. Part 2: Control of Oxidative Stress Induced Damage, Increase of Antioxidant Enzyme Activities and Attenuation of Inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 Signalling by Manuka Honey Protects Human Dermal Fibroblasts against Oxidative Damage by Improving Antioxidant Response and Mitochondrial Function Promoting Wound Healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An Overview of Physicochemical Characteristics and Health-Promoting Properties of Honeydew Honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ölmez, Ç. Some Qualitative Properties of Different Monofloral Honeys. Food Chem. 2014, 163, 212–218. [Google Scholar] [CrossRef]
- Starowicz, M.; Ostaszyk, A.; Zielí Nski, H.; Miguel, C.G. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967. [Google Scholar] [CrossRef]
- Patouna, A.; Vardakas, P.; Skaperda, Z.O.I.; Spandidos, D.A.; Kouretas, D. Evaluation of the Antioxidant Potency of Greek Honey from the Taygetos and Pindos Mountains Using a Combination of Cellular and Molecular Methods. Mol. Med. Rep. 2023, 27, 1–13. [Google Scholar] [CrossRef]
- Marco, G.D.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, A.; Canini, A. Plant Biosystems-An International Journal Dealing with All Aspects of Plant Biology Official Journal of the Societa Botanica Italiana Geographical, Botanical and Chemical Profile of Monofloral Italian Honeys as Food Quality Guarantee and Territory Brand. Plant Biosyst. Int. J. Deal. all Asp. Plant Biol. 2016, 151, 450–463. [Google Scholar] [CrossRef]
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Marco, G.D.; Fattorini, L.; Canini, A. From Robinia Pseudoacacia L. Nectar to Acacia Monofloral Honey: Biochemical Changes and Variation of Biological Properties. J. Sci. Food Agric. 2018, 98, 4312–4322. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geană, E.I. Phenolic Compounds Profile and Biochemical Properties of Honeys in Relationship to the Honey Floral Sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kaškonien, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical Quality Parameters, Antibacterial Properties and Cellular Antioxidant Activity of Polish Buckwheat Honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction Process, Safety Assessment, Toxicological Evaluations, and Regulatory Issues. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MD, USA, 2020; pp. 341–361. [Google Scholar] [CrossRef]
- Begolli, R.; Patouna, A.; Vardakas, P.; Xagara, A.; Apostolou, K.; Kouretas, D.; Giakountis, A. Deciphering the Landscape of GATA-Mediated Transcriptional Regulation in Gastric Cancer. Antioxidants 2024, 13, 1267. [Google Scholar] [CrossRef]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol Redox State (TRS) and Oxidative Stress in the Mouse Hippocampus after Pentylenetetrazol-Induced Epileptic Seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar]
- Vardakas, P.; Veskoukis, A.S.; Rossiou, D.; Gournikis, C.; Kapetanopoulou, T.; Karzi, V.; Docea, A.O.; Tsatsakis, A.; Kouretas, D. A Mixture of Endocrine Disruptors and the Pesticide Roundup® Induce Oxidative Stress in Rabbit Liver When Administered under the Long-Term Low-Dose Regimen: Reinforcing the Notion of Real-Life Risk Simulation. Toxics 2022, 10, 190. [Google Scholar] [CrossRef]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis the Primary Physiologic Role of Ceruloplasmin (Cp) Involves Plasma Redox Reactions. Ceruloplasmin Permits the Incorporation. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef]
- Vardakas, P.; Skaperda, Z.; Tekos, F.; Trompeta, A.F.; Tsatsakis, A.; Charitidis, C.A.; Kouretas, D. An Integrated Approach for Assessing the in Vitro and in Vivo Redox-Related Effects of Nanomaterials. Environ. Res. 2021, 197, 111083. [Google Scholar] [CrossRef]
- Patouna, A.; Sevdalis, P.; Papanikolaou, K.; Kourti, M.; Skaperda, Z.V.; Jamurtas, Z.A.K.D. Evaluation of the Effects of a Honey-based Gel on Blood Redox Biomarkers and the Physiological Profile of Healthy Adults: A Pilot Study. Available online: https://www.spandidos-publications.com/10.3892/br.2023.1614 (accessed on 25 March 2024).


| Honey Samples | TPC (mg GAE/100 g Honey) |
|---|---|
| Forest | 129.4 |
| Oak | 124 |
| Forest with oak honeydew | 115.4 |
| Flower (2) | 89.5 |
| Flower (1) | 86.1 |
| Eryngium creticum | 83.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patouna, A.; Tekos, F.; Charouli, M.; Vardakas, P.; Kouretas, D. Greek Raw Honey from Pindos Mountain Improves Redox Homeostasis of RAW264.7 Macrophages. Int. J. Mol. Sci. 2025, 26, 2868. https://doi.org/10.3390/ijms26072868
Patouna A, Tekos F, Charouli M, Vardakas P, Kouretas D. Greek Raw Honey from Pindos Mountain Improves Redox Homeostasis of RAW264.7 Macrophages. International Journal of Molecular Sciences. 2025; 26(7):2868. https://doi.org/10.3390/ijms26072868
Chicago/Turabian StylePatouna, Anastasia, Fotis Tekos, Myrto Charouli, Periklis Vardakas, and Demetrios Kouretas. 2025. "Greek Raw Honey from Pindos Mountain Improves Redox Homeostasis of RAW264.7 Macrophages" International Journal of Molecular Sciences 26, no. 7: 2868. https://doi.org/10.3390/ijms26072868
APA StylePatouna, A., Tekos, F., Charouli, M., Vardakas, P., & Kouretas, D. (2025). Greek Raw Honey from Pindos Mountain Improves Redox Homeostasis of RAW264.7 Macrophages. International Journal of Molecular Sciences, 26(7), 2868. https://doi.org/10.3390/ijms26072868







