Proteomics Reveals the Role of PLIN2 in Regulating the Secondary Hair Follicle Cycle in Cashmere Goats
Abstract
1. Introduction
2. Results
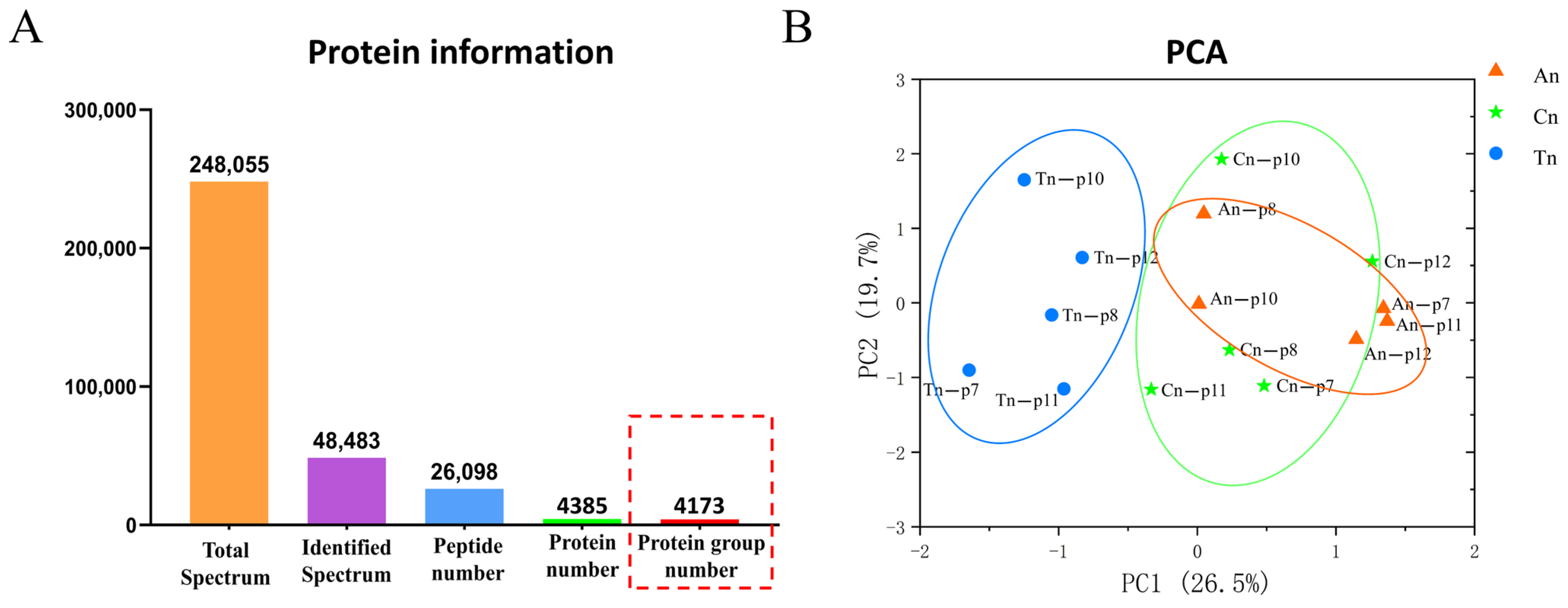
2.1. Proteomic Characterization Within the Skin Tissues of Cashmere Goats
2.2. Functional Classification of Identified Proteins
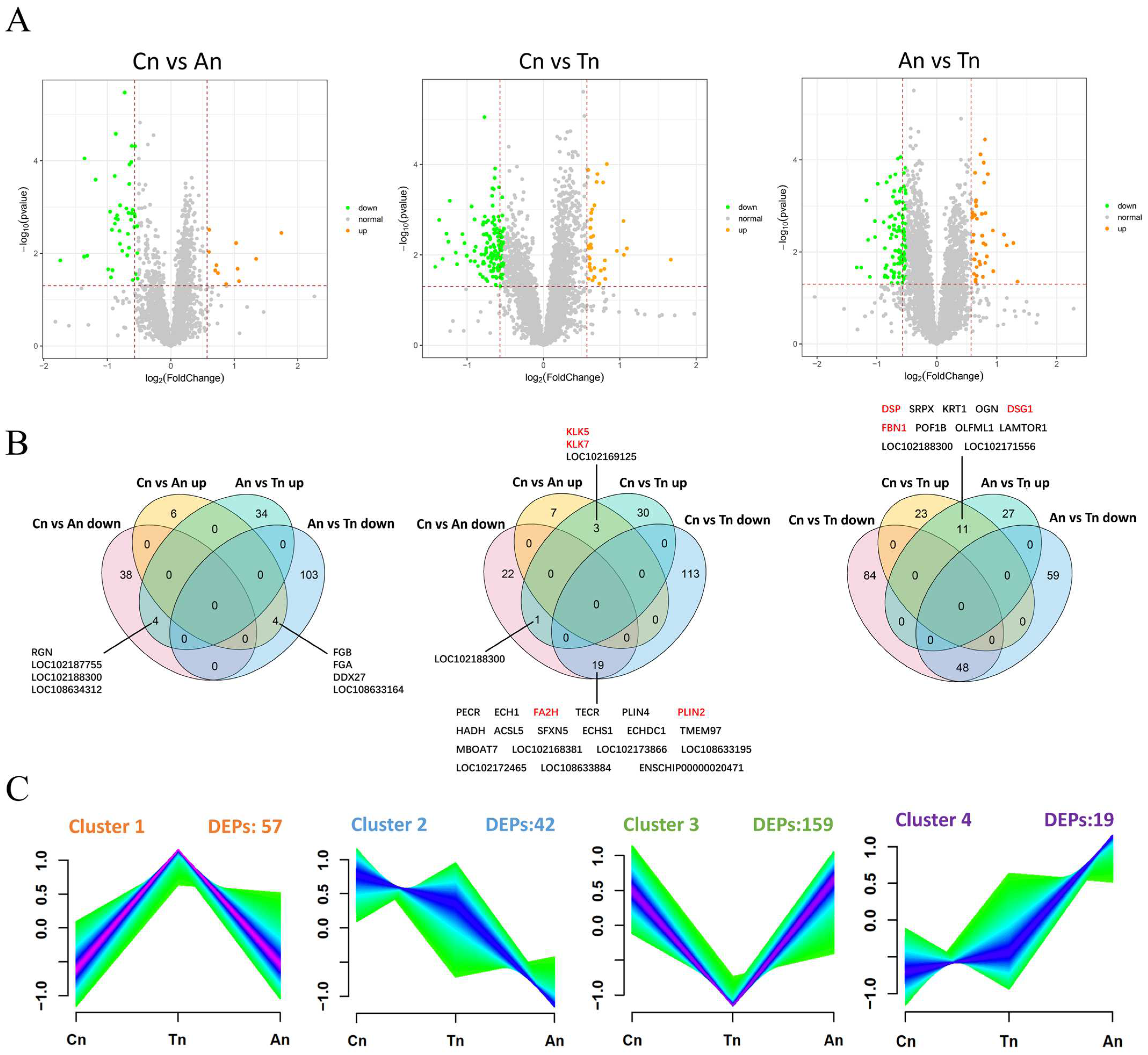
2.3. Differential Expression Analysis of Proteins
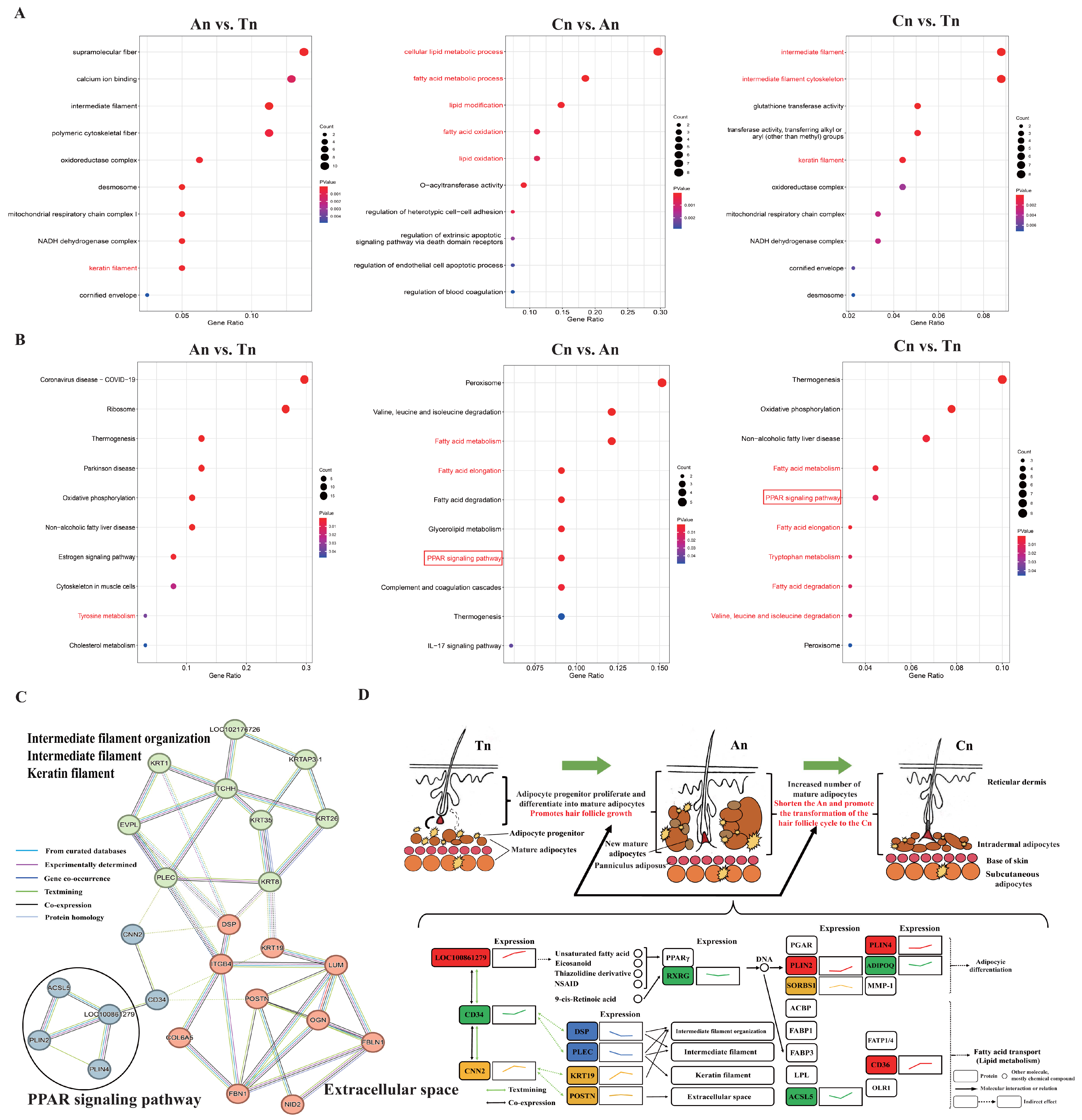
2.4. Functional Enrichment Analysis and PPI (Protein–Protein Interaction) Network Analysis of DEPs
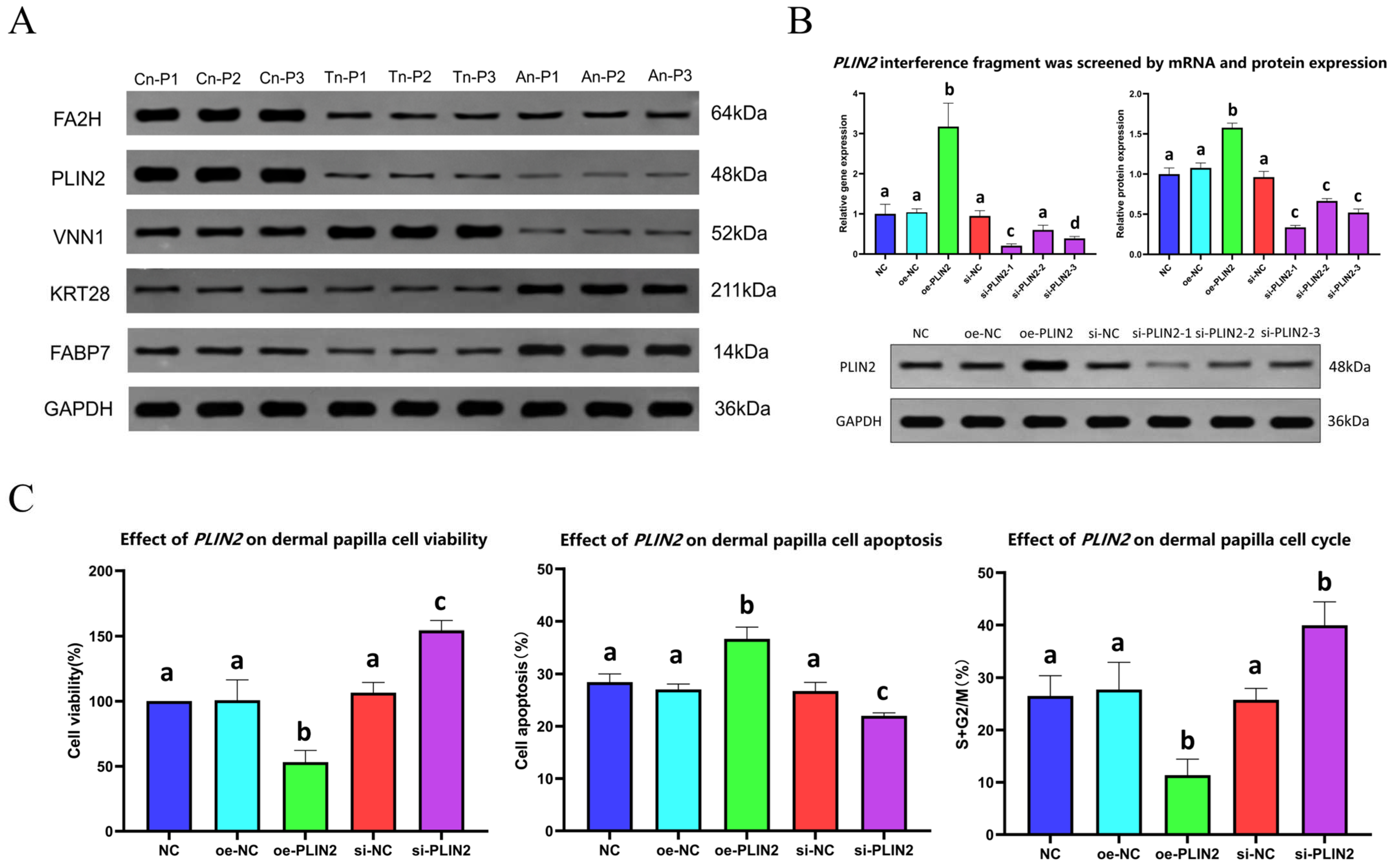
2.5. Protein Western Blot Analysis
2.6. Screening of PLIN2 Gene Interference Fragments
2.7. Effects of the PLIN2 Gene on DPC Proliferation, Apoptosis, and Growth Cycle
3. Discussion
3.1. The Effect of Differentially Expressed Calcium-Binding Proteins on the SHF Cycle
3.2. The Effect of Differentially Expressed Keratins on the SHF Cycle
3.3. The Effect of Differentially Expressed Ribosomal Proteins on the SHF Cycle
3.4. DEPs Linked with Lipid Metabolism Affect SHF Cycle
4. Materials and Methods
4.1. Experimental Animals and Sample Collection
4.2. Protein Extraction and Digestion
4.3. TMT Labeling
4.4. LC–MS/MS Analysis
4.5. Protein Characterisation and Quantitation
4.6. Bioinformatic Analysis
4.7. Western Blot Analysis of DEPs
4.8. Functional Validation of the PLIN2 Gene in DPCs
4.8.1. Synthesis of PLIN2 Gene Interference Fragments
4.8.2. Construction of PLIN2 Gene Overexpression Vector
4.8.3. Analysis of PLIN2 Gene Overexpression and Knockdown Effects
4.8.4. Assessment of PLIN2 Gene Effects on DPC Proliferation, Apoptosis, and Cell Cycle
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, C.; Du, J.; Li, B.; Jia, Y.; Mai’erhaba, A.; Ayinuran, A. Investigation on the Development of Cashmere Goat Industry in Southern Xinjiang of Aksu Region. Appl. Eng. Technol. 2023, 43, 13–15. [Google Scholar]
- Yang, F.; Li, R.; Zhao, C.; Che, T.; Guo, J.; Xie, Y.; Wang, Z.; Li, J.; Liu, Z. Single-cell sequencing reveals the new existence form of dermal papilla cells in the hair follicle regeneration of cashmere goats. J. Genom. 2022, 114, 110316. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, Y.; Wang, Z.; Zhu, Y.; Cong, Y.; Wang, W.; Deng, L.; Liu, H.; Guo, D.; Bai, W. Discovery and molecular analysis of conserved circRNAs from cashmere goat reveal their integrated regulatory network and potential roles in secondary hair follicle. Electron. J. Biotechnol. 2019, 41, 37–47. [Google Scholar] [CrossRef]
- Parry, A.L.; Norton, B.W.; Restall, B.J. Skin follicle development in the Australian cashmere goat. Aust. J. Agric. Res 1992, 43, 857–870. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, X.; Han, W.; Yang, K.; Wang, H.; Zhang, Y.; Su, R.; Liu, Z.; Wang, R.; et al. High-throughput sequencing of hair follicle development-related micrornas in cashmere goat at various fetal periods. Saudi J. Biol. Sci. 2018, 25, 1494–1508. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, L.; Gao, K.; Guo, Y.; Liu, Y.; Zhang, Y. Cashmere production, skin characteristics, and mutated genes in crimped cashmere fibre goats. Animal 2022, 16, 100565. [Google Scholar] [CrossRef]
- Gao, Y.; Duo, L.; Zhe, X.; Hao, L.; Song, W.; Gao, L.; Cai, J.; Liu, D. Developmental mapping of hair follicles in the embryonic stages of cashmere goats using proteomic and metabolomic construction. Animals 2023, 13, 3076. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Rb, W.; Qiao, X.; Yj, Z.; Wang, R.; Su, R.; Wu, J.; Dong, Y.; Li, J. Hair follicle transcriptome profiles during the transition from anagen to catagen in Cashmere goat (Capra hircus). Genet. Mol. Res. 2015, 14, 17904–17915. [Google Scholar] [CrossRef]
- Su, R.; Gong, G.; Zhang, L.; Yan, X.; Wang, F.; Zhang, L.; Qiao, X.; Li, X.; Li, J. Screening the key genes of hair follicle growth cycle in Inner Mongolian Cashmere goat based on RNA sequencing. Arch. Anim. Breed. 2020, 63, 155–164. [Google Scholar] [CrossRef]
- Bai, W.L.; Zhao, S.; Wang, Z.; Zhu, Y.; Dang, Y.L.; Cong, Y.; Xue, H.L.; Wang, W.; Deng, L.; Guo, D.; et al. LncRNAs in Secondary Hair Follicle of Cashmere Goat: Identification, Expression, and Their Regulatory Network in Wnt Signaling Pathway. Anim. Biotechnol. 2018, 29, 199–211. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; Veen, C.V.; Eichmüller, S.B.; Foitzik, K.; McKay, I.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 11, 3–15. [Google Scholar] [CrossRef]
- Peus, D.; Pittelkow, M.R. Growth factors in hair organ development and the hair growth cycle. Dermatol. Clin. 1996, 14, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Mifude, C.; Kaseda, K. PDGF-AA-induced filamentous mitochondria benefit dermal papilla cells in cellular migration. Int. J. Cosmet. Sci. 2015, 37, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Fan, W.; Li, M. Angiogenin is expressed in human dermal papilla cells and stimulates hair growth. Arch. Dermatol. Res. 2009, 301, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kloren, W.R.L.; Norton, B.W.; Waters, M.J. Fleece growth in Australian cashmere goats. III. The seasonal patterns of cashmere and hair growth, and association with growth hormone, prolactin and thyroxine in blood. Aust. J. Agric. Res. 1993, 44, 1035–1050. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, Y.J.; Zhang, J.X.; Chang, Z.L.; Li, J.Q.; Yan, Z.W.; Zhang, W.G. Hoxc13/β-catenin correlation with hair follicle activity in cashmere goat. J. Integr. Agric. 2012, 11, 1159–1166. [Google Scholar] [CrossRef]
- Geng, R.; Yuan, C.; Chen, Y. Exploring differentially expressed genes by RNA-Seq in cashmere goat (Capra hircus) skin during hair follicle development and cycling. PLoS ONE 2013, 8, e62704. [Google Scholar] [CrossRef]
- Liu, N.; Li, H.; Liu, K.; Yu, J.; Cheng, M.; De, W.; Liu, J.; Shi, S.; He, Y.; Zhao, J. Differential expression of genes and proteins associated with wool follicle cycling. Mol. Biol. Rep. 2014, 41, 5343–5349. [Google Scholar] [CrossRef]
- Gao, G.L.; Zhang, Z.Y.; Zhang, Z.W.; Wang, W.Z.; Nai, N.R.; Du, D.C.; Wang, W.L.; Liu, L.Z.; Li, L.J. Analysis of Protein Expression Profile of Hair Follicle Cycle of Inner Mongolia Cashmere Goat (Capra cashmere). J. Agric. Biotechnol. 2014, 22, 727–735. [Google Scholar]
- Yu, M.R.; Liu, Z.H.; Zhang, Y.J.; Zhao, M.; Nai, R.L.; Zheng, Z.Q.; Li, J.Q. Protein mass spectrometry in the Inner Mongolia White Cashmere Goat. Anim. Husb. Vet. Med. 2016, 48, 45–49. [Google Scholar]
- Yang, F. Mechanisms of Follicular Cell Action in Inner Mongolia Cashmere Goats. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2020. [Google Scholar]
- Ansari-Renani, H.R.; Ebadi, Z.; Moradi, S.; Baghershah, H.R.; Ansari-Renani, M.Y.; Ameli, S.H. Determination of hair follicle characteristics, density and activity of Iranian cashmere goat breeds. Small Rumin. Res. 2011, 95, 128–132. [Google Scholar] [CrossRef]
- Kumamoto, T.; Shalhevet, D.; Matsue, H.; Mummert, M.E.; Ward, B.R.; Jester, J.V.; Takashima, A. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood 2003, 102, 1654–1660. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Yu, X.; He, J.; Fan, L.; Dong, Y.; Dong, C.; Liu, T. Immunolocalization of β-catenin and Lef-1 during postnatal hair follicle development in mice. Acta Histochem. 2012, 114, 773–778. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Xu, X.; Xu, Q.; Qin, C.; Liu, G.; Wei, C.; Zhang, G.; Tian, K.; Fu, X. Effect of the FA2H Gene on cashmere fineness of Jiangnan cashmere goats based on transcriptome sequencing. BMC Genom. 2022, 23, 527. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Gong, H.; Wu, D.; Li, C.; Liu, B.; Ding, L. Molecular Basis of Maintaining Circannual Rhythm in the Skin of Cashmere Goat. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wu, C.; Qin, C.; Fu, X.; Huang, X.; Tian, K. Integrated analysis of lncRNAs and mRNAs by RNA-Seq in secondary hair follicle development and cycling (anagen, catagen and telogen) of Jiangnan cashmere goat (Capra hircus). BMC Vet. Res. 2022, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Nogueira da Costa, A.; Makrantonaki, E.; Hou, X.; Almansouri, D.; Dudley, J.T.; Edwards, H.; Readhead, B.; Balthasar, O.; Jemec, G.B.; et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 846–861. [Google Scholar] [CrossRef]
- Wu, C. Screening of Cashmere Trait Related Genes and Functional Verification of ELOVL3 and FA2H Genes in Jiangnan Cashmere Goats. Ph.D. Thesis, Xinjiang Agricultural University, Urumqi, China, 2022. [Google Scholar]
- Zhang, Y.; Huang, J.; Fu, D.; Liu, Z.; Wang, H.; Wang, J.; Qu, Q.; Li, K.; Fan, Z.; Hu, Z.; et al. Transcriptome analysis reveals an inhibitory effect of Dihydrotestosterone-treated 2D-and 3D-cultured dermal papilla cells on hair follicle growth. Front. Cell Dev. Biol. 2021, 9, 724310. [Google Scholar] [CrossRef]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef]
- Ohnemus, U.; Uenalan, M.; Inzunza, J.; Gustafsson, J.A.; Paus, R. The hair follicle as an estrogen target and source. Endocr. Rev. 2006, 27, 677–706. [Google Scholar] [CrossRef]
- Hearle JW, S. A critical review of the structural mechanics of wool and hair fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. [Google Scholar] [CrossRef]
- Akiba, H.; Ikeuchi, E.; Ganbat, J.; Fujikawa, H.; Arai-Kusano, O.; Iwanari, H.; Nakakido, M.; Hamakubo, T.; Shimomura, Y.; Tsumoto, K. Structural behavior of keratin-associated protein 8.1 in human hair as revealed by a monoclonal antibody. J. Struct. Biol. 2018, 204, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Langbein, L.; Rogers, M.A.; Winter, H.; Praetzel, S.; Beckhaus, U.; Rackwitz, H.R.; Schweizer, J. The catalog of human hair keratins. I. Expression of the nine type I members in the hair follicle. J. Biol. Chem. 1999, 274, 19874–19884. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sakamoto, Y.; Honda, Y.; Koike, K.; Nakamura, H.; Matsumoto, T.; Ando, S. De novo filament formation by human hair keratins K85 and K35 follows a filament development pattern distinct from cytokeratin filament networks. FEBS Open Bio 2021, 11, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Harland, D.P.; Richena, M.; Thomas, A.; Hefer, C.A.; van Koten, C.; Scobie, D.R.; Grosvenor, A.J. Wool fiber curvature is correlated with abundance of K38 and specific keratin-associated proteins. Proteins 2022, 90, 973–981. [Google Scholar] [CrossRef]
- Jin, M.; Wang, J.; Chu, M.X.; Piao, J.; Piao, J.A.; Zhao, F.Q. The study on biological function of keratin 26, a novel member of Liaoning cashmere goat keratin gene family. PLoS ONE 2016, 11, e0168015. [Google Scholar] [CrossRef] [PubMed]
- Duchstein, P.; Clark, T.; Zahn, D. Atomistic modeling of a KRT35/KRT85 keratin dimer: Folding in aqueous solution and unfolding under tensile load. Phys. Chem. Chem. Phys. 2015, 17, 21880–21884. [Google Scholar] [CrossRef]
- Hui, T.; Zheng, Y.; Yue, C.; Wang, Y.; Bai, Z.; Sun, J.; Cai, W.; Zhang, X.; Bai, W.L.; Wang, Z. Screening of cashmere fineness-related genes and their ceRNA network construction in cashmere goats. Sci. Rep. 2021, 11, 21977. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D.; et al. Molecular mechanisms of hair growth and regeneration: Current understanding and novel paradigms. Dermatology 2020, 236, 271–280. [Google Scholar] [CrossRef]
- Ohnemus, U.G.; Uenalan, M.; Conrad, F.; Handjiski, B.; Mecklenburg, L.; Nakamura, M.; Inzunza, J.; Gustafsson, J.; Paus, R. Hair cycle control by estrogens: Catagen induction via estrogen receptor (ER)-α is checked by ERβ signaling. Endocrinology 2005, 146, 1214–1225. [Google Scholar] [CrossRef]
- Moraleva, A.A.; Deryabin, A.; Rubtsov, Y.P.; Rubtsova, M.P.; Dontsova, O.A. Eukaryotic ribosome biogenesis: The 40S subunit. Acta Naturae 2022, 14, 14. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Q.; Liu, Z.; Wang, Y.; Lan, M.; Zhao, D.; Zhang, J.; Wang, Z.; Li, J.; Liu, Z. Combining multiomics to analyze the molecular mechanism of hair follicle cycle change in cashmere goats from Inner Mongolia. Front. Vet. Sci. 2024, 11, 1405355. [Google Scholar]
- Festa, E.; Fretz, J.A.; Berry, R.; Schmidt, B.; Rodeheffer, M.S.; Horowitz, M.C.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Venkataram, A.; Mysore, V. The Role of Adipose Tissue in Hair Regeneration: A Potential Tool for Management? J. Cutan. Aesthet. Surg. 2021, 14, 295–304. [Google Scholar] [PubMed]
- Bai, T.; Liang, B.; Zhao, Y.; Han, J.; Pu, Y.; Wang, C.; Ma, Y.; Jiang, L. Transcriptome analysis reveals candidate genes regulating the skin and hair diversity of Xinji fine-wool sheep and Tan sheep. Agriculture 2021, 12, 15. [Google Scholar] [CrossRef]
- Icre, G.; Wahli, W.; Michalik, L. Functions of the peroxisome proliferator-activated receptor (PPAR) alpha and beta in skin homeostasis, epithelial repair, and morphogenesis. J. Investig. Dermatol. Symp. Proc. 2006, 11, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bao, P.; Zhang, X.; Guo, X.; Liang, C.; Chu, M.; Wu, X.; Yan, P. Genome-wide detection of RNA editing events during the hair follicles cycle of Tianzhu white yak. BMC Genom. 2022, 23, 737. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, J.J. Autotaxin-LPA Axis in Obesity and Obesity-related Diseases. Prog. Biochem.Biophys. 2021, 48, 768–778. [Google Scholar]
- Najt, C.P.; Devarajan, M.; Mashek, D.G.; Devarajan, M.; Mashek, D.G. Perilipins at a glance. J. Cell. Sci. 2022, 135, jcs259501. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Tsai, T.; Chen, E.; Li, L.; Saha, P.K.; Lee, H.; Huang, L.; Shelness, G.S.; Chan, L.; Chang, B.H. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 2017, 13, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Inoguchi, T.; Ikuyama, S.; Taniguchi, S.; Kobayashi, K.; Nakashima, N.; Nawata, H. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E775–E783. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35 (Suppl. S2), W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
| Sequence Name | Primers | Sequence (5′-3′) |
|---|---|---|
| si-PLIN2-1 | Forward Primer | GCAGAGACCUCUCAUCCUUTT |
| Reverse Primer | GUUCAGAAGCCAAGUUAUUAU | |
| si-PLIN2-2 | Forward Primer | AGUCUAACAUAAUAACUUGGC |
| Reverse Primer | UCAAUCAGGUGAACAGUAGAA | |
| si-PLIN2-3 | Forward Primer | CUACUGUUCACCUGAUUGAAU |
| Reverse Primer | AAAGCUCGAGUACACCAGCTT | |
| si-NC | Forward Primer | UUCUCCGAACGUGUCACGUTT |
| Reverse Primer | ACGUGACACGUUCGGAGAATT |
| Gene Name | Primers | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward Primer | 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse Primer | 5′-GGGGTCGTTGATGGCAACA-3′ | |
| PLIN2 | Forward Primer | 5′-GACCTTGTGTCCTCCGCTTAT-3′ |
| Reverse Primer | 5′-CAACCGCAATTTGTGGCTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Lu, Q.; Ma, S.; Mamat, N.; Tang, S.; Liu, W.; Wang, Y.; Anwar, A.; Lu, Y.; Ma, Q.; et al. Proteomics Reveals the Role of PLIN2 in Regulating the Secondary Hair Follicle Cycle in Cashmere Goats. Int. J. Mol. Sci. 2025, 26, 2710. https://doi.org/10.3390/ijms26062710
Wu C, Lu Q, Ma S, Mamat N, Tang S, Liu W, Wang Y, Anwar A, Lu Y, Ma Q, et al. Proteomics Reveals the Role of PLIN2 in Regulating the Secondary Hair Follicle Cycle in Cashmere Goats. International Journal of Molecular Sciences. 2025; 26(6):2710. https://doi.org/10.3390/ijms26062710
Chicago/Turabian StyleWu, Cuiling, Qingwei Lu, Shengchao Ma, Nuramina Mamat, Sen Tang, Wenna Liu, Yaqian Wang, Asma Anwar, Yingjie Lu, Qiangqiang Ma, and et al. 2025. "Proteomics Reveals the Role of PLIN2 in Regulating the Secondary Hair Follicle Cycle in Cashmere Goats" International Journal of Molecular Sciences 26, no. 6: 2710. https://doi.org/10.3390/ijms26062710
APA StyleWu, C., Lu, Q., Ma, S., Mamat, N., Tang, S., Liu, W., Wang, Y., Anwar, A., Lu, Y., Ma, Q., Aimaier, G., & Fu, X. (2025). Proteomics Reveals the Role of PLIN2 in Regulating the Secondary Hair Follicle Cycle in Cashmere Goats. International Journal of Molecular Sciences, 26(6), 2710. https://doi.org/10.3390/ijms26062710






