StTCTP Positively Regulates StSN2 to Enhance Drought Stress Tolerance in Potato by Scavenging Reactive Oxygen Species
Abstract
:1. Introduction
2. Results
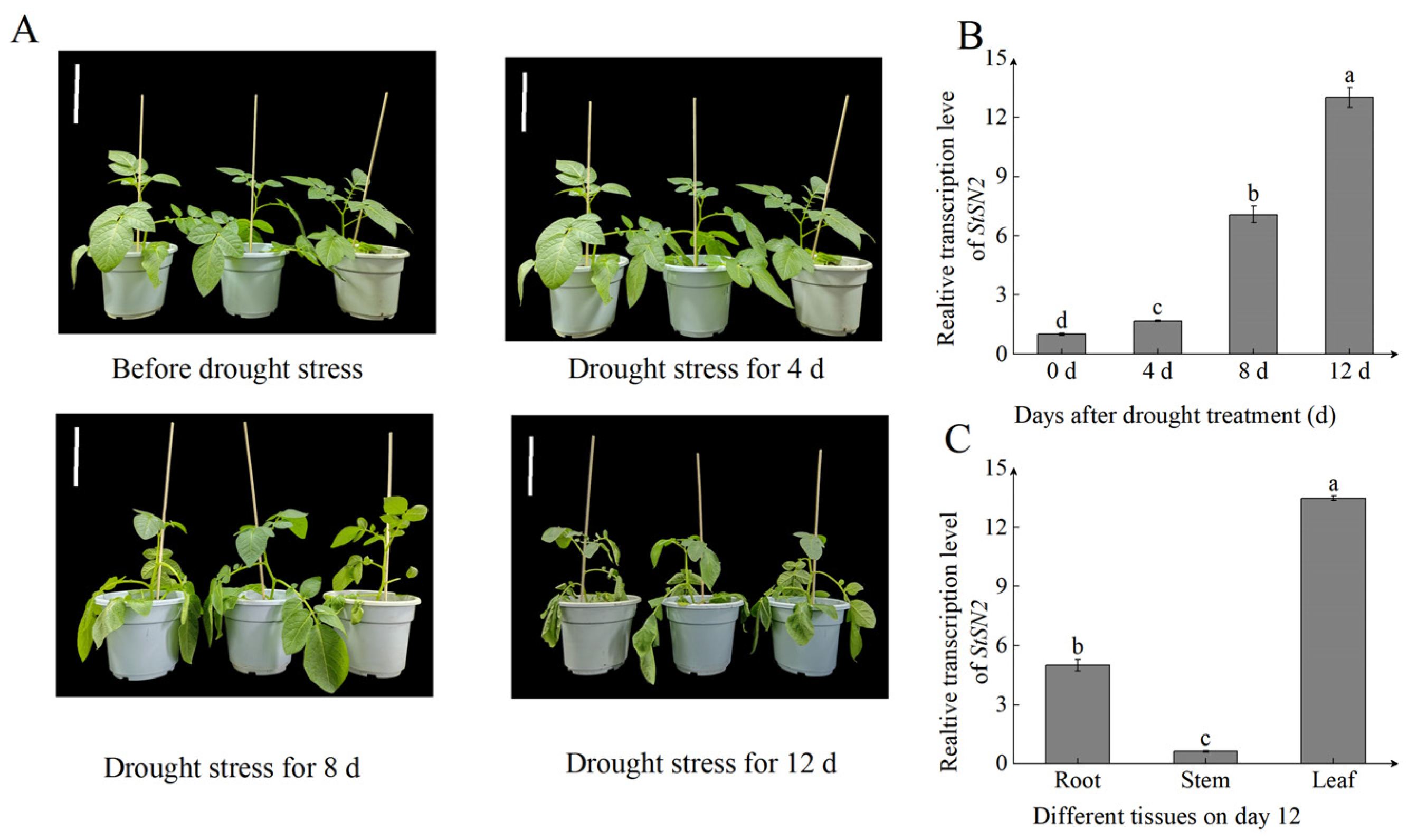
2.1. StSN2 Was Upregulated by Drought Stress
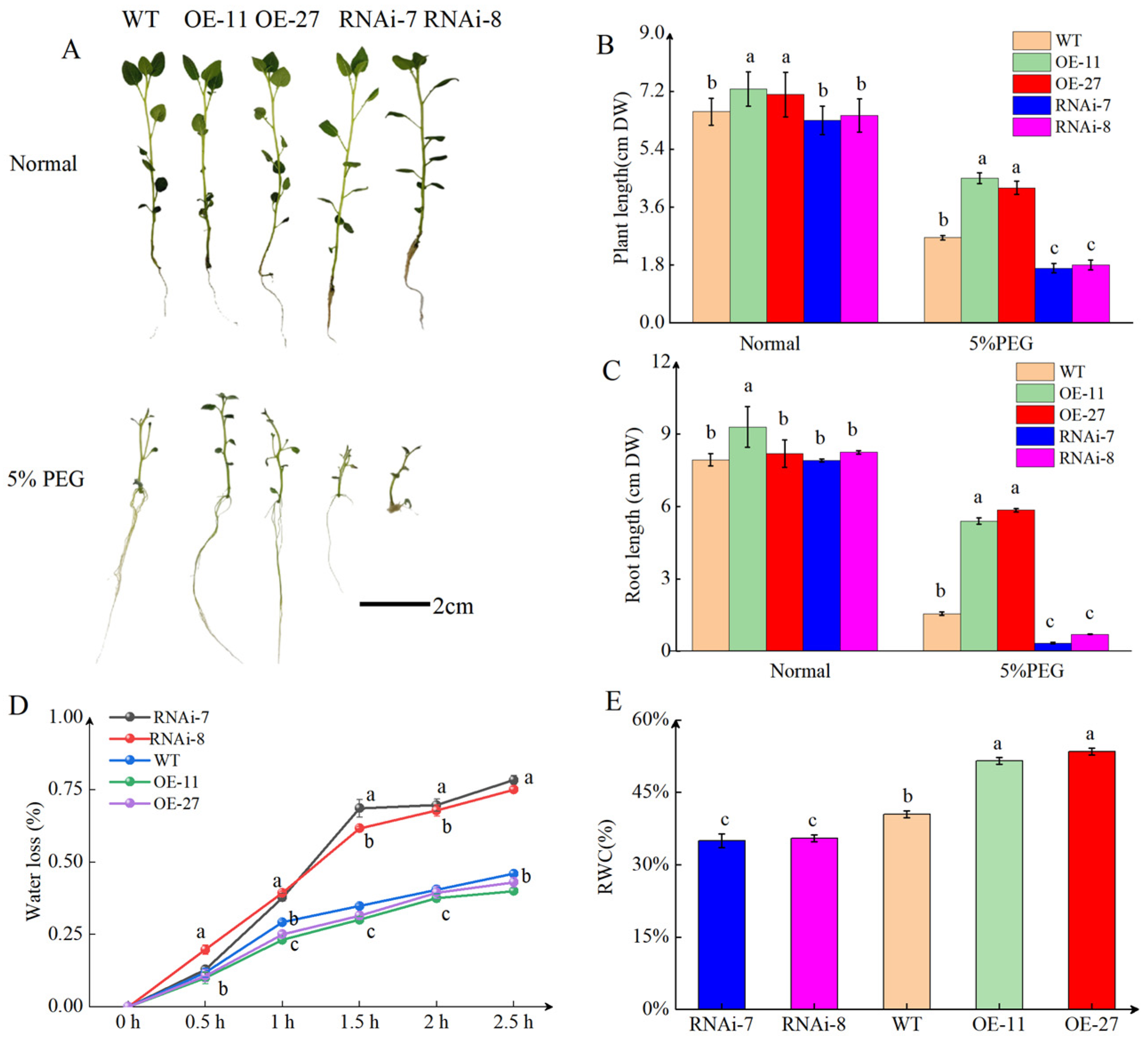
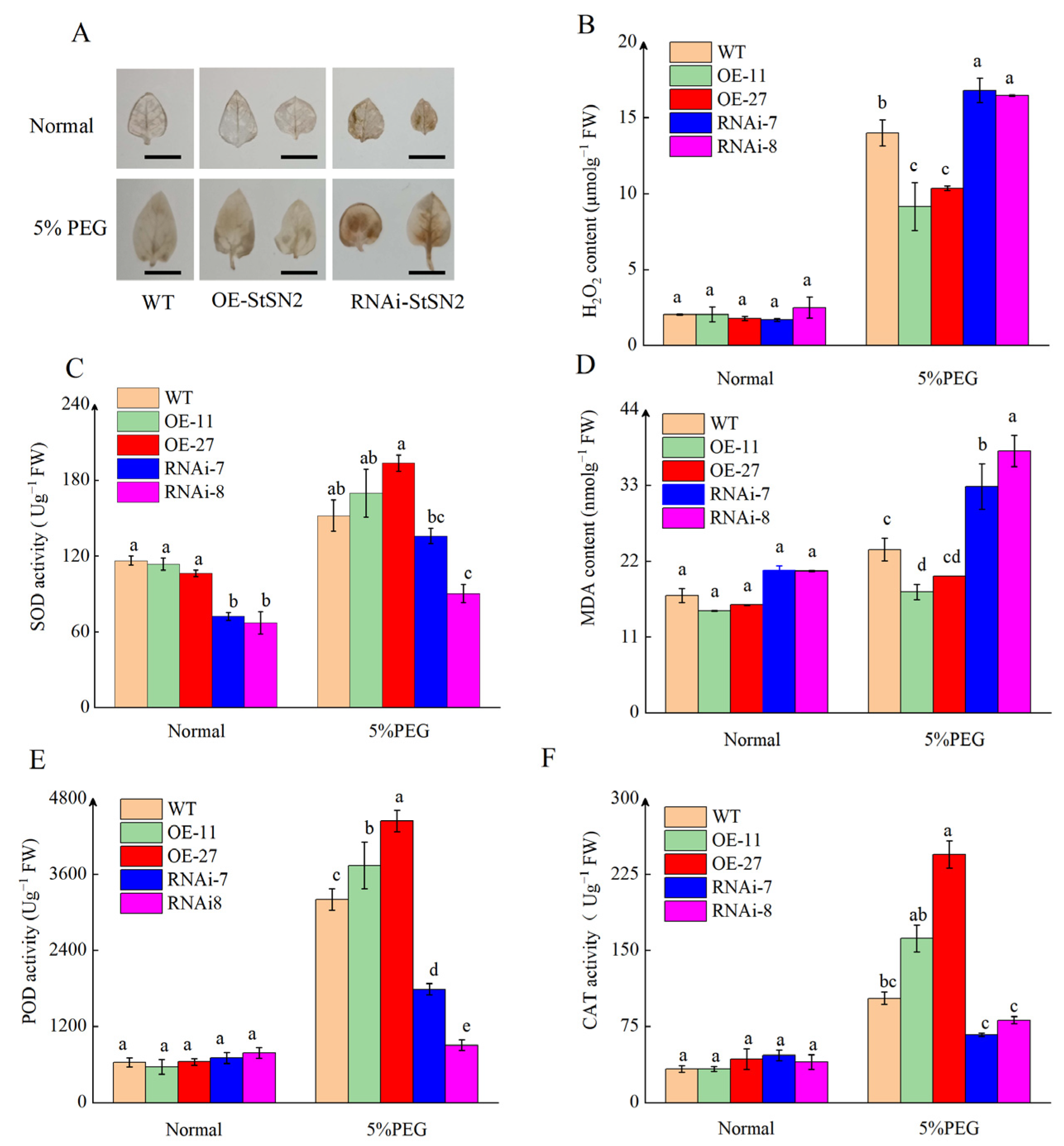
2.2. StSN2 Enhances Drought Tolerance in Potato
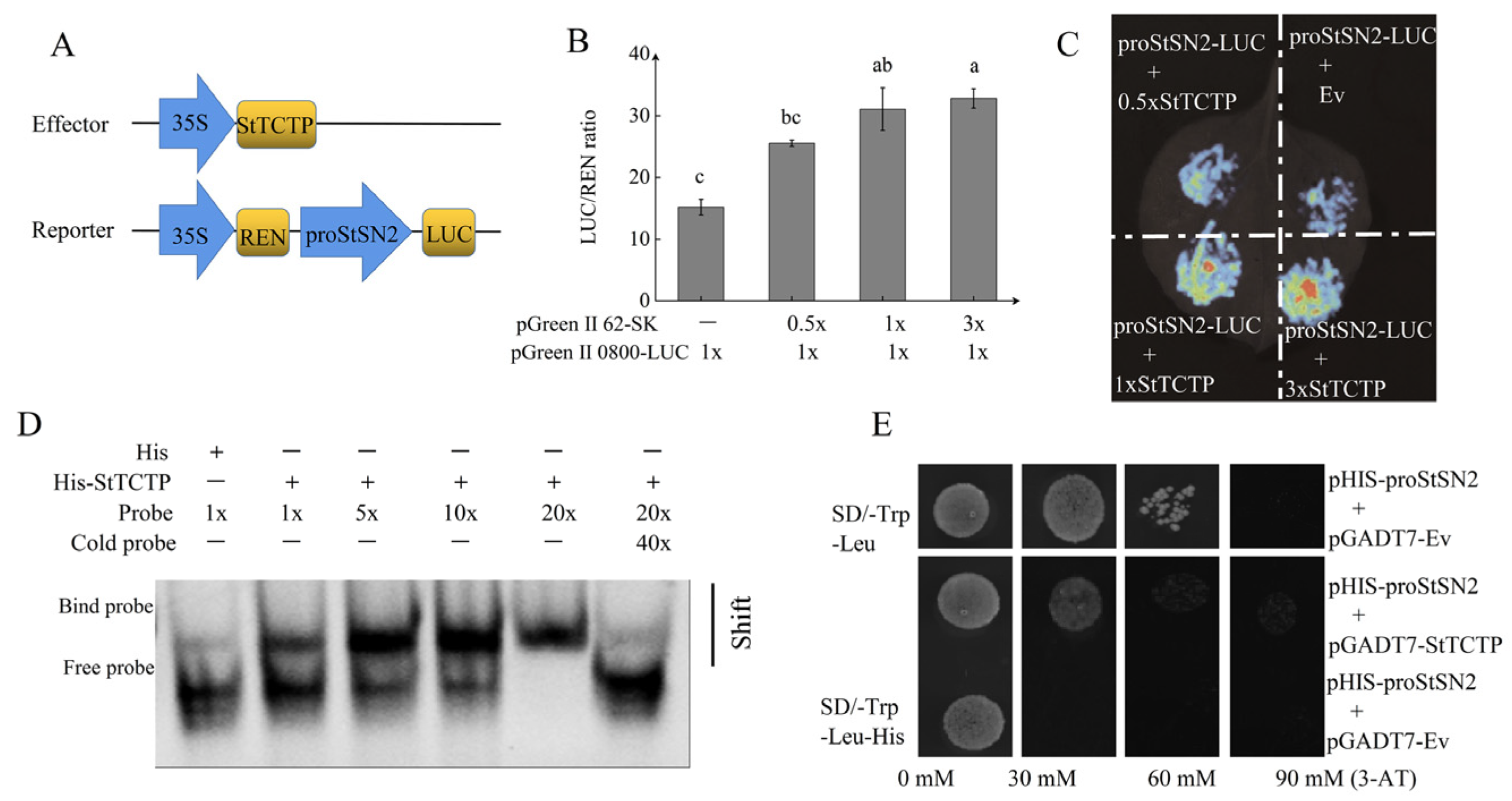
2.3. StTCTP Is an Upstream Regulatory Factor of StSN2
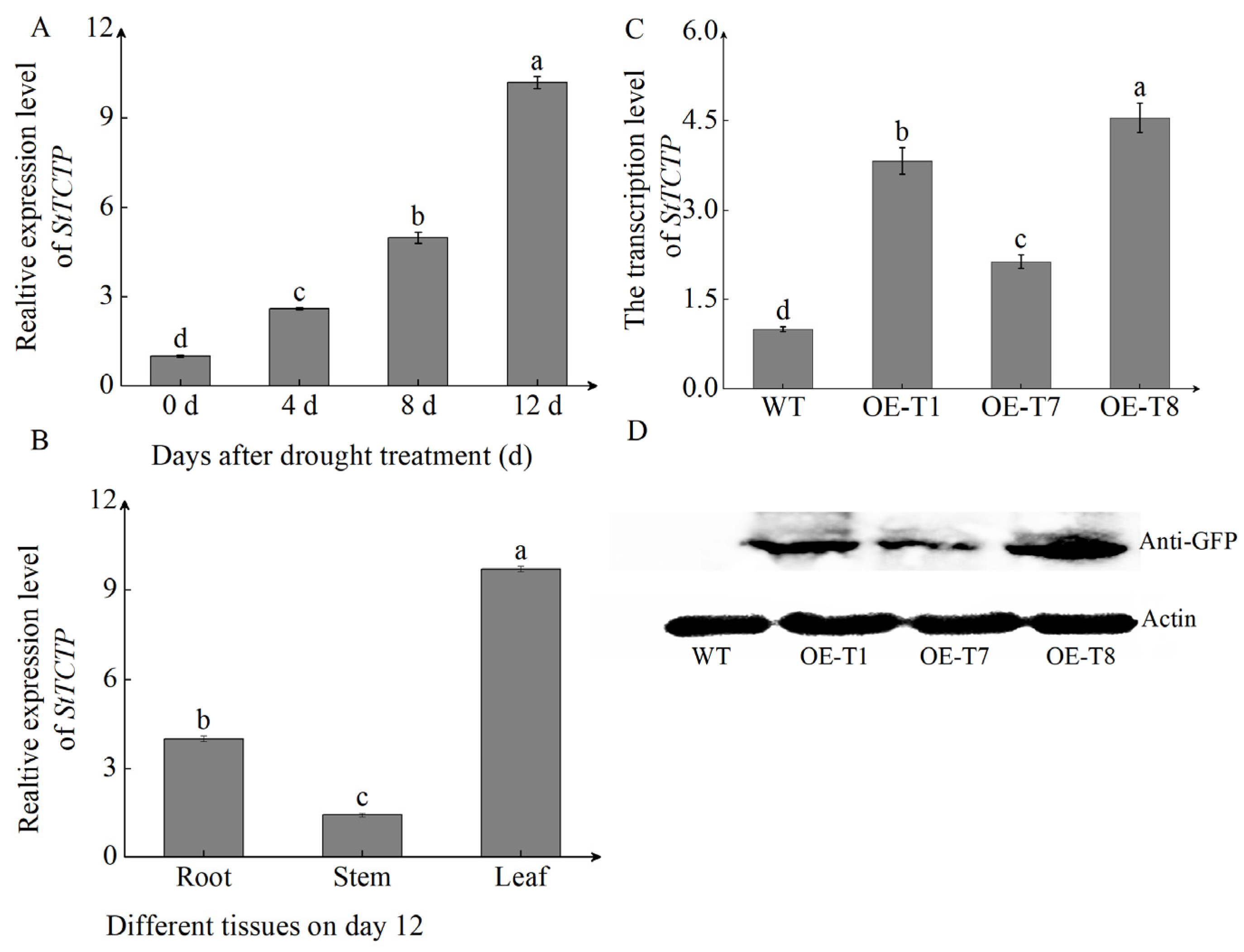
2.4. StTCTP Was Upregulated by Drought Stress
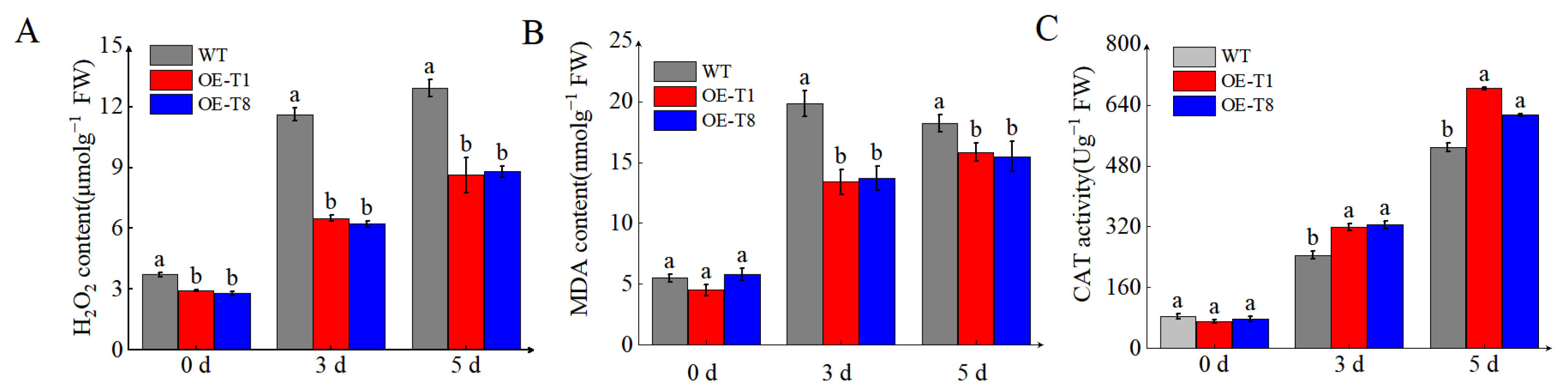
2.5. Overexpression of StTCTP Enhances Tolerance to Drought
3. Discussion
4. Material and Method
4.1. Plant Materials
4.2. Drought Tolerance Experiment
4.3. PEG-Treated Seedlings Experiment
4.4. Water Loss and Relative Water Content Measurements
4.5. Measurement of Indices of Drought Stress Tolerance
4.6. DNA Pull-Down
4.7. Luciferase Reporting Assay
4.8. Yeast One-Hybrid Assays
4.9. Electrophoretic Mobility Shift Assays
4.10. Bioinformatics Analysis
4.11. Expression Analysis
4.12. Western Blotting
4.13. Measurement of Chlorophyll a Fluorescence
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brody, H. Water: A source of life and strife. Nature 2023, 10, 1038. [Google Scholar]
- Ramachandra, R.A.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar]
- Gervais, T.; Creelman, A.; Li, X.Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato response to drought stress: Physiological and growth basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar]
- Tomlekova, N.; Mladenov, P.; Dincheva, I.; Nacheva, E. Metabolic profiling of bulgarian potato cultivars. Foods 2022, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Nahirñak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA proteins: Involvement in hormone crosstalk and redox homeostasis. Plant Signal. Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef]
- Wigoda, N.; Ben-Nissan, G.; Granot, D.; Schwartz, A.; Weiss, D. The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J. 2006, 48, 796–805. [Google Scholar] [CrossRef]
- Roxrud, I.; Lid, S.E.; Fletcher, J.C.; Schmidt, E.D.; Opsahl-Sorteberg, H.G. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol. 2007, 48, 471–483. [Google Scholar]
- Zhang, S.C.; Wang, X.J. Expression pattern of GASA, downstream genes of DELLA, in Arabidopsis. Chin. Sci. Bull. 2008, 53, 3839–3846. [Google Scholar] [CrossRef]
- Almasia, N.I.; Molinari, M.P.; Maroniche, G.A. Successful production of the potato antimicrobial peptide Snakin-1 in baculovirus-infected insect cells and development of specific antibodies. BMC Biotechnol. 2017, 17, 75. [Google Scholar]
- Deng, M.; Peng, J.; Zhang, J. The cysteine-rich peptide Snakin-2 negatively regulates tubers sprouting through modulating lignin biosynthesis and H2O2 accumulation in potato. Int. J. Mol. Sci. 2021, 22, 2287. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lyu, C.; Chen, J.; Lu, Y.; Yang, S.; Ni, S.; Zheng, S.; Yu, L.; Wang, X.; Wang, Q.; et al. Snakin-2 interacts with cytosolic glyceraldehyde-3-phosphate dehydrogenase 1 to inhibit sprout growth in potato tubers. Hortic. Res. 2022, 9, uhab060. [Google Scholar]
- Tao, J.J.; Cao, Y.R.; Chen, H.W.; Wei, W.; Li, Q.T.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. Tobacco translationally controlled tumor protein interacts with ethylene receptor tobacco histidine kinase1 and enhances plant growth through promotion of cell proliferation. Plant Physiol. 2015, 169, 96–114. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, Y.J.; Hwang, O.J.; Lee, S.S.; Shin, A.Y.; Kim, S.Y.; Kim, J.I. Overexpression of Arabidopsis translationally controlled tumor protein gene AtTCTP enhances drought tolerance with rapid ABA-induced stomatal closure. Mol. Cells 2012, 33, 617–626. [Google Scholar]
- Gu, H.; Wang, Y.; Xie, H.; Qiu, C.; Zhang, S.; Xiao, J.; Li, H.; Chen, L.; Li, X.; Ding, Z. Drought stress triggers proteomic changes involving lignin, flavonoids and fatty acids in tea plants. Sci. Rep. 2020, 10, 15504. [Google Scholar]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar]
- Yang, W.; Zhou, Z.; Chu, Z. Emerging roles of salicylic acid in plant saline stress tolerance. Int. J. Mol. Sci. 2023, 24, 3388. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar]
- Qin, X.; Gao, F.; Zhang, J.; Gao, J.; Lin, S.; Wang, Y.; Jiang, L.; Liao, Y.; Wang, L.; Jia, Y.; et al. Molecular cloning, characterization and expression of cDNA encoding translationally controlled tumor protein (TCTP) from Jatropha curcas L. Mol. Biol. Rep. 2011, 38, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Deng, Z.; Liu, X.; Qin, B. Molecular cloning, expression profiles and characterization of a novel translationally controlled tumor protein in rubber tree (Hevea brasiliensis). J. Plant Physiol. 2013, 170, 497–504. [Google Scholar]
- Koziol, M.J.; Garrett, N.; Gurdon, J.B. Tpt1 activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr. Biol. 2007, 17, 801–807. [Google Scholar]
- Cheng, X.; Li, J.; Deng, J.; Li, Z.; Meng, S.; Wang, H. Translationally controlled tumor protein (TCTP) downregulates Oct4 expression in mouse pluripotent cells. BMB Rep. 2012, 45, 20–25. [Google Scholar] [PubMed]
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition-a review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar]
- Panji, A.; Ismaili, A.; Sohrabi, S.M. Genome-wide identification and expression profiling of Snakin/GASA genes under drought stress in barley (Hordeum vulgare L.). 3 Biotech 2023, 13, 126. [Google Scholar] [CrossRef]
- Nahirak, V.; Rivarola, M.; MGD Urreta, N.; Vazquez-Rovere, C. Genome-wide analysis of the Snakin/GASA gene family in Solanum tuberosum cv. Kennebec. Am. J. Potato Res. 2016, 93, 172–188. [Google Scholar]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-Stimulated drought stress improves the attributes of in vitro growth, steviol glycosides production, and antioxidant activities in stevia rebaudiana bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, Q.; Liu, W.; Zhang, J.; Chen, Y.; Jia, Z.; Shao, Y.; Wang, H. Responses of photosynthetic characteristics of oat flag leaf and spike to drought stress. Front. Plant Sci. 2020, 13, 917528. [Google Scholar]
- Bhatt, I.; Tripathi, B.N. Plant peroxiredoxins: Catalytic mechanisms, functional significance and future. Biotechnol. Adv. 2011, 29, 850–859. [Google Scholar] [CrossRef]
- de Carvalho, M.; Acencio, M.L.; Laitz, A.V.N.; de Araújo, L.M.; de Lara Campos Arcuri, M.; do Nascimento, L.C.; Maia, I.G. Impacts of the overexpression of a tomato translationally controlled tumor protein (TCTP) in tobacco revealed by phenotypic and transcriptomic analysis. Plant Cell Rep. 2017, 36, 887–900. [Google Scholar] [PubMed]
- Dong, C.; Wang, Q.; Wang, Y.; Qin, L.; Shi, Y.; Wang, X.; Wang, R. NtDREB-1BL1 enhances carotenoid biosynthesis by regulating phytoene synthase in nicotiana tabacum. Genes 2022, 13, 1134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Li, X.; Gao, X.; Dai, Z.; Cui, Y.; Zhi, Y.; Liu, Q.; Zhai, H.; Gao, S.; et al. The IbBBX24-IbTOE3-IbPRX17 module enhances abiotic stress tolerance by scavenging reactive oxygen species in sweet potato. New Phytol. 2022, 233, 1133–1152. [Google Scholar]
- Su, B.; Huang, J.; Fischer, T.; Wang, Y.; Kundzewicz, Z.W.; Zhai, J.; Sun, H.; Wang, A.; Zeng, X.; Wang, G.; et al. Drought losses in China might double between the 1.5°C and 2.0°C warming. Proc. Natl. Acad. Sci. USA 2018, 115, 10600–10605. [Google Scholar]
- Jia, Y.; Gu, X.; Chai, J.; Yao, X.; Cheng, S.; Liu, L.; He, S.; Peng, Y.; Zhang, Q.; Zhu, Z. Rice OsANN9 enhances drought tolerance through modulating ROS scavenging systems. Int. J. Mol. Sci. 2023, 24, 17495. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Gómez-Cadenas, A.; Nicolás, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; García-Olmedo, F.; Molina, A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002, 128, 951–961. [Google Scholar]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar]
- Liu, S.; Cai, C.; Li, L.; Wen, H.; Liu, J.; Li, L.; Wang, Q.; Wang, X. StSN2 interacts with the brassinosteroid signaling suppressor StBIN2 to maintain tuber dormancy. Hortic. Res. 2023, 10, uhad228. [Google Scholar]
- Cao, B.; Lu, Y.; Chen, G.; Lei, J. Functional characterization of the translationally controlled tumor protein (TCTP) gene associated with growth and defense response in cabbage. Plant Cell Tissue Organ Cult. 2010, 103, 217–226. [Google Scholar] [CrossRef]
- Mu, D.W.; Feng, N.J.; Zheng, D.F.; Zhou, H.; Liu, L.; Chen, G.J.; Mu, B. Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci. Rep. 2022, 12, 20439. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Zou, X.; Lu, L.; Liu, F. Ectopic expression of AtCIPK23 enhances tolerance against low-K+ stress in transgenic potato. Am. J. Potato Res. 2011, 88, 153–159. [Google Scholar] [CrossRef]
- Huang, S.; Nie, S.; Wang, S.; Liu, J.; Zhang, Y.; Wang, X. SlBIR3 negatively regulates PAMP responses and cell death in tomato. Int. J. Mol. Sci. 2017, 18, 1966. [Google Scholar] [CrossRef]
- Mehmandar, M.N.; Rasouli, F.; Giglou, M.T.; Zahedi, S.M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Tajaragh, R.P.; Ryant, P.; Mlcek, J. Polyethylene glycol and sorbitol-mediated in vitro screening for drought stress as an efficient and rapid tool to reach the tolerant Cucumis melo L. genotypes. Plants 2023, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, T.; Kan, J.; Yao, Y.; Guo, D.; Yang, Y.; Ling, X.; Wang, J.; Zhang, B. The GhMYB36 transcription factor confers resistance to biotic and abiotic stress by enhancing PR1 gene expression in plants. Plant Biotechnol. J. 2022, 20, 722–735. [Google Scholar] [CrossRef]
- Song, X.; Fang, J.; Han, X.; He, X.; Liu, M.; Hu, J.; Zhuo, R. Overexpression of quinone reductase from Salix matsudana Koidz enhances salt tolerance in transgenic Arabidopsis thaliana. Gene 2016, 576 Pt 3, 520–527. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Liu, Z.; Wen, H.; Jiang, N.; Shi, H.; Kou, Y. The application of zinc oxide nanoparticles: An effective strategy to protect rice from rice blast and abiotic stresses. Environ. Pollut. 2023, 331 Pt 1, 121925. [Google Scholar] [CrossRef]
- Li, P.; Lin, P.; Zhao, Z.; Li, Z.; Liu, Y.; Huang, C.; Huang, G.; Xu, L.; Deng, Z.; Zhang, Y.; et al. Gene co-expression analysis reveals transcriptome divergence between wild and cultivated sugarcane under drought stress. Int. J. Mol. Sci. 2022, 23, 569. [Google Scholar] [CrossRef]
- Sun, G.; Geng, S.; Zhang, H.; Jia, M.; Wang, Z.; Deng, Z.; Tao, S.; Liao, R.; Wang, F.; Kong, X.; et al. Matrilineal empowers wheat pollen with haploid induction potency by triggering postmitosis reactive oxygen species activity. New Phytol. 2022, 233, 2405–2414. [Google Scholar] [CrossRef]
- Kumar, G. Principle and method of silver staining of proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Mol. Biol. 2018, 1853, 231–236. [Google Scholar]
- Qin, X.; Li, Y.; Li, C.; Li, X.; Wu, Y.; Wu, Q.; Wen, H.; Jiang, D.; Liu, S.; Nan, W.; et al. A rapid and simplified method to isolate specific regulators based on biotin-avidin binding affinities in crops. J. Agric. Food Chem. 2024, 72, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Chu, Y.; Liu, Y.; Cao, X.; Zhang, H.; Huang, Y.; Gong, A.; Liao, X.; Wang, D.; et al. O-GlcNAcylation of ZEB1 facilitated mesenchymal pancreatic cancer cell ferroptosis. Int. J. Biol. Sci. 2022, 18, 4135–4150. [Google Scholar] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Yom, H.C.; Bremel, R.D. Xerographic paper as a transfer medium for western blots: Quantification of bovine alpha S1-casein by western blot. Anal. Biochem. 1992, 200, 249–253. [Google Scholar] [PubMed]
- Hnasko, T.S.; Hnasko, R.M. The Western Blot. Methods Mol. Biol. 2015, 1318, 87–96. [Google Scholar]
- Stirbet, A. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Zhou, S.; Xu, B.; Chen, P.; Ma, F.; Mao, K. The ABA receptor gene MdPYL9 confers tolerance to drought stress in transgenic apple (Malus domestica). Environ. Exp. Bot. 2022, 194, 104695. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, F.; Feng, H.; Wang, X.; Wang, Q.; Lai, X.; Yan, L. StTCTP Positively Regulates StSN2 to Enhance Drought Stress Tolerance in Potato by Scavenging Reactive Oxygen Species. Int. J. Mol. Sci. 2025, 26, 2796. https://doi.org/10.3390/ijms26062796
Liu S, Zhang F, Feng H, Wang X, Wang Q, Lai X, Yan L. StTCTP Positively Regulates StSN2 to Enhance Drought Stress Tolerance in Potato by Scavenging Reactive Oxygen Species. International Journal of Molecular Sciences. 2025; 26(6):2796. https://doi.org/10.3390/ijms26062796
Chicago/Turabian StyleLiu, Shifeng, Feng Zhang, Haojie Feng, Xiyao Wang, Qiang Wang, Xianjun Lai, and Lang Yan. 2025. "StTCTP Positively Regulates StSN2 to Enhance Drought Stress Tolerance in Potato by Scavenging Reactive Oxygen Species" International Journal of Molecular Sciences 26, no. 6: 2796. https://doi.org/10.3390/ijms26062796
APA StyleLiu, S., Zhang, F., Feng, H., Wang, X., Wang, Q., Lai, X., & Yan, L. (2025). StTCTP Positively Regulates StSN2 to Enhance Drought Stress Tolerance in Potato by Scavenging Reactive Oxygen Species. International Journal of Molecular Sciences, 26(6), 2796. https://doi.org/10.3390/ijms26062796





