Role of miRNAs in Bovine Oocyte Maturation and Reproductive Regulation
Abstract
1. Introduction
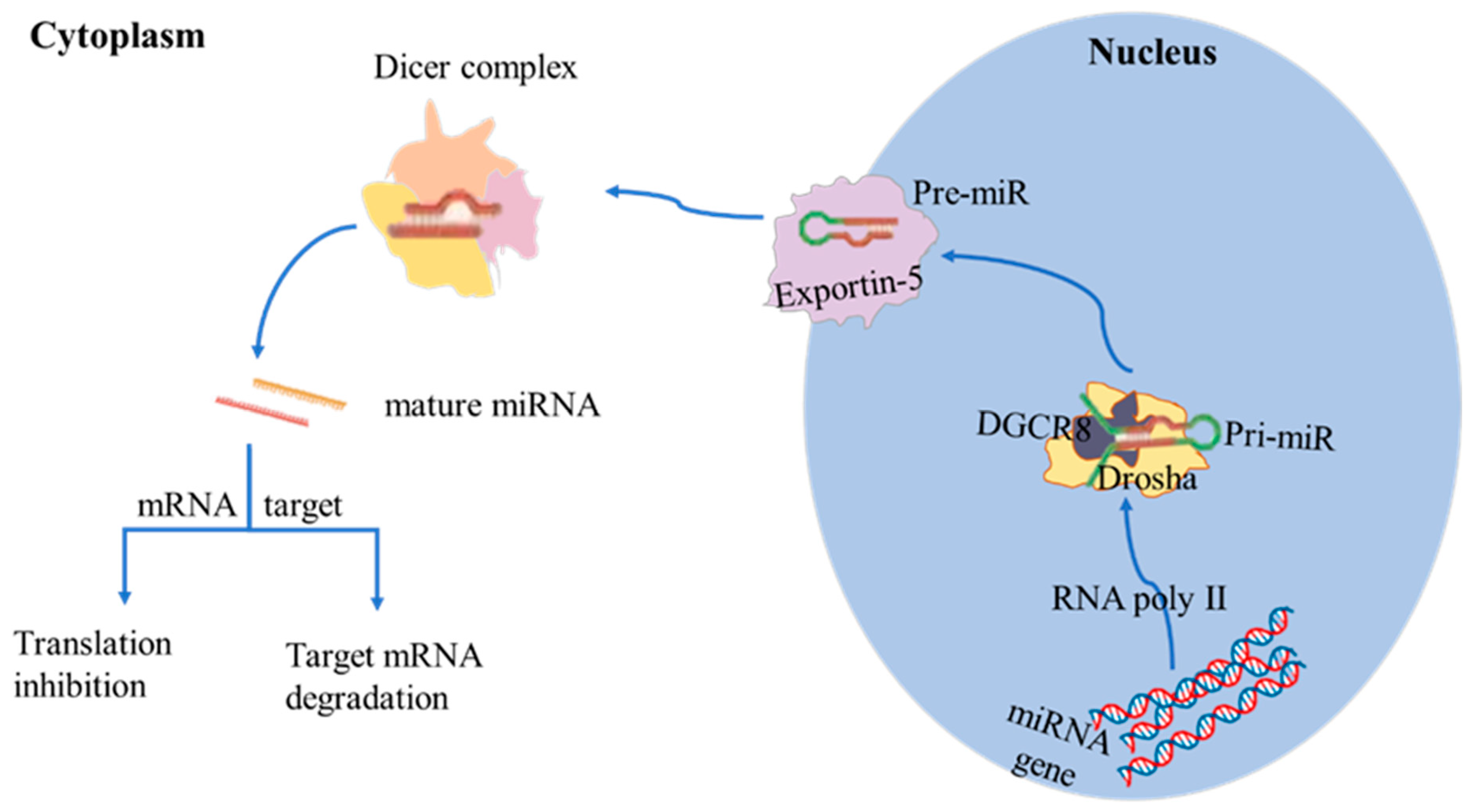
2. Biosynthesis of miRNAs
3. Regulation of miRNAs in Germ Cells
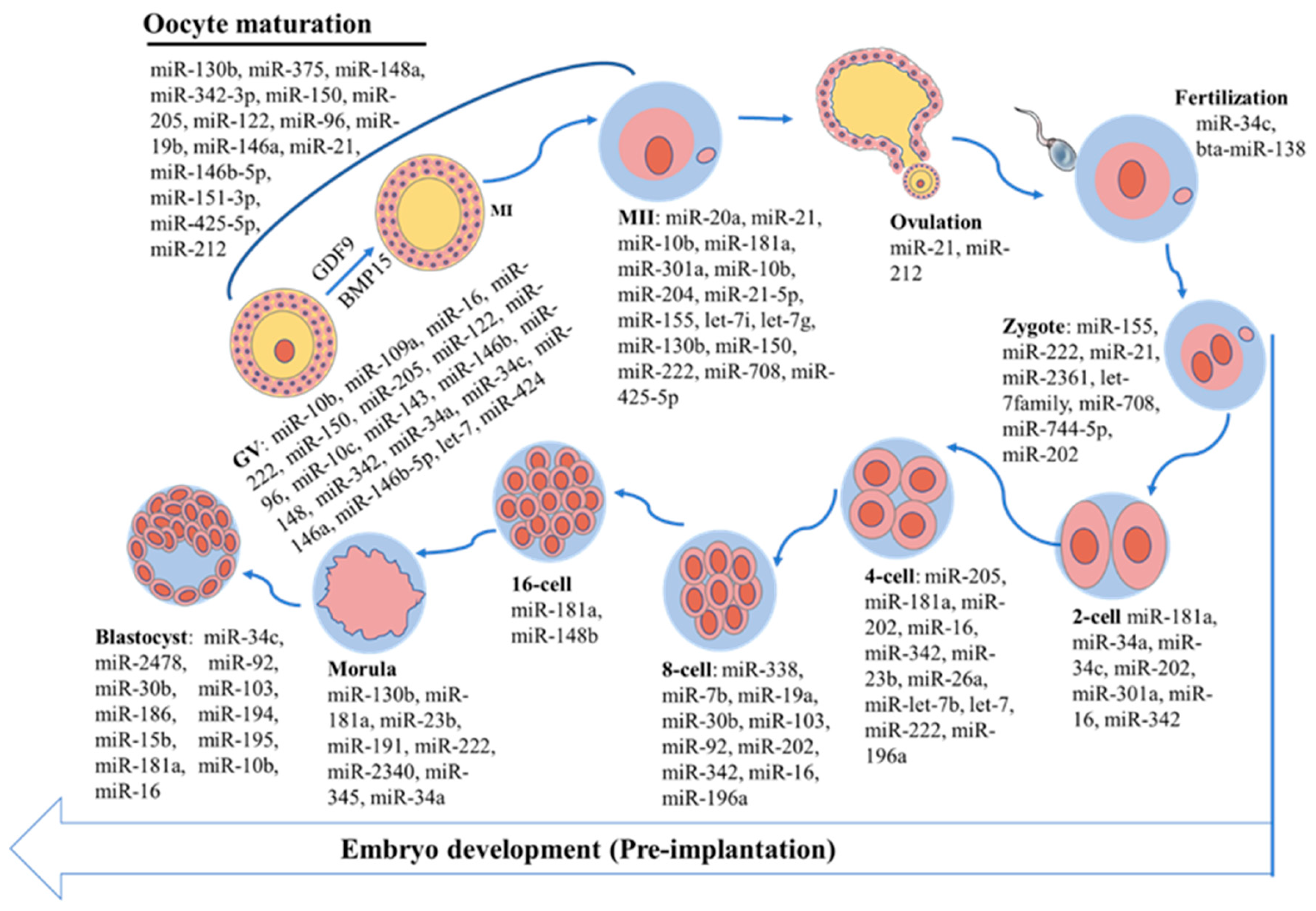
3.1. Expression Patterns of miRNAs During Bovine Oocyte Maturation
3.2. Role of miRNAs in Bovine Oocyte Maturation
3.3. Expression Patterns of miRNAs in Early Bovine Embryo Development
3.4. Role of miRNAs in Early Bovine Embryo Development
| MiRNAs | Function | References |
|---|---|---|
| bta-miR-183 | Regulates microvilli formation and improve early embryonic development by targeting EZRIN | [90] |
| miR-29b | Regulates the expression of Dnmt3a/3b and Dnmt1 in bovine SCNT embryos | [91] |
| miR-34c | Modulates blastocyst quality | [92] |
| bta-miR-301a | Influences cleavage time and blastocyst formation rate of early embryos by targeting ACVR1 | [93] |
| miR-151-3p, miR-425-5p | Improved embryonic development to the blastocyst stage. | [70] |
| miR-202 | Targets SEPT7 and regulates first cleavage of bovine embryos via cytoskeletal remodeling | [94] |
| miR-449b | Improves the first cleavage division, epigenetic reprogramming and apoptotic of SCNT embryos in bovine | [95] |
4. Potential Applications of miRNAs in Bovine ART
4.1. Improving Embryo Quality and Development
4.2. MiRNAs as Biomarkers in Bovine ART
4.3. Sex Determination
| MiRNAs | Function | Application | Species | References |
|---|---|---|---|---|
| bta-miR-140, bta-miR-92a, bta-miR-222, bta-miR-2285a | Negative association with blastocyst development | Improve embryonic development potential by adding miRNA inhibitors. | Bovine | [109] |
| miR-320a | High expression in high-quality embryo culture medium as a marker of embryo quality | For embryo quality assessment and selection | Human | [110] |
| miR-124 | miR-124 regulates Sox-9 gene affects embryo sex determination | MiRNA-mediated sex-regulation technology to improve sex-specific embryo productivity | Mice | [107] |
| miR-34c | Positive association with embryonic development | Use miR-34c as an indicator of IVF success | Human | [111] |
| miR-210 | Inhibit cell migration and trophoblast invasion and angiogenesis | Monitor pregnancy with pre-eclampsia. | Human | [112] |
| miR-155 | Negative association with embryonic development | Add miRNA-155 inhibitors to enhance embryonic development | Porcine | [113] |
| Let-7 family, miR-106a | relate genes for regulating oocyte maturation and embryo development | optimize conditions for oocyte maturation and embryo development | Bovine | [114] |
| miR-21 | Regulate cell proliferation and apoptosis, improve embryo survival rate | optimize embryo culture conditions and improving embryo quality | Bovine | [115] |
5. Application of miRNA in ART: Limitations and Solutions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Wee, G.; Zhang, K.; Folger, J.K.; Knott, J.G.; Smith, G.W. Functional role of the bovine oocyte-specific protein JY-1 in meiotic maturation, cumulus expansion, and subsequent embryonic development. Biol. Reprod. 2014, 90, 69. [Google Scholar] [CrossRef]
- Liang, L.; He, X. A narrative review of microRNA therapeutics: Understanding the future of microRNA research. Precis. Cancer Med. 2021, 4, 33. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Kropp, J.; Salih, S.M.; Khatib, H. Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front. Genet. 2014, 5, 91. [Google Scholar] [CrossRef]
- Cook, M.S.; Blelloch, R. Chapter Six -Small RNAs in germline development. Curr. Top. Dev. Biol. 2013, 102, 159–205. [Google Scholar]
- Dallaire, A.; Simard, M.J. The implication of microRNAs and endo-siRNAs in animal germline and early development. Dev. Biol. 2016, 416, 18–25. [Google Scholar] [CrossRef]
- Reza, A.; Choi, Y.J.; Han, S.G.; Song, H.; Park, C.; Hong, K.; Kim, J.H. Roles of microRNAs in mammalian reproduction: From the commitment of germ cells to peri-implantation embryos. Biol. Rev. Camb. Philos. Soc. 2019, 94, 415–438. [Google Scholar] [CrossRef]
- Tesfaye, D.; Worku, D.; Rings, F.; Phatsara, C.; Tholen, E.; Schellander, K.; Hoelker, M. Identification and expression profiling of microRNAs during bovine oocyte maturation using heterologous approach. Mol. Reprod. Dev. 2009, 76, 665–677. [Google Scholar] [CrossRef]
- Sohel, M.M.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and Non-Exosomal Transport of Extra-Cellular microRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef]
- Ma, T.; Jiang, H.; Gao, Y.; Zhao, Y.; Dai, L.; Xiong, Q.; Xu, Y.; Zhao, Z.; Zhang, J. Microarray analysis of differentially expressed microRNAs in non-regressed and regressed bovine corpus luteum tissue; microRNA-378 may suppress luteal cell apoptosis by targeting the interferon gamma receptor 1 gene. J. Appl. Genet. 2011, 52, 481–486. [Google Scholar] [CrossRef]
- Zielak-Steciwko, A.E.; Browne, J.A.; Mcgettigan, P.A.; Gajewska, M.; Dzięcioł, M.; Szulc, T.; Evans, A.C. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol. Genom. 2014, 46, 735–745. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Salilew-Wondim, D.; Ahmad, I.; Sahadevan, S.; Hossain, M.M.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Looft, C.; et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS ONE 2015, 10, e0125912. [Google Scholar] [CrossRef]
- Hossain, M.M.; Salilew-Wondim, D.; Schellander, K.; Tesfaye, D. The role of microRNAs in mammalian oocytes and embryos. Anim. Reprod. Sci. 2012, 134, 36–44. [Google Scholar] [CrossRef]
- Abd EL Naby, W.S.; Hagos, T.H.; Hossain, M.M.; Salilew-Wondim, D.; Gad, A.; Rings, F.; Cinar, M.U.; Tholen, E.; Looft, C.; Schellander, K.; et al. Expression analysis of regulatory microRNAs in bovine cumulus oocyte complex and preimplantation embryos. Zygote 2013, 21, 31–51. [Google Scholar] [CrossRef]
- Mondou, E.; Dufort, I.; Gohin, M.; Fournier, E.; Sirard, M.A. Analysis of microRNAs and their precursors in bovine early embryonic development. Mol. Hum. Reprod. 2012, 18, 425–434. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- De Rooij, L.A.; Mastebroek, D.J.; Ten Voorde, N.; Van Der Wall, E.; Van Diest, P.J.; Moelans, C.B. The microRNA Lifecycle in Health and Cancer. Cancers 2022, 14, 5748. [Google Scholar] [CrossRef]
- Alexandri, C.; Daniel, A.; Bruylants, G.; Demeestere, I. The role of microRNAs in ovarian function and the transition toward novel therapeutic strategies in fertility preservation: From bench to future clinical application. Hum. Reprod. Update 2020, 26, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Cairns, M.J. Identifying miRNAs, targets and functions. Brief. Bioinform. 2014, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Chen, J.; Han, C. In vivo functions of miRNAs in mammalian spermatogenesis. Front. Cell Dev. Biol. 2023, 11, 1154938. [Google Scholar] [CrossRef]
- Walker, W.H. Regulation of mammalian spermatogenesis by miRNAs. Semin. Cell Dev. Biol. 2022, 121, 24–31. [Google Scholar] [CrossRef]
- Marcon, E.; Babak, T.; Chua, G.; Hughes, T.; Moens, P.B. MiRNA and piRNA localization in the male mammalian meiotic nucleus. Chromosome Res. 2008, 16, 243–260. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, D.; Gao, J.; Dou, X.; Cheng, P.; Peng, D.; Zhang, Y.; Mao, J.; Zhang, L.; Zhang, X. MiR-34c disrupts spermatogonial stem cell homeostasis in cryptorchid testes by targeting Nanos2. Reprod. Biol. Endocrinol. 2018, 16, 97. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Mielnik, A.; Robinson, B.D.; Wosnttzer, M.S.; Schlegel, P.N.; Paduch, D.A. Possible germ cell-sertoli cell interactions are critical for establishing appropriate expression levels for the Sertoli cell-specific MicroRNA, miR-202-5p, in human testis. Basic Clin. Androl. 2015, 25, 2. [Google Scholar] [CrossRef]
- Menezes, E.S.B.; Badial, P.R.; EL Debaky, H.; Husna, A.U.; Ugur, M.R.; Kaya, A.; Topper, E.; Bulla, C.; Grant, K.E.; Bolden-Tiller, O.; et al. Sperm miR-15a and miR-29b are associated with bull fertility. Andrologia 2020, 52, e13412. [Google Scholar] [CrossRef]
- Gao, H.; Wen, H.; Cao, C.; Dong, D.; Yang, C.; Xie, S.; Zhang, J.; Huang, X.; Huang, X.; Yuan, S.; et al. Overexpression of microRNA-10a in germ cells causes male infertility by targeting rad51 in mouse and human. Front. Physiol. 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Di Giacomo, M.; Rasmussen, K.D.; Much, C.; Azzi, C.; Perlas, E.; Morgan, M.; O’Carroll, D. Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genet. 2014, 10, e1004597. [Google Scholar] [CrossRef]
- Guo, W.; Xie, B.; Xiong, S.; Liang, X.; Gui, J.F.; Mei, J. MiR-34a regulates sperm motility in zebrafish. Int. J. Mol. Sci. 2017, 18, 2676. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Nadri, P.; Nadri, T.; Gholami, D.; Zahmatkesh, A.; Hosseini Ghaffari, M.; Savvulidi Vargova, K.; Georgijevic Savvulidi, F.; LaMarre, J. Role of miRNAs in assisted reproductive technology. Gene 2024, 927, 148703. [Google Scholar] [CrossRef] [PubMed]
- Siristatidis, C.; Vogiatzi, P.; Brachnis, N.; Liassidou, A.; Iliodromiti, Z.; Bettocchi, S.; Chrelias, C. Review: MicroRNAs in assisted reproduction and their potential role in IVF failure. In Vivo 2015, 29, 169–175. [Google Scholar]
- Gross, N.; Kropp, J.; Khatib, H. MicroRNA Signaling in Embryo Development. Biology 2017, 6, 34. [Google Scholar] [CrossRef]
- Nagaraja, A.K.; Andreu-Vieyra, C.; Franco, H.L.; Ma, L.; Chen, R.; Han, D.Y.; Zhu, H.; Agno, J.E.; Gunaratne, P.H.; DeMayo, F.J.; et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol. Endocrinol. 2008, 22, 2336–2352. [Google Scholar] [CrossRef]
- Murchison, E.P.; Stein, P.; Xuan, Z.; Pan, H.; Zhang, M.Q.; Schultz, R.M.; Hannon, G.J. Critical roles for Dicer in the female germline. Genes Dev. 2007, 21, 682–693. [Google Scholar] [CrossRef]
- Labrecque, R.; Sirard, M.A. The study of mammalian oocyte competence by transcriptome analysis: Progress and challenges. Mol. Hum. Reprod. 2014, 20, 103–116. [Google Scholar] [CrossRef]
- Fair, T.; Carter, F.; Park, S.; Evans, A.C.; Lonergan, P. Global gene expression analysis during bovine oocyte in vitro maturation. Theriogenology 2007, 68 (Suppl. 1), S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, H.; Lin, S.; Zhu, X.; Shen, Z.; Lu, G.; Poon, W.S.; Xie, D.; Lin, M.C.; Kung, H.F. Transcriptional and epigenetic regulation of human microRNAs. Cancer Lett. 2013, 331, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Six, R.; Benedetti, C.; Fan, Y.; Guan, X.; Gansemans, Y.; Hedia, M.; Bogado Pascottini, O.; Pavani, K.C.; Van Nieuwerburgh, F.; Deforce, D.; et al. Expression profile and gap-junctional transfer of microRNAs in the bovine cumulus-oocyte complex. Front. Cell Dev. Biol. 2024, 12, 1404675. [Google Scholar] [CrossRef] [PubMed]
- Fair, T. Follicular oocyte growth and acquisition of developmental competence. Anim. Reprod. Sci. 2003, 78, 203–216. [Google Scholar] [CrossRef]
- Xiong, X.R.; Lan, D.L.; Li, J.; Zi, X.D.; Li, M.Y. Identification of candidate miRNAs and expression profile of yak oocytes before and after in vitro maturation by high-throughput sequencing. Reprod. Domest. Anim. 2016, 51, 886–894. [Google Scholar] [CrossRef]
- Gilchrist, G.C.; Tscherner, A.; Nalpathamkalam, T.; Merico, D.; Lamarre, J. MicroRNA Expression during Bovine Oocyte Maturation and Fertilization. Int. J. Mol. Sci. 2016, 17, 396. [Google Scholar] [CrossRef]
- Eppig, J.J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 485–489. [Google Scholar] [CrossRef]
- Andreas, E.; Pandey, H.O.; Hoelker, M.; Salilew-Wondim, D.; Gebremedhns, S.; Schellander, K.; Tesfaye, D. The regulatory role of miR-20a in bovine cumulus cells and its contribution to oocyte maturation. Zygote 2021, 29, 435–444. [Google Scholar] [CrossRef]
- Sinha, P.B.; Tesfaye, D.; Rings, F.; Hossien, M.; Hoelker, M.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Salilew-Wondim, D. MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation. J. Ovarian Res. 2017, 10, 37. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, M.; Yu, H.; Hu, Y.; Yang, L.; Zhu, Y.; Fei, X.; Pan, B.; Xiong, Y.; Fu, W.; et al. MicroRNA-342-3p regulates yak oocyte meiotic maturation by targeting DNA methyltransferase 1. Reprod. Domest. Anim. 2022, 57, 761–770. [Google Scholar] [CrossRef] [PubMed]
- O’connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Tagawa, H.; Takahashi, N.; Watanabe, A.; Guo, Y.-M.; Iwamoto, K.; Yamashita, J.; Saitoh, H.; Kameoka, Y.; Shimizu, N.; et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood 2009, 114, 3265–3275. [Google Scholar] [CrossRef] [PubMed]
- Tomek, W.; Smiljakovic, T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reproduction 2005, 130, 423–430. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, B.; Chen, H.; Xu, M.; Sun, X.; Xu, J.; Gao, Y.; Chen, C.; Jiang, H.; Zhang, J. Effects of MiR-375-BMPR2 as a Key Factor Downstream of BMP15/GDF9 on the Smad1/5/8 and Smad2/3 Signaling Pathways. Cell Physiol. Biochem. 2018, 46, 213–225. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, Y.; Shen, C.; Zhang, L.; Wang, X. MicroRNA-375 regulates oocyte in vitro maturation by targeting ADAMTS1 and PGR in bovine cumulus cells. Biomed. Pharmacother. 2019, 118, 109350. [Google Scholar] [CrossRef]
- Tscherner, A.; Gilchbist, G.; Smith, N.; Blondin, P.; Gillis, D.; Lamarre, J. MicroRNA-34 family expression in bovine gametes and preimplantation embryos. Reprod. Biol. Endocrinol. 2014, 12, 85. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zheng, Y.; Zhao, G.; Jiang, H.; Yuan, B. miR-302d Targeting of CDKN1A Regulates DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells. Genes 2023, 14, 2195. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Jiang, H.; Gao, Y.; Xu, M.; Wang, J.; Liu, S.; Fu, Y.; Sun, X.; Xu, J.; et al. Regulatory Role of miRNA-375 in Expression of BMP15/GDF9 Receptors and its Effect on Proliferation and Apoptosis of Bovine Cumulus Cells. Cell Physiol. Biochem. 2017, 41, 439–450. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Y.; Tang, X.; Gao, H.; Liu, N.; Gao, Y.; Hao, L.; Liu, S.; Jiang, Z. MiR-21-3p inhibits autophagy of bovine granulosa cells by targeting VEGFA via PI3K/AKT signaling. Reproduction 2019, 158, 441–452. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Salilew-Wondim, D.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D. MicroRNA-183-96-182 Cluster Regulates Bovine Granulosa Cell Proliferation and Cell Cycle Transition by Coordinately Targeting FOXO1. Biol. Reprod. 2016, 94, 127. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Yunqi, Z.; Deji, L.; Jian, K.; Fusheng, Q. Follicular fluid HD-sevs-mir-128-3p is a key molecule in regulating bovine granulosa cells autophagy. Theriogenology 2024, 226, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Andreas, E.; Hoelker, M.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D.; Salilew-Wondim, D. MicroRNA 17-92 cluster regulates proliferation and differentiation of bovine granulosa cells by targeting PTEN and BMPR2 genes. Cell Tissue Res. 2016, 366, 219–230. [Google Scholar] [CrossRef]
- Han, X.; Xue, R.; Yuan, H.-J.; Wang, T.-Y.; Lin, J.; Zhang, J.; Liang, B.; Tan, J.H. MicroRNA-21 plays a pivotal role in the oocyte-secreted factor-induced suppression of cumulus cell apoptosis. Biol. Reprod. 2017, 96, 1167–1180. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, J.; Sizhu, S.; Li, B.; Chen, Y.; Li, R.; Yangzong, Q.; Li, Q.; Xu, Y. MicroRNA-125b Regulates Apoptosis by Targeting Bone Morphogenetic Protein Receptor 1B in Yak Granulosa Cells. DNA Cell Biol. 2018, 37, 878–887. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.Z.; Xu, M.Q.; Zhang, L.Q.; Liu, J.B.; Gao, Y.; Jiang, H.; Yuan, B.; Zhang, J.-B. MiR-31 and miR-143 affect steroid hormone synthesis and inhibit cell apoptosis in bovine granulosa cells through FSHR. Theriogenology 2019, 123, 45–53. [Google Scholar] [CrossRef]
- Pande, H.O.; Tesfaye, D.; Hoelker, M.; Gebremedhn, S.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Wondim, D.S. MicroRNA-424/503 cluster members regulate bovine granulosa cell proliferation and cell cycle progression by targeting SMAD7 gene through activin signalling pathway. J. Ovarian Res. 2018, 11, 34. [Google Scholar] [CrossRef]
- McBride, D.; Carré, W.; Sontakke, S.D.; Hogg, C.O.; Law, A.; Donadeu, F.X.; Clinton, M. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction 2012, 144, 221–233. [Google Scholar] [CrossRef]
- Tripurani, S.K.; Xiao, C.; Salem, M.; Yao, J. Cloning and analysis of fetal ovary microRNAs in cattle. Anim. Reprod. Sci. 2010, 120, 16–22. [Google Scholar] [CrossRef]
- Aoki, S.; Inoue, Y.; Hara, S.; Itou, J.; Shirasuna, K.; Iwata, H. microRNAs associated with the quality of follicular fluids affect oocyte and early embryonic development. Reprod. Med. Biol. 2024, 23, e12559. [Google Scholar] [CrossRef]
- Tripurani, S.K.; Wee, G.; Lee, K.B.; Smith, G.W.; Wang, L.; Yao, J. MicroRNA-212 post-transcriptionally regulates oocyte-specific basic-helix-loop-helix transcription factor, factor in the germline alpha (FIGLA), during bovine early embryogenesis. PLoS ONE 2013, 8, e76114. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Inoue, Y.; Sato, T.; Tanaka, K.; Shinozawa, A.; Shirasuna, K.; Iwata, H. Age-associated changes in miRNA profile of bovine follicular fluid. Reproduction 2022, 164, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Bogliotti, Y.S.; Chung, N.; Paulson, E.E.; Chitwood, J.; Halstead, M.; Kern, C.; Schultz, R.M.; Ross, P.J. Transcript profiling of bovine embryos implicates specific transcription factors in the maternal-to-embryo transition. Biol. Reprod. 2020, 102, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, F.V.; Caetano, A.R.; Watanabe, Y.F.; Ripamonte, P.; Carambula, S.F.; Merighe, G.K.; Garcia, S.M. Genome activation and developmental block in bovine embryos. Anim. Reprod. Sci. 2004, 82–83, 13–20. [Google Scholar] [CrossRef]
- Bilodeau-Goeseels, S.; Schultz, G.A. Changes in ribosomal ribonucleic acid content within in vitro-produced bovine embryos. Biol. Reprod. 1997, 56, 1323–1329. [Google Scholar] [CrossRef]
- Kues, W.A.; Sudheer, S.; Herrmann, D.; Carnwath, J.W.; Havlicek, V.; Besenfelder, U.; Lehrach, H.; Adjaye, J.; Niemann, H. Genome-wide expression profiling reveals distinct clusters of transcriptional regulation during bovine preimplantation development in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 19768–19773. [Google Scholar] [CrossRef]
- Paulson, E.E.; Fishman, E.L.; Schultz, R.M.; Ross, P.J. Embryonic microRNAs are essential for bovine preimplantation embryo development. Proc. Natl. Acad. Sci. USA 2022, 119, e2212942119. [Google Scholar] [CrossRef]
- Lingenfelter, B.M.; Tripurani, S.K.; Tejomurtula, J.; Smith, G.W.; Yao, J. Molecular cloning and expression of bovine nucleoplasmin 2 (NPM2): A maternal effect gene regulated by miR-181a. Reprod. Biol. Endocrinol. 2011, 9, 40. [Google Scholar] [CrossRef]
- Goossens, K.; Mestdagh, P.; Lefever, S.; Van Poucke, M.; Van Zeveren, A.; Van Soom, A.; Vandesompele, J.; Peelman, L. Regulatory microRNA network identification in bovine blastocyst development. Stem Cells Dev. 2013, 22, 1907–1920. [Google Scholar] [CrossRef]
- Flynt, A.S.; Lai, E.C. Biological principles of microRNA-mediated regulation: Shared themes amid diversity. Nat. Rev. Genet. 2008, 9, 831–842. [Google Scholar] [CrossRef]
- Siomi, H.; Siomi, M.C. On the road to reading the RNA-interference code. Nature 2009, 457, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Clarkin, K.C.; Wahl, G.M. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc. Natl. Acad. Sci. USA 1996, 93, 4827–4832. [Google Scholar] [CrossRef] [PubMed]
- Matwee, C.; Betts, D.H.; King, W.A. Apoptosis in the early bovine embryo. Zygote 2000, 8, 57–68. [Google Scholar] [CrossRef]
- Guan, X.; Fan, Y.; Six, R.; Benedetti, C.; Raes, A.; Fernandez Montoro, A.; Cui, X.; Azari Dolatabad, N.; Van Soom, A.; Pavani, K.C.; et al. Bta-miR-665 improves bovine blastocyst development through its influence on microtubule dynamics and apoptosis. Front. Genet. 2024, 15, 1437695. [Google Scholar] [CrossRef] [PubMed]
- Cañón-Beltrán, K.; Cajas, Y.N.; Almpanis, V.; Egido, S.G.; Gutierrez-Adan, A.; González, E.M.; Rizos, D. MicroRNA-148b secreted by bovine oviductal extracellular vesicles enhance embryo quality through BPM/TGF-beta pathway. Biol. Res. 2024, 57, 11. [Google Scholar] [CrossRef]
- Aoki, S.; Inoue, Y.; Shinozawa, A.; Tanaka, K.; Shirasuna, K.; Iwata, H. MiR-17-5p in bovine oviductal fluid affects embryo development. Mol. Cell Endocrinol. 2022, 551, 111651. [Google Scholar] [CrossRef]
- Mazzarella, R. Pregnancy Effects in the Bovine Oviduct During Early Embryonic Development In Vivo: Modulation of the MicroRNA Profile in Small Extracellular Vesicles of the Oviductal Fluid and Oviductal Epithelial Cells; Universidade de São Paulo: São Paulo, Brazil, 2021. [Google Scholar]
- Pavani, K.C.; Xuefeng, G.; Chunduru, J.; Meese, T.; Peelman, L.J.; Van Nieuwerburgh, F.; Deforce, D.; Hendrix, A.; Tilleman, K.; Van Soom, A.; et al. MicroRNA-146b negatively affects bovine embryo development and quality. Reproduction 2023, 167, e230155. [Google Scholar] [CrossRef]
- Kropp, J.; Khatib, H. Characterization of microRNA in bovine in vitro culture media associated with embryo quality and development. J. Dairy Sci. 2015, 98, 6552–6563. [Google Scholar] [CrossRef]
- Wu, Y.; Zuo, Z.; Wang, Z.; Liu, H.; Zhou, Q.; Ren, S.; Lan, X.; Zhang, Y.; Wang, Y. Bta-miR-183 targets EZRIN to regulate microvilli formation and improve early development of bovine embryos. Reproduction 2023, 165, 363–371. [Google Scholar] [CrossRef]
- Liang, S.; Nie, Z.W.; Guo, J.; Niu, Y.J.; Shin, K.T.; Ock, S.A.; Cui, X.S. Overexpression of MicroRNA-29b Decreases Expression of DNA Methyltransferases and Improves Quality of the Blastocysts Derived from Somatic Cell Nuclear Transfer in Cattle. Microsc. Microanal. 2018, 24, 29–37. [Google Scholar] [CrossRef]
- Benedetti, C.; Pavani, K.C.; Gansemans, Y.; Azari-Dolatabad, N.; Pascottini, O.B.; Peelman, L.; Six, R.; Fan, Y.; Guan, X.; Deserranno, K.; et al. From follicle to blastocyst: MicroRNA-34c from follicular fluid-derived extracellular vesicles modulates blastocyst quality. J. Anim. Sci. Biotechnol. 2024, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, J.; Wang, J.; Zhao, B.; Ma, X.; Zhang, B.; Lv, C.; Qiao, H.; Wang, Y.; Qing, S. Bta-miR-301a targets ACVR1 to influence cleavage time and blastocyst formation rate of early embryos in cattle. Biol. Reprod. 2024, 110, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Du, Y.; Gao, S.; Wang, Z.; Qu, P.; Gao, Y.; Wang, J.; Liu, Z.; Zhang, J.; Zhang, Y.; et al. Sperm-borne miR-202 targets SEPT7 and regulates first cleavage of bovine embryos via cytoskeletal remodeling. Development 2021, 148, dev189670. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Y.; Qu, P.; Qing, S.; Qiao, F.; Zhang, Y.; Mager, J.; Wang, Y. Sperm-borne miR-449b influences cleavage, epigenetic reprogramming and apoptosis of SCNT embryos in bovine. Sci. Rep. 2017, 7, 13403. [Google Scholar] [CrossRef]
- Rizos, D.; Maillo, V.; Sánchez-Calabuig, M.J.; Lonergan, P. The Consequences of Maternal-Embryonic Cross Talk During the Periconception Period on Subsequent Embryonic Development. Adv. Exp. Med. Biol. 2017, 1014, 69–86. [Google Scholar]
- Lonergan, P.; Rizos, D.; Gutiérrez-Adán, A.; Fair, T.; Boland, M.P. Effect of culture environment on embryo quality and gene expression—Experience from animal studies. Reprod. Biomed. Online 2003, 7, 657–663. [Google Scholar] [CrossRef]
- Hansen, P.J. The incompletely fulfilled promise of embryo transfer in cattle-why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef]
- Schmaltz-Panneau, B.; Cordova, A.; Dhorne-Pollet, S.; Hennequet-Antier, C.; Uzbekova, S.; Martinot, E.; Doret, S.; Martin, P.; Mermillod, P.; Locatelli, Y. Early bovine embryos regulate oviduct epithelial cell gene expression during in vitro co-culture. Anim. Reprod. Sci. 2014, 149, 103–116. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, B.; Sánchez, J.M.; González, E.; Lonergan, P.; Rizos, D. Challenges in studying preimplantation embryo-maternal interaction in cattle. Theriogenology 2020, 150, 139–149. [Google Scholar] [CrossRef]
- Kamijo, S.; Hamatani, T.; Sasaki, H.; Suzuki, H.; Abe, A.; Inoue, O.; Iwai, M.; Ogawa, S.; Odawara, K.; Tanaka, K.; et al. MicroRNAs secreted by human preimplantation embryos and IVF outcome. Reprod. Biol. Endocrinol. 2022, 20, 130. [Google Scholar] [CrossRef]
- Lin, X.; Beckers, E.; Mc Cafferty, S.; Gansemans, Y.; Joanna SzymaŃska, K.; Chaitanya Pavani, K.; Catani, J.P.; Van Nieuwerburgh, F.; Deforce, D.; De Sutter, P.; et al. Bovine Embryo-Secreted microRNA-30c Is a Potential Non-invasive Biomarker for Hampered Preimplantation Developmental Competence. Front. Genet. 2019, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.R.; Kasimanickam, R.K. Differentially Expressed Candidate miRNAs of Day 16 Bovine Embryos on the Regulation of Pregnancy Establishment in Dairy Cows. Animals 2023, 13, 3052. [Google Scholar] [CrossRef] [PubMed]
- Klees, C.; Alexandri, C.; Demeestere, I.; Lybaert, P. The Role of microRNA in Spermatogenesis: Is There a Place for Fertility Preservation Innovation? Int. J. Mol. Sci. 2023, 25, 460. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.W.; Mccallie, B.; Alvero, R.; Schoolcraft, W.B.; Minjarez, D.; Katz-Jaffe, M.G. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2014, 31, 355–362. [Google Scholar] [CrossRef]
- Eggers, S.; Ohnesorg, T.; Sinclair, A. Genetic regulation of mammalian gonad development. Nat. Rev. Endocrinol. 2014, 10, 673–683. [Google Scholar] [CrossRef]
- Sontakke, S.D.; Mohammed, B.T.; Mcneilly, A.S.; Donadeu, F.X. Characterization of microRNAs differentially expressed during bovine follicle development. Reproduction 2014, 148, 271–283. [Google Scholar] [CrossRef]
- Melo-Baez, B.; Wong, Y.S.; Aguilera, C.J.; Cabezas, J.; Mançanares, A.C.F.; Riadi, G.; Castro, F.O.; Rodriguez-Alvarez, L. MicroRNAs from extracellular vesicles secreted by bovine embryos as early biomarkers of developmental competence. Int. J. Mol. Sci. 2020, 21, 8888. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Keijser, R.; Repping, S.; Lambalk, C.B.; Afink, G.B.; Mastenbroek, S.; Hamer, G. High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a. Hum. Reprod. 2020, 35, 1797–1807. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, M.; Guo, T.; Sui, Y.; Tian, Z.; Ni, X.; Chen, X.; Jiang, M.; Jiang, J.; Lu, Y.; et al. MicroRNAs in spermatogenesis dysfunction and male infertility: Clinical phenotypes, mechanisms and potential diagnostic biomarkers. Front. Endocrinol. 2024, 15, 1293368. [Google Scholar] [CrossRef]
- Jaszczuk, I.; Koczkodaj, D.; Kondracka, A.; Kwaśniewska, A.; Winkler, I.; Filip, A. The role of miRNA-210 in pre-eclampsia development. Ann. Med. 2022, 54, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Tanga, B.M.; Fang, X.; Bang, S.; Seong, G.; De Zoysa, M.; Saadeldin, I.M.; Lee, S.; Cho, J. MiRNA-155 inhibition enhances porcine embryo preimplantation developmental competence by upregulating ZEB2 and downregulating ATF4. Theriogenology 2022, 183, 90–97. [Google Scholar] [CrossRef]
- Miles, J.R.; Mcdaneld, T.G.; Wiedmann, R.T.; Cushman, R.A.; Echternkamp, S.E.; Vallet, J.L.; Smith, T.P. MicroRNA expression profile in bovine cumulus-oocyte complexes: Possible role of let-7 and miR-106a in the development of bovine oocytes. Anim. Reprod. Sci. 2012, 130, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Ghanem, N.; Hoelker, M.; Rings, F.; Phatsara, C.; Tholen, E.; Schellander, K.; Tesfaye, D. Identification and characterization of miRNAs expressed in the bovine ovary. BMC Genom. 2009, 10, 443. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T.; Yeste, M. The expression of miRNAs in human ovaries, oocytes, extracellular vesicles, and early embryos: A Systematic Review. Cells 2019, 8, 1564. [Google Scholar] [CrossRef]
- Toporcerová, S.; Špaková, I.; Šoltys, K.; Klepcová, Z.; Kľoc, M.; Bohošová, J.; Trachtová, K.; Peterová, L.; Mičková, H.; Urdzík, P.; et al. Small non-coding RNAs as new biomarkers to evaluate the quality of the embryo in the IVF process. Biomolecules 2022, 12, 1687. [Google Scholar] [CrossRef]
- Fang, F.; Li, Z.; Yu, J.; Long, Y.; Zhao, Q.; Ding, X.; Wu, L.; Shao, S.; Zhang, L.; Xiang, W. MicroRNAs secreted by human embryos could be potential biomarkers for clinical outcomes of assisted reproductive technology. J. Adv. Res. 2021, 31, 25–34. [Google Scholar] [CrossRef]
| MiRNAs | Function | References |
|---|---|---|
| miR-302d | Regulates DNA damage and steroid hormone secretion in bovine cumulus cells by targeting CDKN1A | [58] |
| miR-375 | Proliferation and apoptosis in cumulus cells and oocyte maturation | [56,59] |
| miR-21-3p | Inhibits bovine granulosa cell autophagy | [60] |
| miR-183-96-182 cluster | Promote bovine granulosa cells proliferation and cell cycle transition | [61] |
| miR-128-3p | Targets TFEB and FoxO4 and activates bovine granulosa cells autophagy | [62] |
| mir-17-92 cluster | regulates proliferation and differentiation of bovine granulosa cells | [63] |
| miR-21 | Prevented apoptosis via the PI3K/Akt signaling in bovine cumulus cells | [64] |
| miR-125b | Regulates apoptosis by targeting BMPR1B in yak granulosa cells | [65] |
| miR-31 miR-143 | Regulate steroid hormone synthesis and inhibit cell apoptosis in granulosa cells by targeting FSHR gene | [66] |
| miR-424/503 | Bovine granulosa cell proliferation and cell cycle progression | [67] |
| miR-145, miR-125b, miR-199a-3p | Involved in follicle-luteal transition | [68] |
| miR-424, miR-10b | Abundant in GV oocytes’ role in zygotic genome activation | [69] |
| miR-212, miR-151-3p, miR-425-5p | Regulation of oocyte maturation | [70,71] |
| miR-19b | increased the diameter, acetylation levels, and fertilization ability of the oocytes | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; He, H.; Wang, P.; Wang, Y.; Wang, L.; Yang, F.; Li, J.; Zhang, H. Role of miRNAs in Bovine Oocyte Maturation and Reproductive Regulation. Int. J. Mol. Sci. 2025, 26, 2828. https://doi.org/10.3390/ijms26072828
Yang X, He H, Wang P, Wang Y, Wang L, Yang F, Li J, Zhang H. Role of miRNAs in Bovine Oocyte Maturation and Reproductive Regulation. International Journal of Molecular Sciences. 2025; 26(7):2828. https://doi.org/10.3390/ijms26072828
Chicago/Turabian StyleYang, Xiaogeng, Honghong He, Peng Wang, Yaying Wang, Linlin Wang, Falong Yang, Jian Li, and Huizhu Zhang. 2025. "Role of miRNAs in Bovine Oocyte Maturation and Reproductive Regulation" International Journal of Molecular Sciences 26, no. 7: 2828. https://doi.org/10.3390/ijms26072828
APA StyleYang, X., He, H., Wang, P., Wang, Y., Wang, L., Yang, F., Li, J., & Zhang, H. (2025). Role of miRNAs in Bovine Oocyte Maturation and Reproductive Regulation. International Journal of Molecular Sciences, 26(7), 2828. https://doi.org/10.3390/ijms26072828






