Genome-Wide Identification and Expression Assessment for the Phosphate Transporter 2 Gene Family Within Sweet Potato Under Phosphorus Deficiency Stress
Abstract
1. Introduction
2. Results
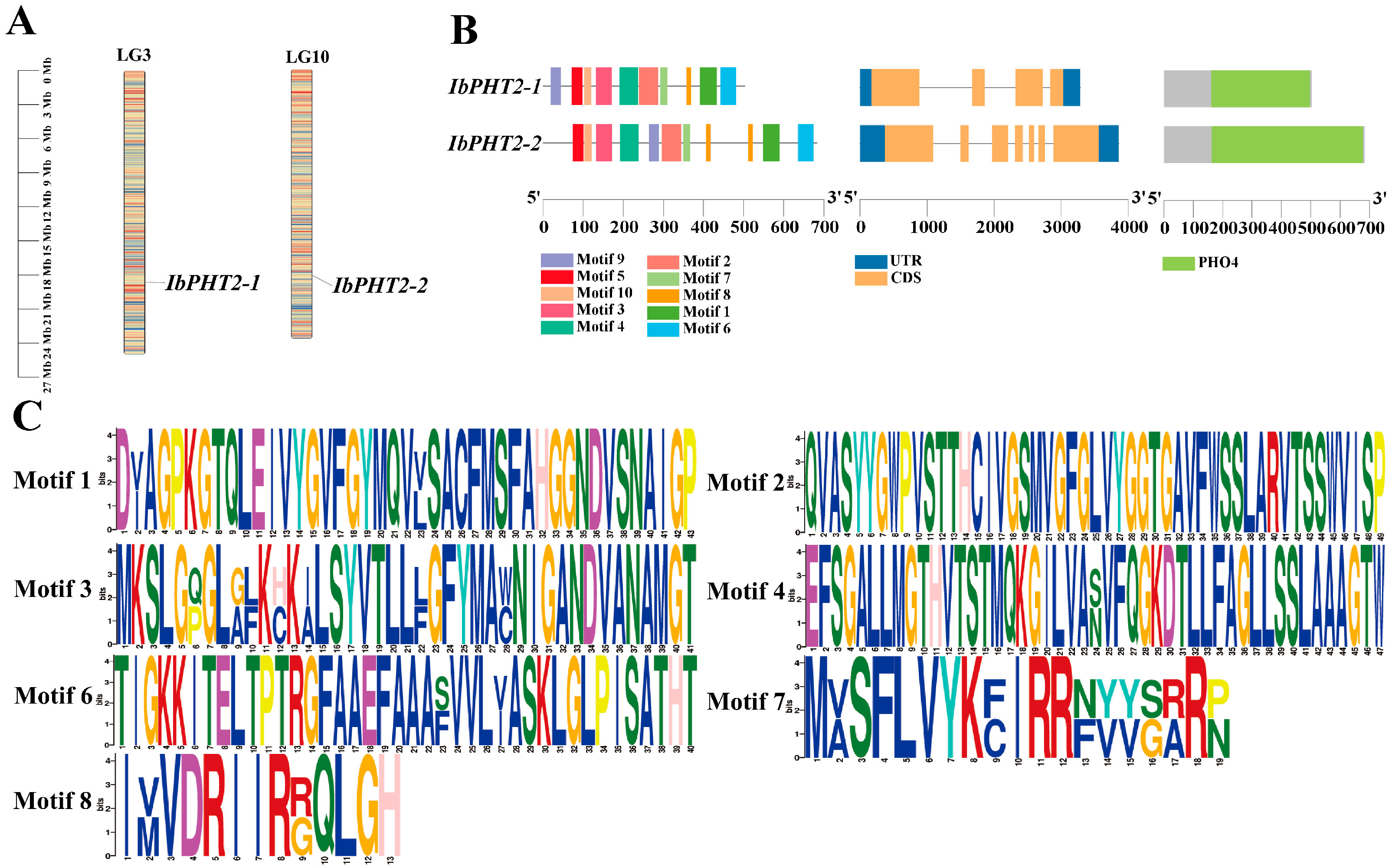
2.1. Characterization and Examination of the PHT2 Family Genes
2.2. Predictive Analysis of PHT2 Protein Structure and Interaction Network in Sweet Potato
2.3. Phylogenetic Analysis of PHT2 in Sweet Potato and Multiple Species: Interspecific Collinearity Analysis of PHT2 Gene
2.4. Analysis of PHT2 Phosphorus Transporter Promoter, Phosphorylation Sites, and Trans-Membrane Structural Regions
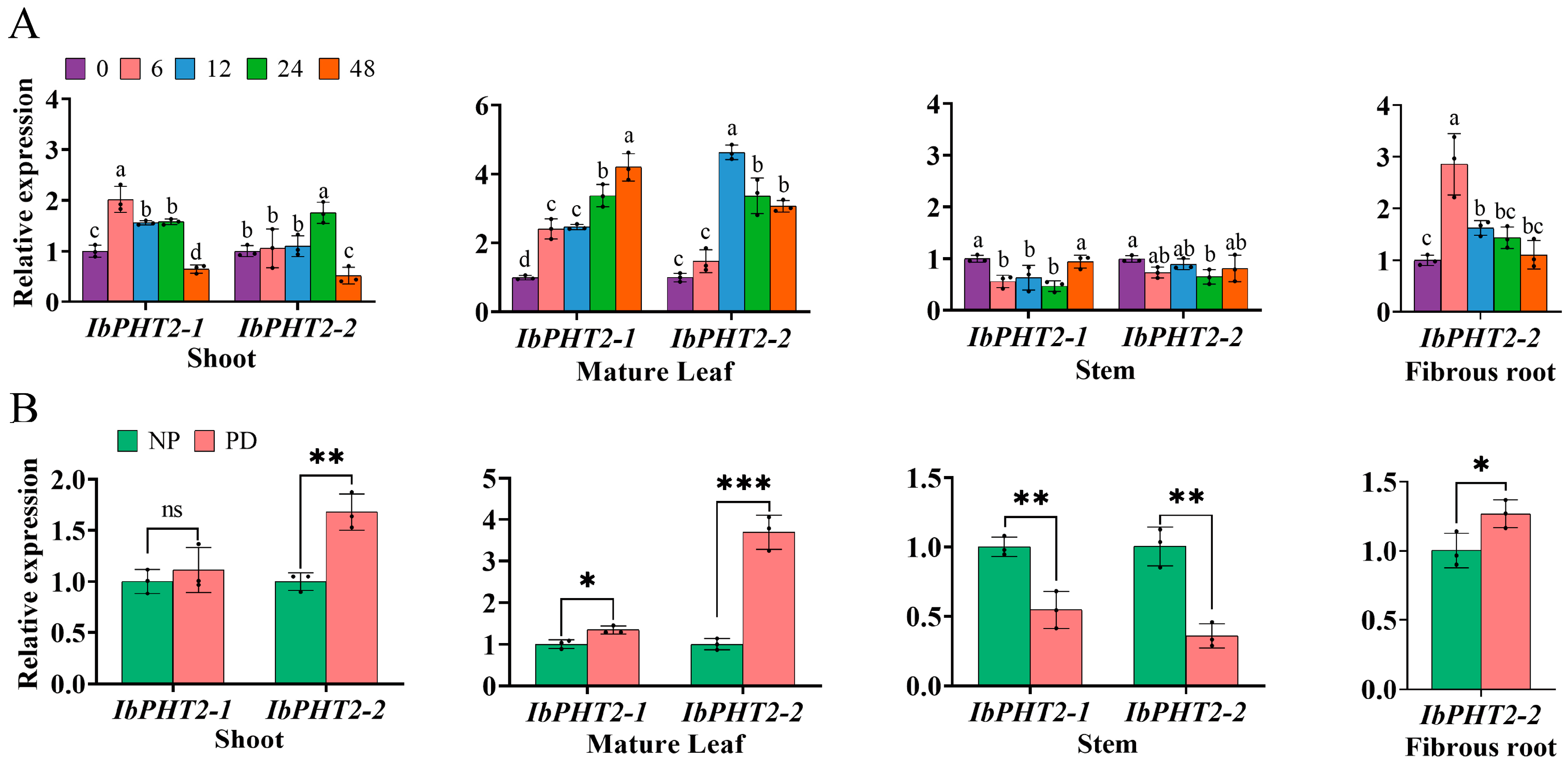
2.5. Construction and Analysis of IbPHT2 Gene Expression Profile in Sweet Potato
2.6. Consequences of Phosphorus Deficiency Stress on the Development of Sweet Potato Seedlings
2.7. Analysis of Gene Expression Under Phosphorus Deficiency Stress
2.8. Correlation Between IbPHT2 Gene Expression and Agronomic Traits
3. Discussion
3.1. Characteristic Analysis of PHT2 Gene Family
3.2. Impact of Phosphorus Deficiency Stress on Sweet Potato Seedling Growth
3.3. Analysis of IbPHT2 Expression Patterns and Phosphorus Deficiency Stress Response
4. Materials and Methods
4.1. Identification of Sweet Potato PHT2 Family
4.2. Physicochemical Properties and Subcellular Localization Prediction of Sweet Potato PHT2 Family Proteins
4.3. Chromosomal Location, Gene Structure, Conserved Motifs, and Domains Prediction of Sweet Potato PHT2 Genes
4.4. Secondary and Tertiary Structure Prediction and Protein Interaction Network of Sweet Potato PHT2 Proteins
4.5. Construction and Collinearity Assessment of the PHT2 Family Phylogenetic Tree in Sweet Potato
4.6. Prediction of Cis-Acting Elements, Trans-Membrane Domains, and Phosphorylation Sites in the Sweet Potato PHT2 Protein Promoter
4.7. Experimental Design
4.8. PHT2 Gene Transcriptome Data Download and RT-qPCR Analysis Under Phosphorus Deficiency Stress
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Hu, S.; Wang, S.; Yin, T.; Liu, Q.; Li, H. Sweet potato yield and quality characteristics affected by different late-season irrigation levels. J. Sci. Food Agric. 2024, 104, 5207–5218. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wu, Z.; Chen, B.; Yang, C.; Qi, D.; Lan, G.; Fraedrich, K. Effects of land-use change on eco-environmental quality in Hainan Island, China. Ecol. Indic. 2020, 109, 105777. [Google Scholar] [CrossRef]
- Ji, C.; Liu, H.; Cha, Z.; Lin, Q.; Feng, G. Spatial-Temporal Variation of N, P, and K Stoichiometry in Cropland of Hainan Island. Agriculture 2022, 12, 39. [Google Scholar] [CrossRef]
- Wang, D.; Huang, H.; Feng, H.; Wang, H.; Han, M.; Qi, Z. Distribution of copper in soil and rice system of Hainan Island, China. Braz. Arch. Biol. Technol. 2016, 59, e16160516. [Google Scholar]
- Zhang, Q.; Chu, Y.; Xue, Y.; Ying, H.; Chen, X.; Zhao, Y.; Ma, W.; Ma, L.; Zhang, J.; Yin, Y.; et al. Outlook of China’s agriculture transforming from smallholder operation to sustainable production. Glob. Food Secur. 2020, 26, 100444. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.Y.; Hsiao, Y.M.; Chiou, T.J. Milestones in understanding transport, sensing, and signaling of the plant nutrient phosphorus. Plant Cell 2024, 36, 1504–1523. [Google Scholar] [CrossRef]
- Li, T.; Hong, X.; Liu, S.; Wu, X.; Fu, S.; Liang, Y.; Li, J.; Li, R.; Zhang, C.; Song, X.; et al. Cropland degradation and nutrient overload on Hainan Island: A review and synthesis. Environ. Pollut. 2022, 313, 120100. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Jing, X.; Chen, P.; Jin, X.; Lei, J.; Wang, L.; Chai, S.; Yang, X. Physiological, Photosynthetic, and Transcriptomics Insights into the Influence of Shading on Leafy Sweet Potato. Genes 2023, 14, 2112. [Google Scholar] [CrossRef]
- Minemba, D. The Root and Shoot Characteristics That Enable Sweet Potato (Ipomoea batatas (L.) Lam.) to Grow in Low Phosphorus Soils. 2020. Available online: https://research-repository.uwa.edu.au/en/publications/the-root-and-shoot-characteristics-that-enable-sweet-potato-iipom (accessed on 8 December 2024).
- Liu, M.; Fan, W.; Jin, R.; Zhao, P.; Zhang, Q.; Zhu, X.; Wang, J.; Zhang, A.; Tang, Z. Effect of phosphorus application on economic yield, quality and phosphorus utilisation efficiency of purple-fleshed sweetpotato. Plant Soil Environ. 2022, 68, 451–458. [Google Scholar] [CrossRef]
- Mani, B.; Maurya, K.; Kohli, P.S.; Giri, J. Chickpea (Cicer arietinum) PHO1 family members function redundantly in Pi transport and root nodulation. Plant Physiol. Biochem. 2024, 211, 108712. [Google Scholar] [CrossRef]
- Versaw, W.K.; Harrison, M.J. A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 2002, 14, 1751–1766. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Xu, L.; Sun, M.; Yi, K.; Zhao, H. Functional Analysis of Phosphate Transporter OsPHT4 Family Members in Rice. Rice Sci. 2020, 27, 493–503. [Google Scholar]
- Młodzińska, E.; Zboińska, M. Phosphate Uptake and Allocation—A Closer Look at Arabidopsis thaliana L. and Oryza sativa L. Front. Plant Sci. 2016, 7, 1198. [Google Scholar] [CrossRef]
- Daram, P.; Brunner, S.; Rausch, C.; Steiner, C.; Amrhein, N.; Bucher, M. Pht2;1 encodes a low-affinity phosphate transporter from Arabidopsis. Plant Cell 1999, 11, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wang, L.; Wang, X.W.; Yan, Y.; Yang, X.L.; Xie, M.Y.; Hu, Z.; Shen, X.; Ai, H.; Lin, H.H.; et al. Mutation of the chloroplast-localized phosphate transporter OsPHT2;1 reduces flavonoid accumulation and UV tolerance in rice. Plant J. Cell Mol. Biol. 2020, 102, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, S.; Wu, X.; Wang, X.; Nan, Y.; Wang, D.; Chen, Q. Identification and characterization of phosphate transporter genes in potato. J. Biotechnol. 2017, 264, 17–28. [Google Scholar] [CrossRef]
- Rui, W.; Ma, J.; Wei, N.; Zhu, X.; Li, Z. Genome-Wide Analysis of the PHT Gene Family and Its Response to Mycorrhizal Symbiosis in Tomatoes under Phosphate Starvation Conditions. Int. J. Mol. Sci. 2023, 24, 10246. [Google Scholar] [CrossRef]
- Wei, X.; Fu, Y.; Yu, R.; Wu, L.; Wu, Z.; Tian, P.; Li, S.; Yang, X.; Yang, M. Comprehensive sequence and expression profile analysis of the phosphate transporter gene family in soybean. Sci. Rep. 2022, 12, 20883. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, S.; Li, M.; Zhao, Z. Genomic Identification and Expression Analysis of the Phosphate Transporter Gene Family in Poplar. Front. Plant Sci. 2016, 7, 1398. [Google Scholar] [CrossRef]
- Zhao, L.; Versaw, W.K.; Liu, J.; Harrison, M.J. A phosphate transporter from Medicago truncatula is expressed in the photosynthetic tissues of the plant and located in the chloroplast envelope. New Phytol. 2003, 157, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, X.; Sun, D.; Chen, Y.; Xu, G.; Cao, Y.; Ma, L.Q. Expressing Phosphate Transporter PvPht2;1 Enhances P Transport to the Chloroplasts and Increases Arsenic Tolerance in Arabidopsis thaliana. Environ. Sci. Technol. 2021, 55, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.; Zimmermann, P.; Amrhein, N.; Bucher, M. Expression analysis suggests novel roles for the plastidic phosphate transporter Pht2;1 in auto- and heterotrophic tissues in potato and Arabidopsis. Plant J. Cell Mol. Biol. 2004, 39, 13–28. [Google Scholar] [CrossRef]
- Pinard, D.; Mizrachi, E. Unsung and understudied: Plastids involved in secondary growth. Curr. Opin. Plant Biol. 2018, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhao, X.; Liu, X.; Zhang, L.; Gu, J.; Li, X.; Lu, W.; Xiao, K. Function of wheat phosphate transporter gene TaPHT2;1 in Pi translocation and plant growth regulation under replete and limited Pi supply conditions. Planta 2013, 237, 1163–1178. [Google Scholar] [CrossRef]
- Wei, X.; Xu, X.; Fu, Y.; Yang, X.; Wu, L.; Tian, P.; Yang, M.; Wu, Z. Effects of Soybean Phosphate Transporter Gene GmPHT2 on Pi Transport and Plant Growth under Limited Pi Supply Condition. Int. J. Mol. Sci. 2023, 24, 11115. [Google Scholar] [CrossRef]
- López-Maury, L.; Marguerat, S.; Bähler, J. Tuning gene expression to changing environments: From rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008, 9, 583–593. [Google Scholar] [CrossRef]
- Kareem, I.; Akinrinde, E.; Yusuff, O.; Eifediyi, E.K.; Abdulmaliq, S.Y.; Sikiru Yusuf, A.; Kareem, S.A.; Adekola, O. Enhancement of phosphorus uptake, growth and yield of sweet potato (Ipomoea batatas) with phosphorus fertilizers. J. Appl. Sci. Environ. Manag. 2020, 24, 79. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Liu, G.; Lu, X.; Chen, K.; Tian, J.; Liang, C. A Berberine Bridge Enzyme-Like Protein, GmBBE-like43, Confers Soybean’s Coordinated Adaptation to Aluminum Toxicity and Phosphorus Deficiency. Front. Plant Sci. 2022, 13, 947986. [Google Scholar] [CrossRef]
- Chen, W.; Tang, L.; Wang, J.; Zhu, H.; Jin, J.; Yang, J.; Fan, W. Research Advances in the Mutual Mechanisms Regulating Response of Plant Roots to Phosphate Deficiency and Aluminum Toxicity. Int. J. Mol. Sci. 2022, 23, 1137. [Google Scholar] [CrossRef]
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles, Regulation, and Agricultural Application of Plant Phosphate Transporters. Front. Plant Sci. 2017, 8, 817. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Z.; Xia, J.; Shen, S.; Guan, M.; Zhu, M.; Qiao, C.; Sun, F.; Liang, Y.; Li, J.; et al. Genome-Wide Analysis of Phosphorus Transporter Genes in Brassica and Their Roles in Heavy Metal Stress Tolerance. Int. J. Mol. Sci. 2020, 21, 2209. [Google Scholar] [CrossRef]
- Versaw, W.K.; Metzenberg, R.L. Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. USA 1995, 92, 3884–3887. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Xie, Y.; Pan, X.; Zhang, H.; Cao, P.; Su, X.; Chang, W.; Li, M. Photosynthetic Phosphoribulokinase Structures: Enzymatic Mechanisms and the Redox Regulation of the Calvin-Benson-Bassham Cycle. Plant Cell 2020, 32, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, P.; Liu, S.; Wang, C.; Liu, J. Evaluation of the Methods for Estimating Leaf Chlorophyll Content with SPAD Chlorophyll Meters. Remote Sens. 2022, 14, 5144. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Wang, N.; Zhang, H.Y.; Xu, R.; Si, C.C. Genome-Wide Analysis of Sweet Potato Ammonium Transporter (AMT): Influence on Nitrogen Utilization, Storage Root Development and Yield. Int. J. Mol. Sci. 2023, 24, 17424. [Google Scholar] [CrossRef]
- Ahmad, I.; Rawoof, A.; Islam, K.; Momo, J.; Ramchiary, N. Identification and expression analysis of phosphate transporter genes and metabolites in response to phosphate stress in Capsicum annuum. Environ. Exp. Bot. 2021, 190, 104597. [Google Scholar] [CrossRef]
- Mo, C.; Wan, S.; Xia, Y.; Ren, N.; Zhou, Y.; Jiang, X. Expression Patterns and Identified Protein-Protein Interactions Suggest That Cassava CBL-CIPK Signal Networks Function in Responses to Abiotic Stresses. Front. Plant Sci. 2018, 9, 269. [Google Scholar] [CrossRef]
- Ding, N.; Wang, A.; Zhang, X.; Wu, Y.; Wang, R.; Cui, H.; Huang, R.; Luo, Y. Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol. 2017, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]






| Gene Name | Gene ID | Protein Size (aa) | MW (kDa) | Isoelectric Point | Instability Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| IbPHT2-1 | g11922.t1 | 502 | 52.30 | 9.13 | 25.44 | 0.521 | Chloroplast |
| IbPHT2-2 | g40754.t1 | 682 | 72.98 | 9.52 | 35.26 | 0.305 | Chloroplast |
| Gene Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| actin | TATGGTTGGGATGGGACAGAA | CGGTAAGAAGGACAGGGTGCT |
| IbPHT2-1 | GACAGAAACTACCCAGACCAAGAAC | ACCCAAACACGCCGTAAACTATC |
| IbPHT2-2 | AATGATGTCTCCAACGCAATAGGC | ACAATCTGAGTCCCGCTTAGTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Bao, C.; Xing, H.; Guo, X.; Wang, S.; Zhou, X.; Lin, Y.; Si, C. Genome-Wide Identification and Expression Assessment for the Phosphate Transporter 2 Gene Family Within Sweet Potato Under Phosphorus Deficiency Stress. Int. J. Mol. Sci. 2025, 26, 2681. https://doi.org/10.3390/ijms26062681
Li H, Bao C, Xing H, Guo X, Wang S, Zhou X, Lin Y, Si C. Genome-Wide Identification and Expression Assessment for the Phosphate Transporter 2 Gene Family Within Sweet Potato Under Phosphorus Deficiency Stress. International Journal of Molecular Sciences. 2025; 26(6):2681. https://doi.org/10.3390/ijms26062681
Chicago/Turabian StyleLi, Hongyang, Cici Bao, Huixian Xing, Xin Guo, Shujuan Wang, Xianming Zhou, Yanhui Lin, and Chengcheng Si. 2025. "Genome-Wide Identification and Expression Assessment for the Phosphate Transporter 2 Gene Family Within Sweet Potato Under Phosphorus Deficiency Stress" International Journal of Molecular Sciences 26, no. 6: 2681. https://doi.org/10.3390/ijms26062681
APA StyleLi, H., Bao, C., Xing, H., Guo, X., Wang, S., Zhou, X., Lin, Y., & Si, C. (2025). Genome-Wide Identification and Expression Assessment for the Phosphate Transporter 2 Gene Family Within Sweet Potato Under Phosphorus Deficiency Stress. International Journal of Molecular Sciences, 26(6), 2681. https://doi.org/10.3390/ijms26062681





