β-hydroxy-β-methylbutyrate Attenuates Age-Dependent Loss of Flight Ability and Extends Lifespan in Drosophila
Abstract
1. Introduction
2. Results
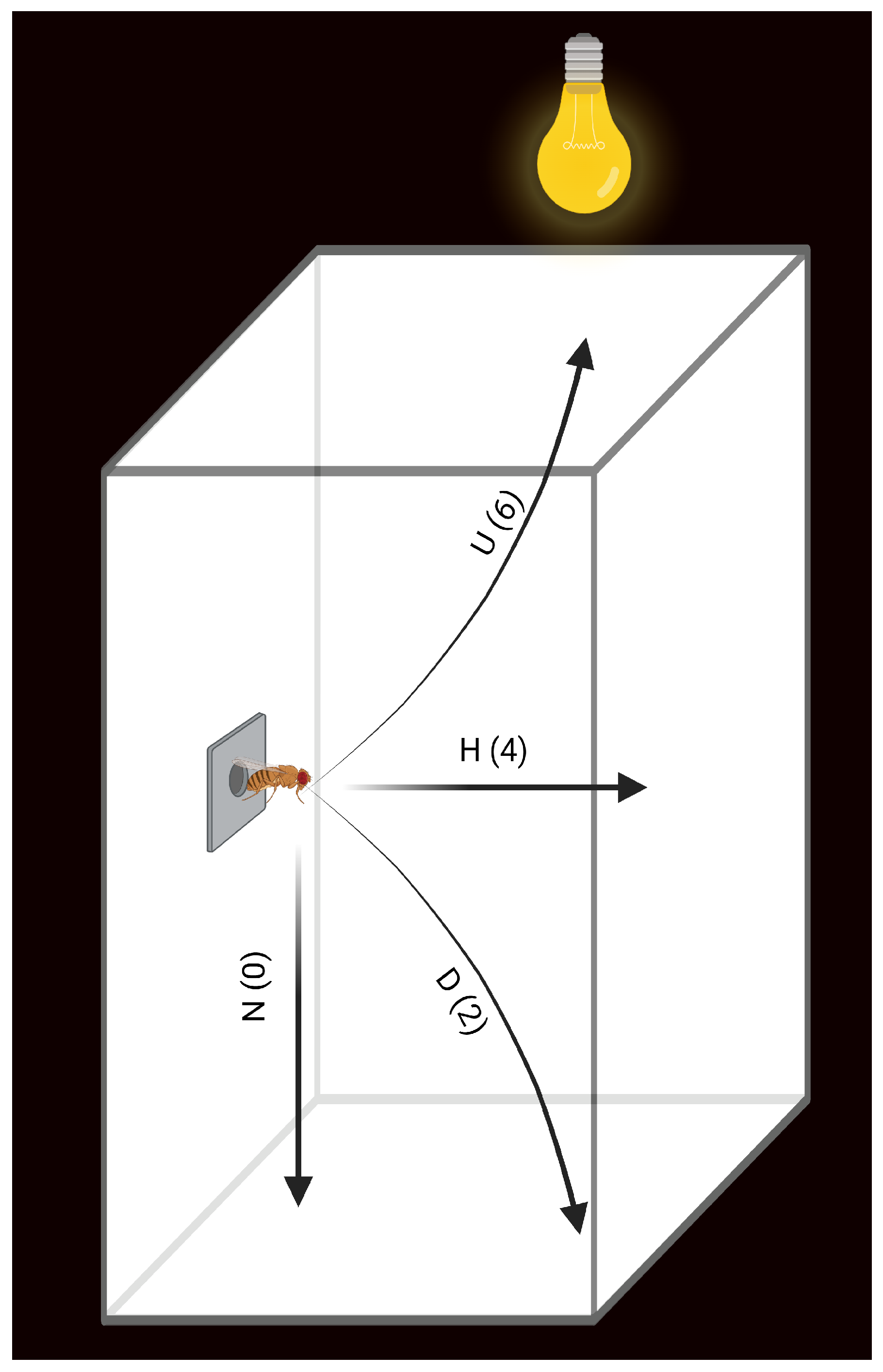
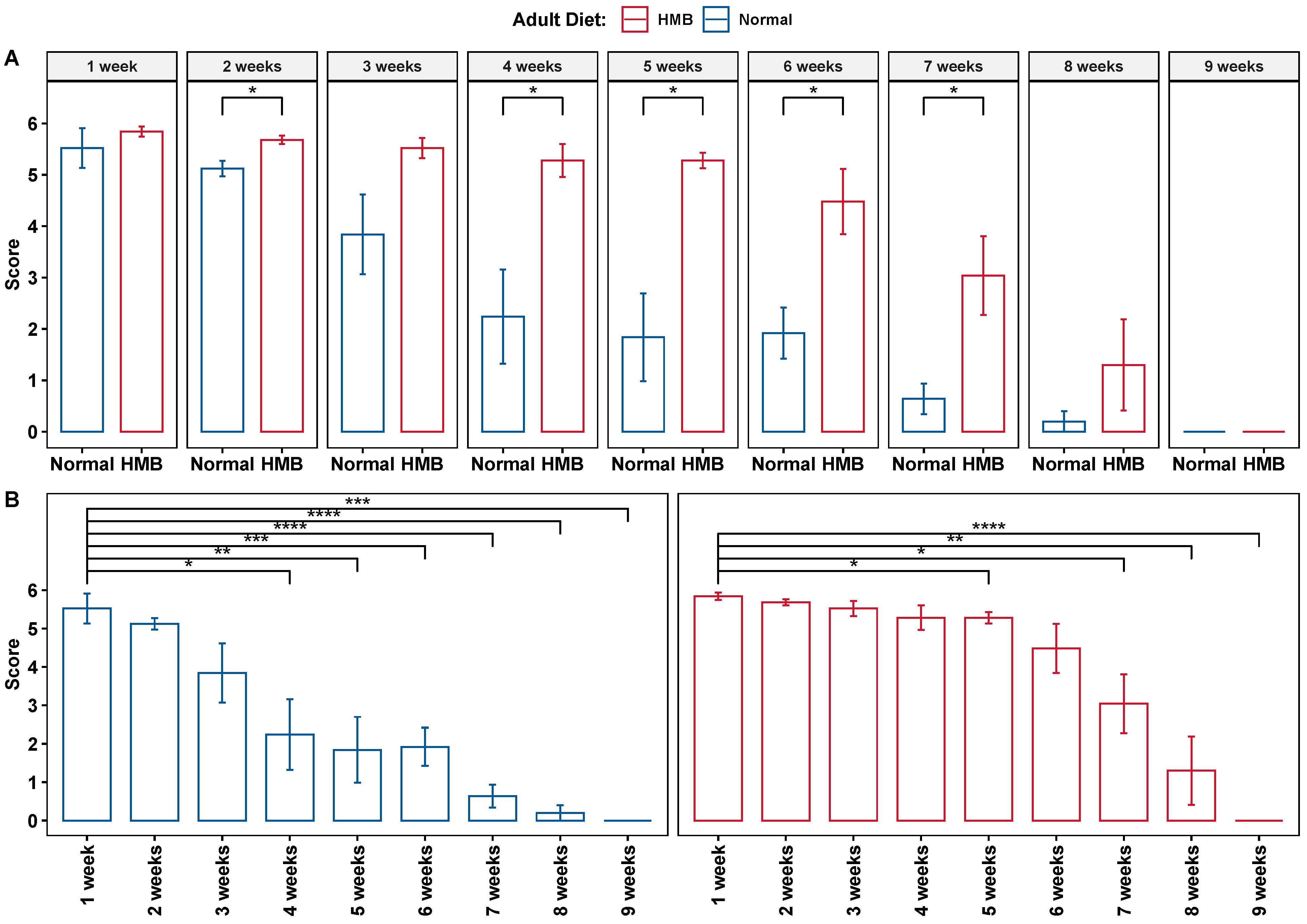
2.1. HMB Supplementation Attenuates Aging-Associated Loss of Flight Ability
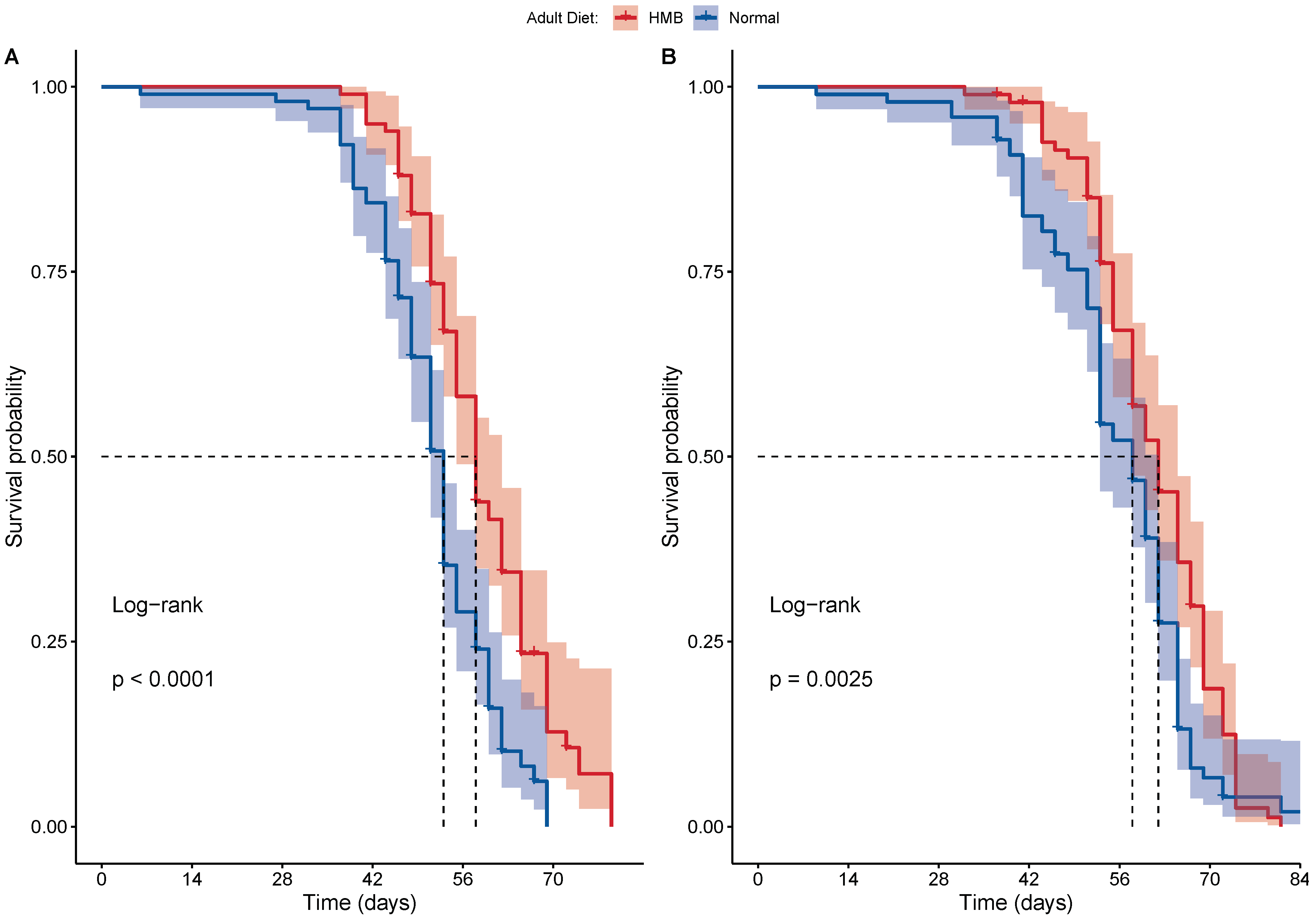
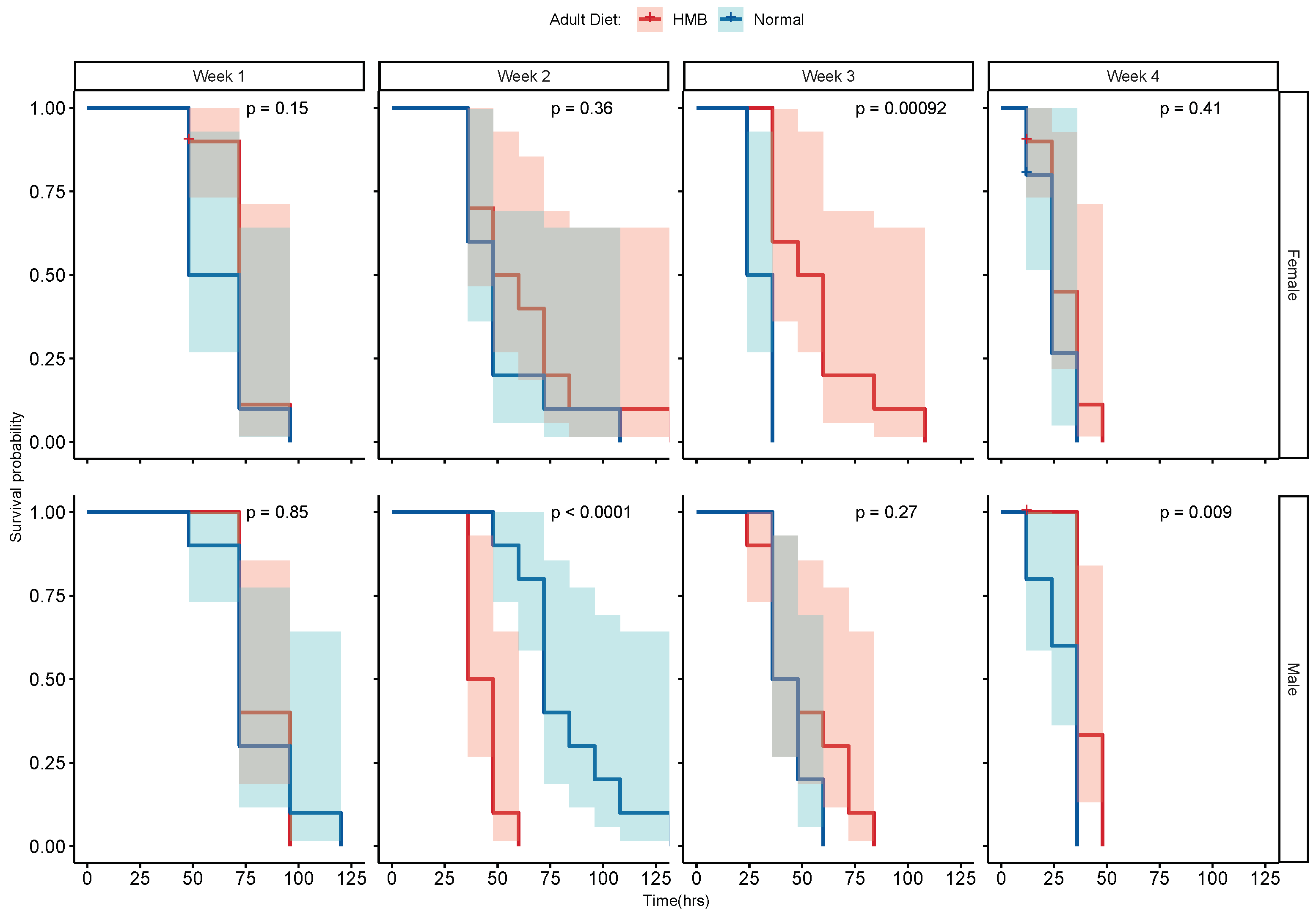
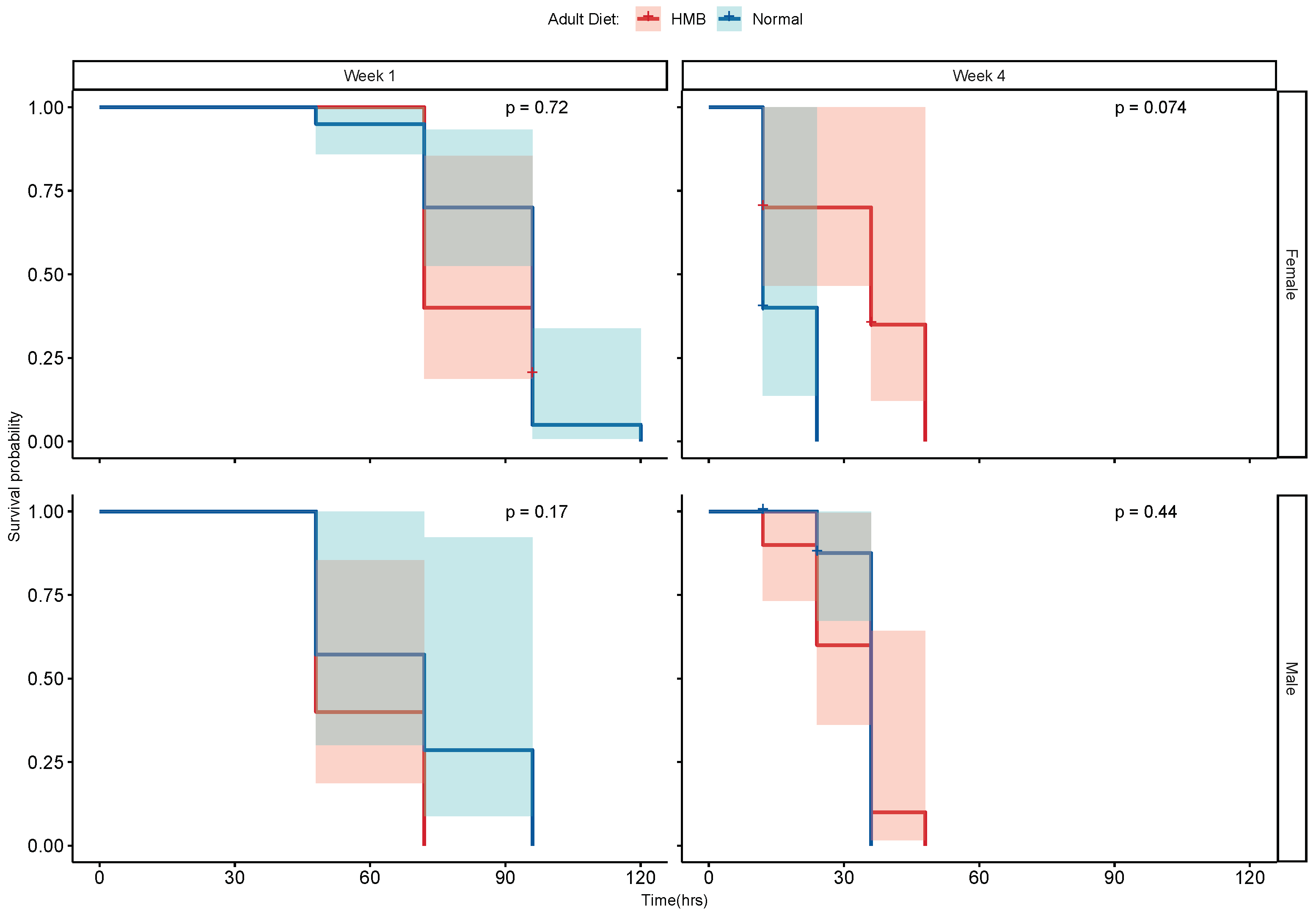
2.2. HMB Supplementation Extends Lifespan in D. melanogaster
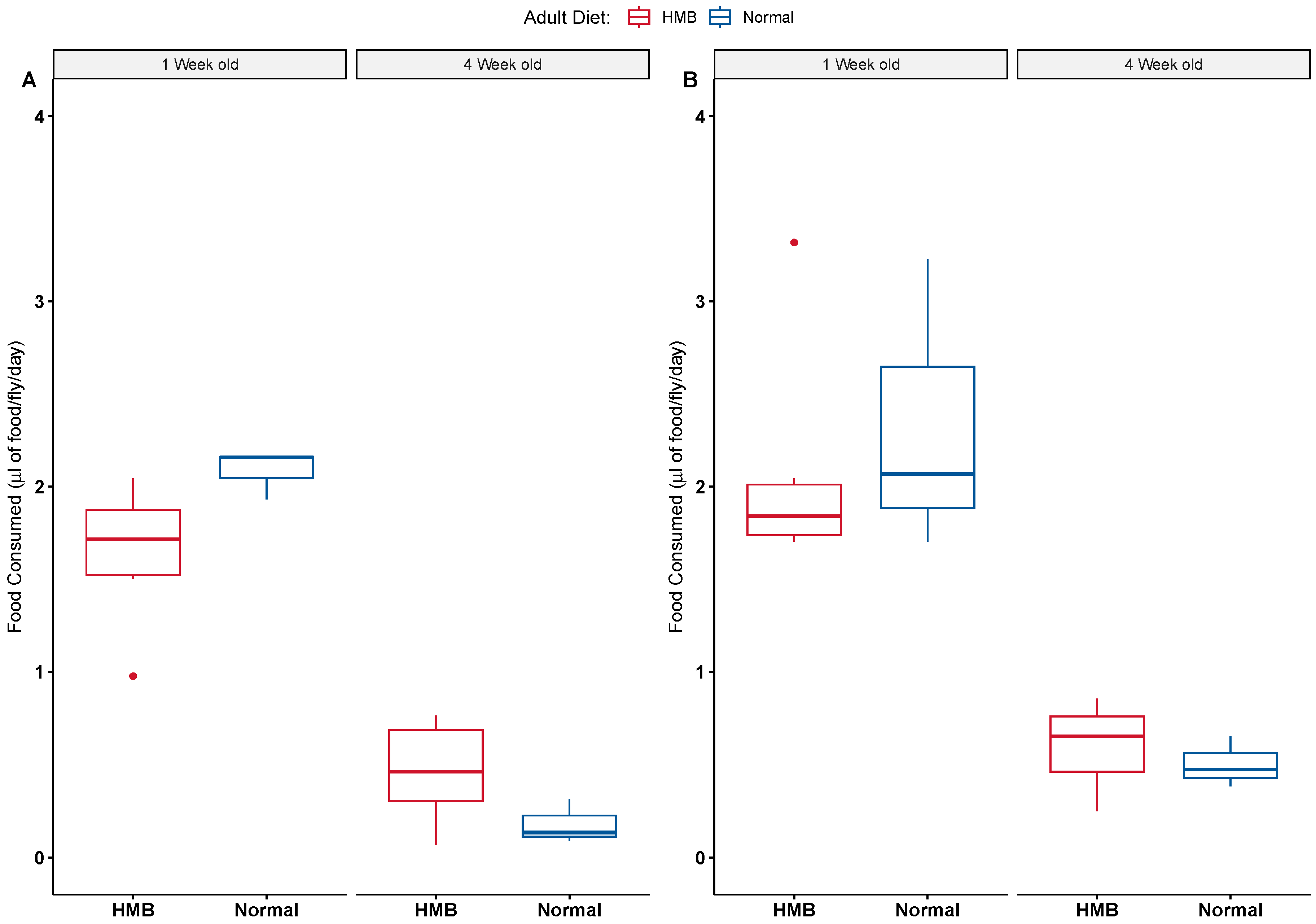
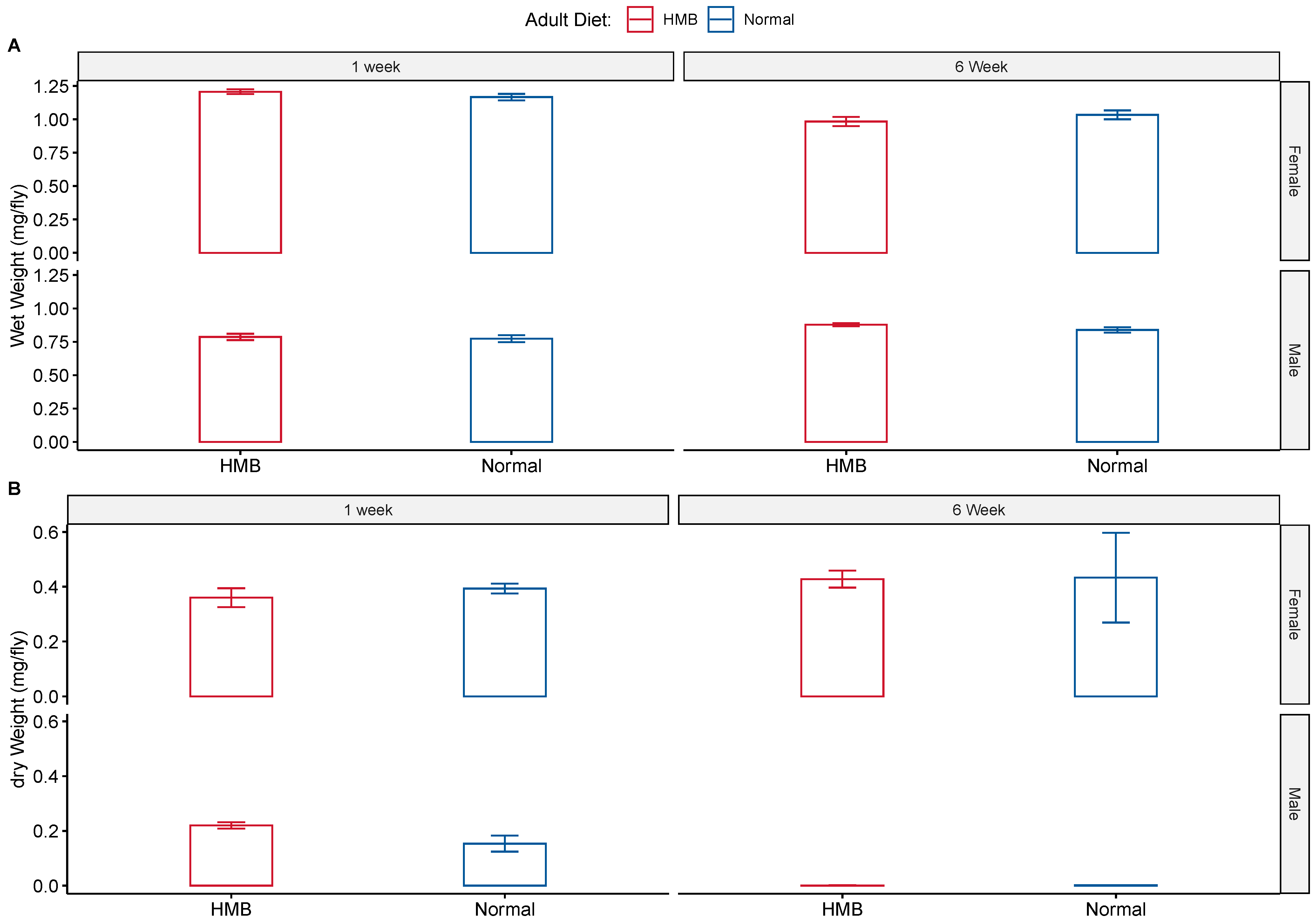
2.3. HMB Supplementation Does Not Alter Feeding Behavior
2.4. HMB Supplementation Confers No Resistance to Oxidative Stress
2.4.1. Resistance to Paraquat Stress
2.4.2. Resistance to Hydrogen Peroxide
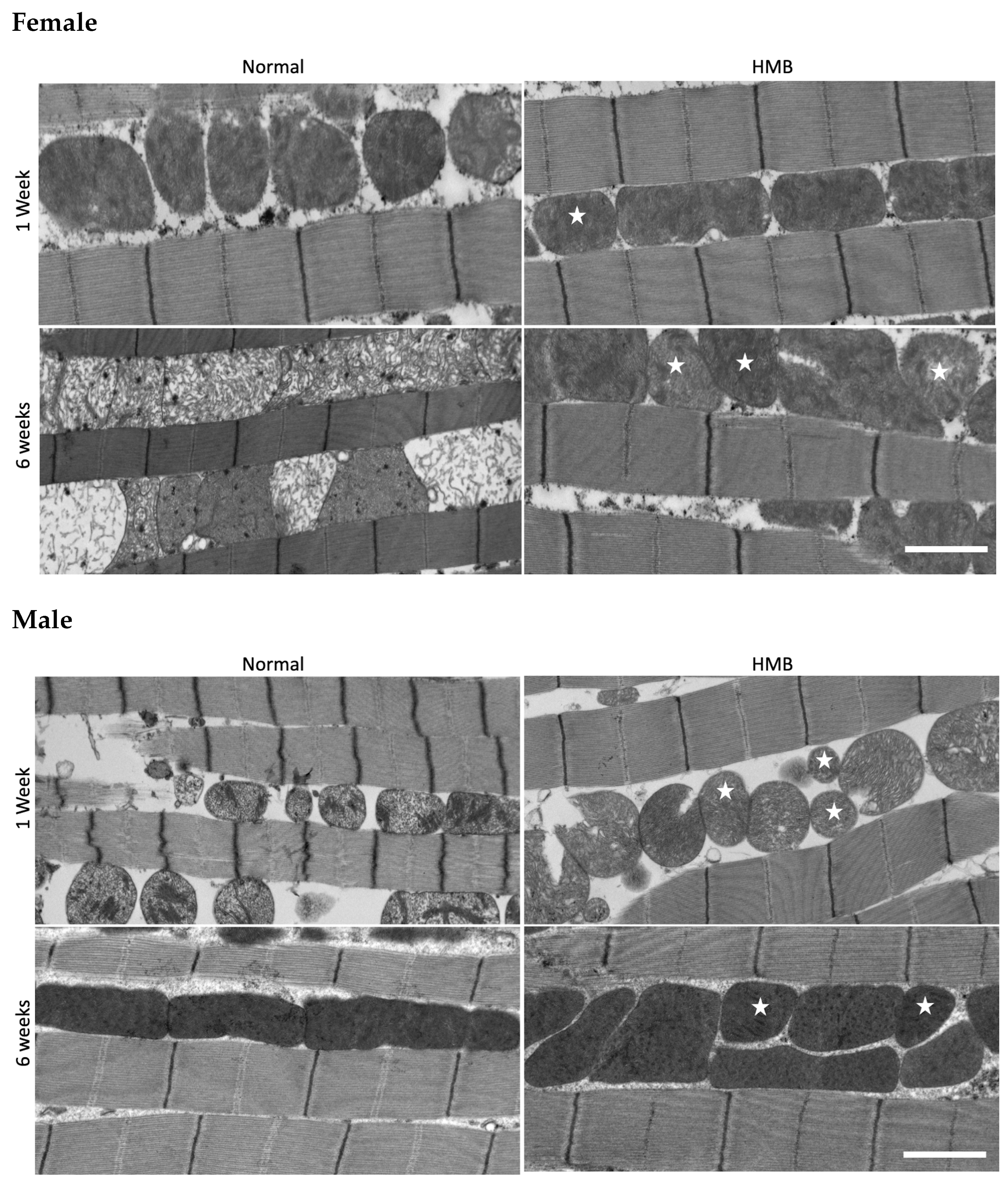
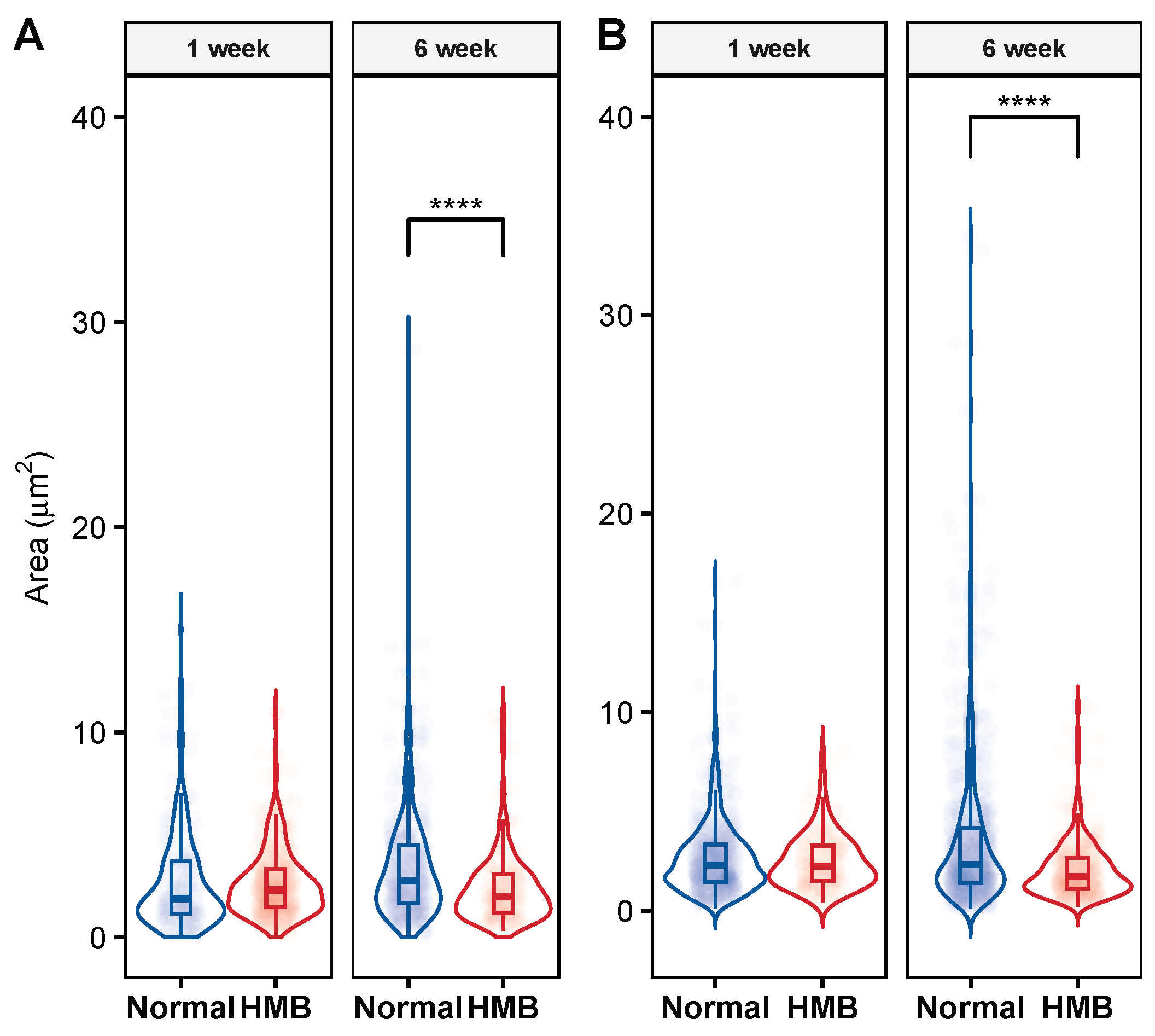
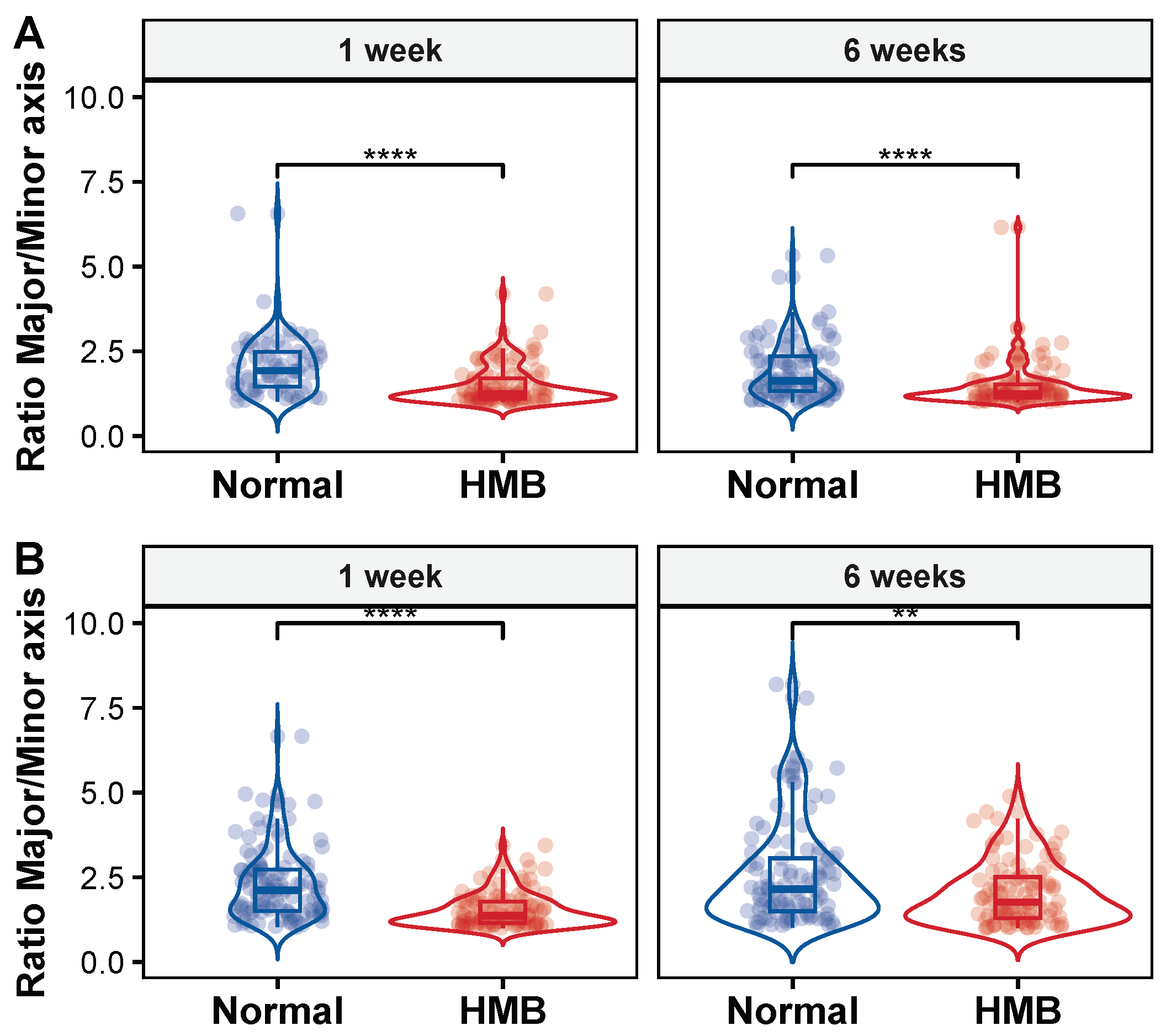
2.5. HMB Supplementation Improves Mitochondrial Morphology in Aging Flies
2.6. HMB Supplementation Does Not Improve Citrate Synthase Activity in Aging Flies
3. Discussion
4. Materials and Methods
4.1. D. melanogaster Rearing and Lifespan Assay
4.2. Flight Performance
4.3. Body Weight Measurements
4.4. CAFE Assay
4.5. Stress-Resistance Assay
4.6. Electron Microscopy
4.7. Image Analysis
4.8. Citrate Synthase Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | branched-chain amino acid |
| CI | confidence interval |
| CSY medium | cornmeal, sugar, and yeast medium |
| HMB | -hydroxy- -methylbutyrate |
| IFM | indirect flight muscle |
| TOR | target of rapamycin |
Appendix A
| Diet | Flight Score (±SE) | Age |
|---|---|---|
| Normal | 5.52 (±0.388) | 1 week |
| HMB | 5.84 (±0.098) | 1 week |
| Normal | 5.12 (±0.15) | 2 week |
| HMB | 5.68 (±0.08) | 2 week |
| Normal | 3.84 (±0.776) | 3 week |
| HMB | 5.52 (±0.196) | 3 week |
| Normal | 2.24 (±0.917) | 4 week |
| HMB | 5.28 (±0.32) | 4 week |
| Normal | 1.84 (±0.854) | 5 week |
| HMB | 5.28 (±0.15) | 5 week |
| Normal | 1.92 (±0.496) | 6 week |
| HMB | 4.48 (±0.637) | 6 week |
| Normal | 0.64 (±0.299) | 7 week |
| HMB | 3.04 (±0.765) | 7 week |
| Normal | 0.2 (±0.2) | 8 week |
| HMB | 1.3 (±0.889) | 8 week |
| Normal | 0 (±0) | 9 week |
| HMB | 0 (±0) | 9 week |
| Diet | Flight Score (±SE) | Age |
|---|---|---|
| Normal | 5.76 (±0.16) | 1 Week |
| HMB | 5.76 (±0.098) | 1 Week |
| Normal | 4.5 (±0.576) | 2 Week |
| HMB | 5.6 (±0.126) | 2 Week |
| Normal | 4.08 (±0.774) | 3 Week |
| HMB | 5.44 (±0.299) | 3 Week |
| Normal | 2.72 (±0.388) | 4 Week |
| HMB | 5.28 (±0.528) | 4 Week |
| Normal | 1.12 (±0.637) | 5 Week |
| HMB | 4.4 (±0.716) | 5 Week |
| Normal | 0 (±0) | 6 Week |
| HMB | 3.28 (±0.916) | 6 Week |
| Normal | 0 (±0) | 7 Week |
| HMB | 0.88 (±0.388) | 7 Week |
| Normal | 0 (±0) | 8 Week |
| HMB | 0 (±0) | 8 Week |
| Normal | 0 (±0) | 9 Week |
| HMB | 0 (±0) | 9 Week |
| Term | Contrast | Adj p Value |
|---|---|---|
| Adult Diet | Normal-HMB | 7.59 × 10−1 |
| Age | 4 Week old-1 Week old | 8.89 × 10−6 |
| Adult Diet:Age | Normal:1 Week old-HMB:1 Week old | 8.88 × 10−1 |
| Adult Diet:Age | HMB:4 Week old-HMB:1 Week old | 7.82 × 10−4 |
| Adult Diet:Age | Normal:4 Week old-HMB:1 Week old | 2.66 × 10−3 |
| Adult Diet:Age | HMB:4 Week old-Normal:1 Week old | 1.17 × 10−3 |
| Adult Diet:Age | Normal:4 Week old-Normal:1 Week old | 2.54 × 10−3 |
| Adult Diet:Age | Normal:4 Week old-HMB:4 Week old | 9.92 × 10−1 |
| Term | Contrast | Adj p Value |
|---|---|---|
| Adult Diet:Age | Normal:1 Week old-HMB:1 Week old | 9.448580 × 10−1 |
| Adult Diet:Age | HMB:4 Week old-HMB:1 Week old | 3.093000 × 10−4 |
| Adult Diet:Age | Normal:4 Week old-HMB:1 Week old | 2.264880 × 10−2 |
| Adult Diet:Age | HMB:4 Week old-Normal:1 Week old | 5.646400 × 10−3 |
| Adult Diet:Age | Normal:4 Week old-Normal:1 Week old | 1.209837 × 10−1 |
| Adult Diet:Age | Normal:4 Week old-HMB:4 Week old | 5.878281 × 10−1 |
| Term | Contrast | Adj p Value |
|---|---|---|
| Adult Diet | Normal-HMB | 5.82 × 10−1 |
| Age | 4 Week old-1 Week old | 5.25 × 10−8 |
| Adult Diet:Age | Normal:1 Week old-HMB:1 Week old | 1.77 × 10−1 |
| Adult Diet:Age | HMB:4 Week old-HMB:1 Week old | 2.84 × 10−5 |
| Adult Diet:Age | Normal:4 Week old-HMB:1 Week old | 2.51 × 10−5 |
| Adult Diet:Age | HMB:4 Week old-Normal:1 Week old | 7.6 × 10−6 |
| Adult Diet:Age | Normal:4 Week old-Normal:1 Week old | 6.3 × 10−6 |
| Adult Diet:Age | Normal:4 Week old-HMB:4 Week old | 5.29 × 10−1 |
| Term | Contrast | Adj p Value |
|---|---|---|
| Adult Diet | Normal-HMB | 2.14 × 10−1 |
| Age | 4 Week old-1 Week old | 4.00 × 10−3 |
| Adult Diet:Age | Normal:1 Week old-HMB:1 Week old | 9.63 × 10−1 |
| Adult Diet:Age | HMB:4 Week old-HMB:1 Week old | 3.30 × 10−2 |
| Adult Diet:Age | Normal:4 Week old-HMB:1 Week old | 6.40 × 10−1 |
| Adult Diet:Age | HMB:4 Week old-Normal:1 Week old | 3.90 × 10−2 |
| Adult Diet:Age | Normal:4 Week old-Normal:1 Week old | 4.93 × 10−1 |
| Adult Diet:Age | Normal:4 Week old-HMB:4 Week old | 5.40 × 10−1 |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Adult Diet | 1 | 0.02 | 0.02 | 0.10 | 7.59 × 10−1 |
| Age | 1 | 11.47 | 11.47 | 45.95 | 8.89 × 10−6 |
| Adult Diet:Age | 1 | 0.12 | 0.12 | 0.49 | 4.94 × 10−1 |
| Residuals | 14 | 3.49 | 0.25 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Adult Diet | 1 | 0.09 | 0.09 | 0.27 | 6.13 × 10−1 |
| Age | 1 | 12.43 | 12.43 | 36.06 | 3.23 × 10−5 |
| Adult Diet:Age | 1 | 0.58 | 0.58 | 1.68 | 2.16 × 10−1 |
| Residuals | 14 | 4.82 | 0.34 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Adult Diet | 1 | 0.03 | 0.03 | 0.32 | 5.82 × 10−1 |
| Age | 1 | 9.08 | 9.08 | 109.69 | 5.25 × 10−8 |
| Adult Diet:Age | 1 | 0.52 | 0.52 | 6.34 | 2.46 × 10−2 |
| Residuals | 14 | 1.16 | 0.08 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Adult Diet | 1 | 0.22 | 0.22 | 1.69 | 0.21418 |
| Age | 1 | 1.49 | 1.49 | 11.52 | 0.00436 |
| Adult Diet:Age | 1 | 0.05 | 0.05 | 0.39 | 0.53997 |
| Residuals | 14 | 1.82 | 0.13 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Age | 1 | 0.02 | 0.02 | 14.57 | 0.00411 |
| Adult Diet | 1 | 0.00 | 0.00 | 1.62 | 0.23514 |
| Residuals | 9 | 0.01 | 0.00 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Age | 1 | 0.10 | 0.1 | 99.74 | 3.62 × 10−6 |
| Adult Diet | 1 | 0.00 | 0.0 | 3.13 | 1.11 × 10−1 |
| Residuals | 9 | 0.01 | 0.0 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Age | 1 | 0.10 | 0.1 | 33.94 | 0.000251 |
| Adult Diet | 1 | 0.00 | 0.0 | 0.03 | 0.873853 |
| Residuals | 9 | 0.03 | 0.0 | NA | NA |
| Term | DF | SUMSQ | MEANSQ | Statistic | p Value |
|---|---|---|---|---|---|
| Age | 1 | 0.01 | 0.01 | 0.44 | 0.522 |
| Adult Diet | 1 | 0.00 | 0.00 | 0.06 | 0.816 |
| Residuals | 9 | 0.18 | 0.02 | NA | NA |
| Term | Contrast | Null.Value | Estimate | Conf.Low | Conf.High | Adj p Value |
|---|---|---|---|---|---|---|
| Diet | Normal-HMB | 0 | 0.66 | 0.43 | 0.89 | 2.07 × 10−8 |
| Age | 6 week-1 week | 0 | 0.21 | −0.02 | 0.44 | 0.06905 |
| Diet:Age | Normal:1 week-HMB:1 week | 0 | 0.09 | −0.37 | 0.54 | 0.961 |
| Diet:Age | HMB:6 week-HMB:1 week | 0 | −0.26 | −0.74 | 0.22 | 0.501 |
| Diet:Age | Normal:6 week-HMB:1 week | 0 | 0.82 | 0.46 | 1.18 | 3.1 × 10−8 |
| Diet:Age | HMB:6 week-Normal:1 week | 0 | −0.35 | −0.91 | 0.21 | 0.38 |
| Diet:Age | Normal:6 week-Normal:1 week | 0 | 0.73 | 0.27 | 1.19 | 2.49 × 10−4 |
| Diet:Age | Normal:6 week-HMB:6 week | 0 | 1.08 | 0.60 | 1.56 | 6.5 × 10−8 |
| Term | Contrast | Null.Value | Estimate | Conf.Low | Conf.High | Adj p Value |
|---|---|---|---|---|---|---|
| Diet | Normal-HMB | 0 | 0.89 | 0.64 | 1.14 | 7.19 × 10−11 |
| Age | 6 week-1 week | 0 | 0.46 | 0.23 | 0.69 | 8.91 × 10−5 |
| Diet:Age | Normal:1 week-HMB:1 week | 0 | 0.15 | −0.40 | 0.70 | 0.9033 |
| Diet:Age | HMB:6 week-HMB:1 week | 0 | −0.45 | −1.04 | 0.15 | 0.2089 |
| Diet:Age | Normal:6 week-HMB:1 week | 0 | 0.93 | 0.40 | 1.46 | 4.657 × 10−5 |
| Diet:Age | HMB:6 week-Normal:1 week | 0 | −0.60 | −1.03 | -0.16 | 2.675 × 10−3 |
| Diet:Age | Normal:6 week-Normal:1 week | 0 | 0.78 | 0.43 | 1.14 | 6.9026 × 10−8 |
| Diet:Age | Normal:6 week-HMB:6 week | 0 | 1.38 | 0.96 | 1.80 | 6.43 × 10−11 |
| Age | Diet | Variable | n | Mean | Median | S.E |
|---|---|---|---|---|---|---|
| 1 week | HMB | Area | 201.00 | 2.51 | 2.25 | 0.10 |
| 1 week | Normal | Area | 661.00 | 2.66 | 2.30 | 0.07 |
| 6 week | HMB | Area | 384.00 | 2.06 | 1.72 | 0.07 |
| 6 week | Normal | Area | 874.00 | 3.44 | 2.34 | 0.12 |
| Age | Diet | Variable | n | Mean | Median | S.E |
|---|---|---|---|---|---|---|
| 1 week | HMB | Area | 497.00 | 2.62 | 2.31 | 0.07 |
| 1 week | Normal | Area | 215.00 | 2.71 | 1.89 | 0.16 |
| 6 week | HMB | Area | 186.00 | 2.36 | 1.98 | 0.12 |
| 6 week | Normal | Area | 471.00 | 3.44 | 2.75 | 0.12 |
| Age | Axes | Group1 | Group2 | n1 | n2 | Statistic | df | p | p.adj |
|---|---|---|---|---|---|---|---|---|---|
| 1 week | Major | HMB | Normal | 111 | 69 | −7.30 | 110.55 | 0.00 | 0.00 |
| 6 weeks | Major | HMB | Normal | 102 | 104 | −6.77 | 147.41 | 0.00 | 0.00 |
| 1 week | Minor | HMB | Normal | 111 | 69 | −0.33 | 132.19 | 0.74 | 0.74 |
| 6 weeks | Minor | HMB | Normal | 102 | 104 | −2.80 | 153.80 | 0.01 | 0.01 |
| Age | Axes | Group1 | Group2 | n1 | n2 | Statistic | df | p | p.adj |
|---|---|---|---|---|---|---|---|---|---|
| 1 week | Major | HMB | Normal | 111 | 69 | −7.30 | 110.55 | 0.00 | 0.00 |
| 6 weeks | Major | HMB | Normal | 102 | 104 | −6.77 | 147.41 | 0.00 | 0.00 |
| 1 week | Minor | HMB | Normal | 111 | 69 | −0.33 | 132.19 | 0.74 | 0.74 |
| 6 weeks | Minor | HMB | Normal | 102 | 104 | −2.80 | 153.80 | 0.01 | 0.01 |
References
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Model. Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Berendzen, K.M.; Dillin, A. Systemic stress signalling: Understanding the cell non-autonomous control of proteostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 211–217. [Google Scholar] [CrossRef]
- Goldspink, G. Age-related loss of muscle mass and strength. J. Aging Res. 2012, 2012, 158279. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Pennings, B.; Fry, C.S.; Dhanani, S.; Dillon, E.L.; Sheffield-Moore, M.; Volpi, E.; Rasmussen, B.B. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J. Appl. Physiol. 2008, 104, 1452–1461. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Wolfe, R.; Volpi, E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J. Nutr. Health Aging 2002, 6, 358. [Google Scholar]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.B.; van Dijk, J.W.; de Lange, A.; Kiskini, A.; Kuklinski, M.; Senden, J.M.; Van Loon, L.J. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am. J. Clin. Nutr. 2013, 98, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Butteiger, D.; Cope, M.; Liu, P.; Mukherjea, R.; Volpi, E.; Rasmussen, B.; Krul, E. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin. Nutr. 2013, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef]
- Kanda, A.; Nakayama, K.; Fukasawa, T.; Koga, J.; Kanegae, M.; Kawanaka, K.; Higuchi, M. Post-exercise whey protein hydrolysate supplementation induces a greater increase in muscle protein synthesis than its constituent amino acid content. Br. J. Nutr. 2013, 110, 981–987. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.; Van Loon, L.J. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef]
- Res, P.T.; Groen, B.; Pennings, B.; Beelen, M.; Wallis, G.A.; Gijsen, A.P.; Senden, J.M.; van Loon, L.J. Protein ingestion before sleep improves postexercise overnight recovery. Med. Sci. Sport. Exerc. 2012, 44, 1560–1569. [Google Scholar] [CrossRef]
- Koopman, R.; Verdijk, L.B.; Beelen, M.; Gorselink, M.; Kruseman, A.N.; Wagenmakers, A.J.; Kuipers, H.; Van Loon, L.J. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br. J. Nutr. 2008, 99, 571–580. [Google Scholar] [CrossRef]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.; Senden, J.M.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef]
- Kimball, S.R.; Shantz, L.M.; Horetsky, R.L.; Jefferson, L.S. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J. Biol. Chem. 1999, 274, 11647–11652. [Google Scholar] [CrossRef]
- Boutry, C.; El-Kadi, S.W.; Suryawan, A.; Wheatley, S.M.; Orellana, R.A.; Kimball, S.R.; Nguyen, H.V.; Davis, T.A. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E620–E631. [Google Scholar] [CrossRef] [PubMed]
- Tischler, M.E.; Desautels, M.; Goldberg, A.L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J. Biol. Chem. 1982, 257, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A complex relationship. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Haymond, M.W. Effects of fasting on flux and interconversion of leucine and alpha-ketoisocaproate in vivo. Am. J. Physiol.-Endocrinol. Metab. 1981, 241, E72–E75. [Google Scholar] [CrossRef]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International society of sports nutrition position stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sport. Nutr. 2013, 10, 1–14. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef]
- Aversa, Z.; Bonetto, A.; Costelli, P.; Minero, V.G.; Penna, F.; Baccino, F.M.; Lucia, S.; Rossi Fanelli, F.; Muscaritoli, M. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int. J. Oncol. 2011, 38, 713–720. [Google Scholar]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by β-hydroxy-β-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [CrossRef]
- Supinski, G.S.; Callahan, L.A. β-hydroxy-β-methylbutyrate (HMB) prevents sepsis-induced diaphragm dysfunction in mice. Respir. Physiol. Neurobiol. 2014, 196, 63–68. [Google Scholar] [CrossRef]
- Kovarik, M.; Muthny, T.; Sispera, L.; Holecek, M. Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J. Physiol. Biochem. 2010, 66, 311–319. [Google Scholar] [CrossRef]
- Hao, Y.; Jackson, J.R.; Wang, Y.; Edens, N.; Pereira, S.L.; Alway, S.E. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R701–R715. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Pereira, S.L.; Edens, N.K.; Hao, Y.; Bennett, B.T. β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp. Gerontol. 2013, 48, 973–984. [Google Scholar] [CrossRef]
- Noh, K.K.; Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Park, C.H.; Yoon, C.; Kim, N.D.; Kim, M.K.; Chung, H.Y. β-Hydroxy β-Methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS ONE 2014, 9, e102947. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Henning, P.C.; Grant, S.C.; Lee, W.J.; Lee, S.R.; Arjmandi, B.H.; Kim, J.S. HMB attenuates muscle loss during sustained energy deficit induced by calorie restriction and endurance exercise. Metabolism 2013, 62, 1718–1729. [Google Scholar] [CrossRef]
- Panton, L.B.; Rathmacher, J.A.; Baier, S.; Nissen, S. Nutritional supplementation of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB) during resistance training. Nutrition 2000, 16, 734–739. [Google Scholar] [CrossRef]
- Gallagher, P.M.; Carrithers, J.A.; Godard, M.P.; Schulze, K.E.; Trappe, S.W. β-hydroxy-β-methylbutyrate ingestion, part I: Effects on strength and fat free mass. Med. Sci. Sport. Exerc. 2000, 32, 2109–2115. [Google Scholar] [CrossRef]
- Portal, S.; Eliakim, A.; Nemet, D.; Halevy, O.; Zadik, Z. Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices. J. Pediatr. Endocrinol. Metab. 2010, 23, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Andersen, J.; Wilson, S.M.; Stout, J.R.; Duncan, N.; Fuller, J.C.; Baier, S.M.; Naimo, M.A.; et al. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: A randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2014, 114, 1217–1227. [Google Scholar] [CrossRef]
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.; Rice, D.; Fuller, J., Jr.; Connelly, A.; Abumrad, N. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar]
- Fuller, J.C., Jr.; Baier, S.; Flakoll, P.; Nissen, S.L.; Abumrad, N.N.; Rathmacher, J.A. Vitamin D status affects strength gains in older adults supplemented with a combination of β-hydroxy-β-methylbutyrate, arginine, and lysine: A cohort study. J. Parenter. Enter. Nutr. 2011, 35, 757–762. [Google Scholar]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of β-hydroxy-β-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Stubbs, N.B.; Bohlken, R.M. Body composition in 70-year-old adults responds to dietary β-hydroxy-β-methylbutyrate similarly to that of young adults. J. Nutr. 2001, 131, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of β-hydroxy-β-methylbutyrate (HMB), l-arginine, and l-lysine. J. Parenter. Enter. Nutr. 2009, 33, 71–82. [Google Scholar] [CrossRef]
- Hsieh, L.C.; Chow, C.J.; Chang, W.C.; Liu, T.H.; Chang, C.K. Effect of beta-hydroxy-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac. J. Clin. Nutr. 2010, 19, 200–208. [Google Scholar]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+ yrs: A randomized, double-blind pilot trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef]
- Deutz, N.E.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.D.; Rosa, J.C.; Lira, F.S.; Zanchi, N.E.; Ropelle, E.R.; Oyama, L.M.; Oller do Nascimento, C.M.; de Mello, M.T.; Tufik, S.; Santos, R.V. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr. Metab. 2011, 8, 11. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by β-hydroxy-β-methylbutyrate. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1409–E1416. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.J.; Wyke, S.M.; Tisdale, M.J. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by β-hydroxy-β-methylbutyrate. Cancer Res. 2004, 64, 8731–8735. [Google Scholar] [CrossRef]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Perrimon, N. Of flies and men: Insights on organismal metabolism from fruit flies. BMC Biol. 2013, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Maughan, D.; Moore, J.; Vigoreaux, J.; Barnes, B.; Mulieri, L.A. Work production and work absorption in muscle strips from vertebrate cardiac and insect flight muscle fibers. Adv. Exp. Med. Biol. 1998, 453, 471–480. [Google Scholar] [PubMed]
- Miller, M.S.; Lekkas, P.; Braddock, J.M.; Farman, G.P.; Ballif, B.A.; Irving, T.C.; Maughan, D.W.; Vigoreaux, J.O. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys. J. 2008, 95, 2391–2401. [Google Scholar] [CrossRef]
- Beattie, A.E. An Analysis of the Effect of β-Hydroxy-β-Methylbutyrate on the Flight Ability and Lifespan of Drosophila melanogaster. Master’s Thesis, University of Vermont, Burlington, VT, USA, 2014. Available online: https://scholarworks.uvm.edu/graddis/263 (accessed on 23 September 2021).
- William, W.J.; Carvalho, G.B.; Mak, E.M.; Noelle, N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar]
- Deepashree, S.; Niveditha, S.; Shivanandappa, T.; Ramesh, S. Oxidative stress resistance as a factor in aging: Evidence from an extended longevity phenotype of Drosophila melanogaster. Biogerontology 2019, 20, 497–513. [Google Scholar] [CrossRef]
- Rana, A.; Rera, M.; Walker, D.W. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl. Acad. Sci. USA 2013, 110, 8638–8643. [Google Scholar] [CrossRef]
- Rana, A.; Oliveira, M.P.; Khamoui, A.V.; Aparicio, R.; Rera, M.; Rossiter, H.B.; Walker, D.W. Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat. Commun. 2017, 8, 448. [Google Scholar] [CrossRef]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef]
- Metaxakis, A.; Partridge, L. Dietary restriction extends lifespan in wild-derived populations of Drosophila melanogaster. PloS ONE 2013, 8, e74681. [Google Scholar] [CrossRef]
- Picard, M.; Ritchie, D.; Wright, K.J.; Romestaing, C.; Thomas, M.M.; Rowan, S.L.; Taivassalo, T.; Hepple, R.T. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 2010, 9, 1032–1046. [Google Scholar] [CrossRef]
- Pinheiro, C.H.D.J.; Gerlinger-Romero, F.; Guimaraes-Ferreira, L.; de Souza, A.L., Jr.; Vitzel, K.F.; Nachbar, R.T.; Nunes, M.T.; Curi, R. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur. J. Appl. Physiol. 2012, 112, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Vishnudas, V.; Vigoreaux, J.O. Sustained High Power Performance: Possible Strategies for Integrating Energy Supply and Demand in Flight Muscle. In Nature’s Versatile Engine: Insect Flight Muscle Inside and Out; Springer: New York, NY, USA; Landes Bioscience: Georgetown, Guyana, 2006; pp. 188–196. [Google Scholar]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.G.; Kühlbrandt, W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. eLife 2017, 6, e24662. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Mitochondrial Impairment in Sarcopenia. Biology 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Tinkerhess, M.J.; Healy, L.; Morgan, M.; Sujkowski, A.; Matthys, E.; Zheng, L.; Wessells, R.J. The Drosophila PGC-1α homolog spargel modulates the physiological effects of endurance exercise. PloS ONE 2012, 7, e31633. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Schnuck, J.K.; Johnson, M.A.; Gould, L.M.; Gannon, N.P.; Vaughan, R.A. Acute β-hydroxy-β-methyl butyrate suppresses regulators of mitochondrial biogenesis and lipid oxidation while increasing lipid content in myotubes. Lipids 2016, 51, 1127–1136. [Google Scholar] [CrossRef]
- Standley, R.A.; Distefano, G.; Pereira, S.L.; Tian, M.; Kelly, O.J.; Coen, P.M.; Deutz, N.E.; Wolfe, R.R.; Goodpaster, B.H. Effects of β-hydroxy-β-methylbutyrate on skeletal muscle mitochondrial content and dynamics, and lipids after 10 days of bed rest in older adults. J. Appl. Physiol. 2017, 123, 1092–1100. [Google Scholar] [CrossRef]
- Linford, N.J.; Bilgir, C.; Ro, J.; Pletcher, S.D. Measurement of lifespan in Drosophila melanogaster. J. Vis. Exp. 2013, e50068. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Barton, B.; Ayer, G.; Heymann, N.; Maughan, D.W.; Lehmann, F.O.; Vigoreaux, J.O. Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene. J. Exp. Biol. 2005, 208, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Kosinski, M.; Biecek, P. R Package Version 0.4.8; Survminer: Drawing Survival Curves using ‘ggplot2’. R Package Version 0.4.8. Available online: https://rpkgs.datanovia.com/survminer/index.html (accessed on 20 September 2021).
- Therneau, T.M. R Package Version 3.2-7; A Package for Survival Analysis in R. Available online: https://CRAN.R-project.org/package=survival (accessed on 20 September 2021).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Kassambara, A. R Package Version 0.6.0; rstatix: Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://rpkgs.datanovia.com/rstatix (accessed on 24 September 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. R Package Version 0.4.0; ggpubr: ‘ggplot2’ Based Publication Ready Plots. Available online: https://rpkgs.datanovia.com/ggpubr (accessed on 9 September 2020).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagori, R.; Vigoreaux, J.O. β-hydroxy-β-methylbutyrate Attenuates Age-Dependent Loss of Flight Ability and Extends Lifespan in Drosophila. Int. J. Mol. Sci. 2025, 26, 2664. https://doi.org/10.3390/ijms26062664
Nagori R, Vigoreaux JO. β-hydroxy-β-methylbutyrate Attenuates Age-Dependent Loss of Flight Ability and Extends Lifespan in Drosophila. International Journal of Molecular Sciences. 2025; 26(6):2664. https://doi.org/10.3390/ijms26062664
Chicago/Turabian StyleNagori, Ravi, and Jim O. Vigoreaux. 2025. "β-hydroxy-β-methylbutyrate Attenuates Age-Dependent Loss of Flight Ability and Extends Lifespan in Drosophila" International Journal of Molecular Sciences 26, no. 6: 2664. https://doi.org/10.3390/ijms26062664
APA StyleNagori, R., & Vigoreaux, J. O. (2025). β-hydroxy-β-methylbutyrate Attenuates Age-Dependent Loss of Flight Ability and Extends Lifespan in Drosophila. International Journal of Molecular Sciences, 26(6), 2664. https://doi.org/10.3390/ijms26062664








