Intratracheal Delivery of a Phospholamban Decoy Peptide Attenuates Cardiac Damage Following Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Delivery of dNP2-SE to the Heart via IT Injection
2.2. Delivery of dNP2-SE via IT Injection Exerts Cardio-Protective Effects upon MI
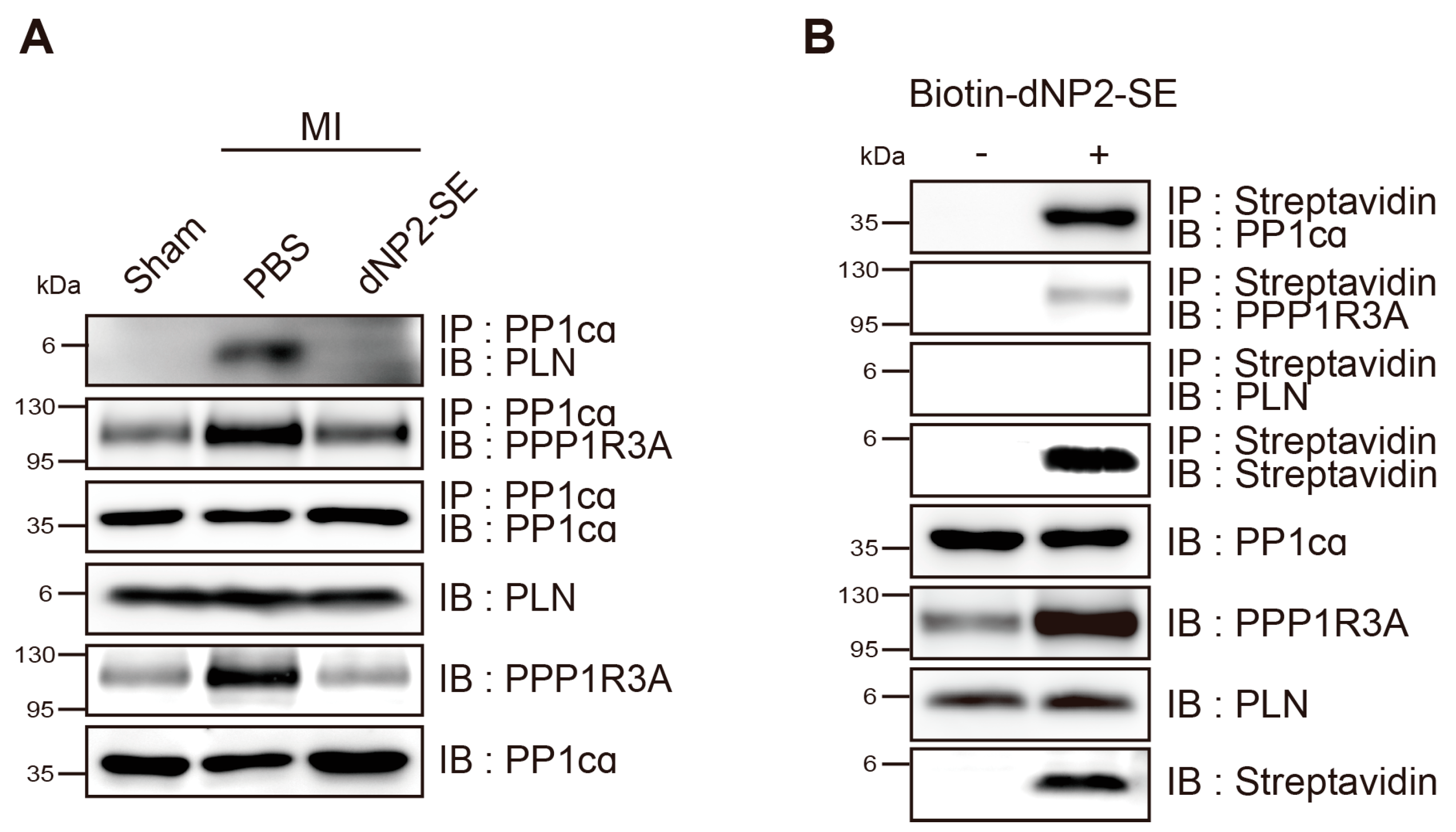
2.3. Delivery of dNP2-SE via IT Injection Prevents Molecular Abnormalities upon MI
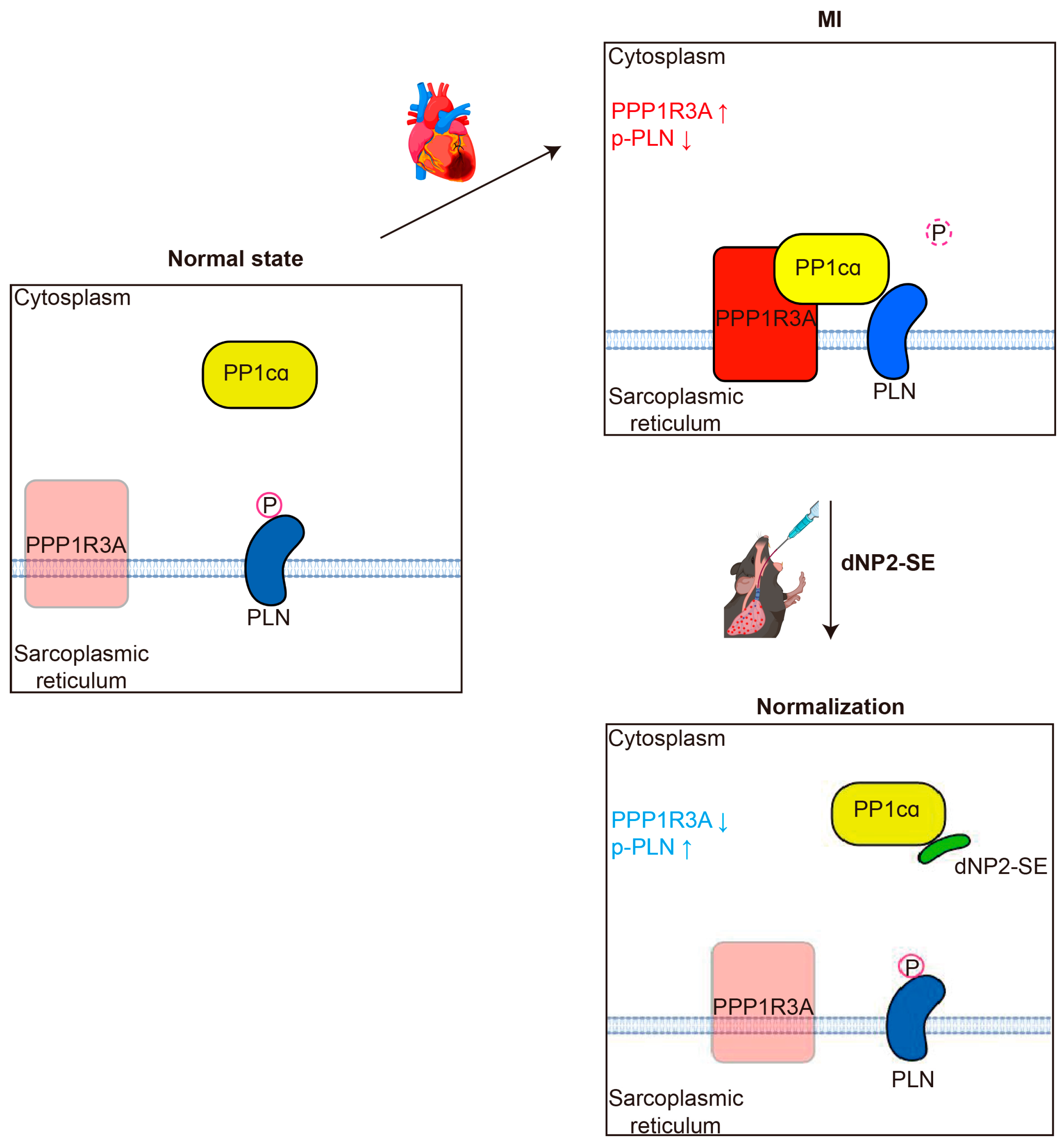
2.4. Inhibitory Mechanism of dNP2-SE on the PP1cα-PPP1R3A-PLN Complex
3. Discussion
4. Materials and Methods
4.1. Design and Synthesis of dNP2-SE
4.2. Animals
4.3. IT Injection
4.4. Fluorescent Slide Scanning
4.5. Measurement of Peptide in the Lung and Heart
4.6. Western Blot
4.7. Myocardial Infarction (MI)
4.8. Histology and TUNEL Assays
4.9. Hemodynamics Analysis
4.10. Immunoprecipitation
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2024, 12, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kariya, T.; Ishikawa, K. Targeted delivery of therapeutic agents to the heart. Nat. Rev. Cardiol. 2021, 18, 389–399. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, K.; Song, J. Targeted Therapy in Cardiovascular Disease: A Precision Therapy Era. Front. Pharmacol. 2021, 12, 623674. [Google Scholar] [CrossRef]
- Pacak, C.A.; Byrne, B.J. AAV Vectors for Cardiac Gene Transfer: Experimental Tools and Clinical Opportunities. Mol. Ther. 2011, 19, 1582–1590. [Google Scholar] [CrossRef]
- Li, D.; Son, Y.; Jang, M.; Wang, S.; Zhu, W. Nanoparticle Based Cardiac Specific Drug Delivery. Biology 2023, 12, 82. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Greish, Y.E.; Beegam, S.; Zaaba, N.E.; Ali, B.H. Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice. Int. J. Mol. Sci. 2023, 24, 8890. [Google Scholar] [CrossRef]
- Lim, G.B. Drug delivery using inhaled nanoparticles. Nat. Rev. Cardiol. 2018, 15, 133. [Google Scholar] [CrossRef]
- Li, C.; Naveed, M.; Dar, K.; Liu, Z.; Baig, M.M.F.A.; Lv, R.; Saeed, M.; Dingding, C.; Feng, Y.; Xiaohui, Z. Therapeutic advances in cardiac targeted drug delivery: From theory to practice. J. Drug Target. 2021, 29, 235–248. [Google Scholar] [CrossRef]
- Alogna, A.; Berboth, L.; Faragli, A.; Ötvös, J.; Muzio, F.P.L.; di Mauro, V.; Modica, J.; Quarta, E.; Semmler, L.; Deißler, P.M.; et al. Lung-to-Heart Nano-in-Micro Peptide Promotes Cardiac Recovery in a Pig Model of Chronic Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Modica, J.; Di Mauro, V.; Barandalla-Sobrados, M.; Chavez, S.E.P.; Carullo, P.; Nemska, S.; Anselmo, A.; Condorelli, G.; Iafisco, M.; Miragoli, M.; et al. Nano-miR-133a Replacement Therapy Blunts Pressure Overload-Induced Heart Failure. Circulation 2021, 144, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Sayers, E.J.; He, L.; Narayan, R.; Williams, T.L.; Mills, E.M.; Allemann, R.K.; Luk, L.Y.P.; Jones, A.T.; Tsai, Y.-H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci. Rep. 2019, 9, 6298. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Lim, S.; Kim, W.-J.; Kim, Y.-H.; Lee, S.; Koo, J.-H.; Lee, J.-A.; Yoon, H.; Kim, D.-H.; Park, H.-J.; Kim, H.-M.; et al. dNP2 is a blood–brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat. Commun. 2015, 6, 8244. [Google Scholar] [CrossRef]
- Koo, J.-H.; Kim, D.-H.; Cha, D.; Kang, M.-J.; Choi, J.-M. LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis. Theranostics 2020, 10, 3138–3150. [Google Scholar] [CrossRef]
- Lee, W.-S.; Nam, K.-H.; Kim, J.H.; Kim, W.-J.; Kim, J.E.; Shin, E.-C.; Kim, G.-R.; Choi, J.-M. Alleviating psoriatic skin inflammation through augmentation of Treg cells via CTLA-4 signaling peptide. Front. Immunol. 2023, 14, 1233514. [Google Scholar] [CrossRef]
- Oh, J.G.; Kim, J.; Jang, S.P.; Nguen, M.; Yang, D.K.; Jeong, D.; Park, Z.Y.; Park, S.-G.; Hajjar, R.J.; Park, W.J. Decoy peptides targeted to protein phosphatase 1 inhibit dephosphorylation of phospholamban in cardiomyocytes. J. Mol. Cell. Cardiol. 2013, 56, 63–71. [Google Scholar] [CrossRef]
- Jang, S.P.; Oh, J.G.; Kang, D.H.; Kang, J.Y.; Kang, S.W.; Hajjar, R.J.; Park, W.J. A Decoy Peptide Targeted to Protein Phosphatase 1 Attenuates Degradation of SERCA2a in Vascular Smooth Muscle Cells. PLoS ONE 2016, 11, e0165569. [Google Scholar] [CrossRef]
- Chaudhri, B.; del Monte, F.; Hajjar, R.J.; Harding, S.E. Contractile effects of adenovirally-mediated increases in SERCA2a activity: A comparison between adult rat and rabbit ventricular myocytes. Mol. Cell. Biochem. 2003, 251, 103–109. [Google Scholar] [CrossRef]
- Gianni, D.; Chan, J.; Gwathmey, J.K.; del Monte, F.; Hajjar, R.J. SERCA2a in Heart Failure: Role and Therapeutic Prospects. J. Bioenerg. Biomembr. 2005, 37, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Zhihao, L.; Jingyu, N.; Lan, L.; Michael, S.; Rui, G.; Xiyun, B.; Xiaozhi, L.; Guanwei, F. SERCA2a: A key protein in the Ca2+ cycle of the heart failure. Heart Fail. Rev. 2019, 25, 523–535. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Kranias, E.G. Phospholamban: A crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell Biol. 2003, 4, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Boens, S.; Szekér, K.; Van Eynde, A.; Bollen, M. Interactor-guided dephosphorylation by protein phosphatase-1. In Phosphatase Modulators; Humana Press: Totowa, NJ, USA, 2013; Volume 1053, pp. 271–281. [Google Scholar] [CrossRef]
- Vafiadaki, E.; Arvanitis, D.E.; Sanoudou, D.; Kranias, E.G. Identification of a protein phosphatase-1/phospholamban complex that is regulated by cAMP-dependent phosphorylation. PLoS ONE 2013, 8, e80867. [Google Scholar] [CrossRef]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodríguez, G.B.; Patrício, T.; Degli Esposti, L.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10, eaan6205. [Google Scholar] [CrossRef]
- Weng, H.; Zou, W.; Tian, F.; Xie, H.; Liu, A.; Liu, W.; Liu, Y.; Zhou, N.; Cai, X.; Wu, J.; et al. Inhalable cardiac targeting peptide modified nanomedicine prevents pressure overload heart failure in male mice. Nat. Commun. 2024, 15, 6058. [Google Scholar] [CrossRef]
- Gorski, P.A.; Jang, S.P.; Jeong, D.; Lee, A.; Lee, P.; Oh, J.G.; Chepurko, V.; Yang, D.K.; Kwak, T.H.; Eom, S.H.; et al. Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca2+ -ATPase in Heart Failure. Circ. Res. 2019, 124, E63–E80. [Google Scholar] [CrossRef]
- Kawase, Y.; Ly, H.Q.; Prunier, F.; Lebeche, D.; Shi, Y.; Jin, H.; Hadri, L.; Yoneyama, R.; Hoshino, K.; Takewa, Y.; et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008, 51, 1112–1119. [Google Scholar] [CrossRef]
- Kranias, E.G.; Hajjar, R.J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 2012, 110, 1646–1660. [Google Scholar] [CrossRef]
- Lipskaia, L.; Chemaly, E.R.; Hadri, L.; Lompre, A.-M.; Hajjar, R.J. Sarcoplasmic reticulum Ca2+ ATPase as a therapeutic target for heart failure. Expert Opin. Biol. Ther. 2010, 10, 29–41. [Google Scholar] [CrossRef]
- Tsuji, T.; Del Monte, F.; Yoshikawa, Y.; Abe, T.; Shimizu, J.; Nakajima-Takenaka, C.; Taniguchi, S.; Hajjar, R.J.; Takaki, M. Rescue of Ca2+ overload-induced left ventricular dysfunction by targeted ablation of phospholamban. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H310–H317. [Google Scholar] [CrossRef]
- Peti, W.; Page, R. Strategies to make protein serine/threonine (PP1, calcineurin) and tyrosine phosphatases (PTP1B) druggable: Achieving specificity by targeting substrate and regulatory protein interaction sites. Bioorganic Med. Chem. 2015, 23, 2781–2785. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kook, T.; Lee, M.-Y.; Kwak, T.H.; Jeong, D.; Sim, D.S.; Jeong, M.H.; Ahn, Y.; Kook, H.; Park, W.J.; Jang, S.P. Intratracheal Delivery of a Phospholamban Decoy Peptide Attenuates Cardiac Damage Following Myocardial Infarction. Int. J. Mol. Sci. 2025, 26, 2649. https://doi.org/10.3390/ijms26062649
Kook T, Lee M-Y, Kwak TH, Jeong D, Sim DS, Jeong MH, Ahn Y, Kook H, Park WJ, Jang SP. Intratracheal Delivery of a Phospholamban Decoy Peptide Attenuates Cardiac Damage Following Myocardial Infarction. International Journal of Molecular Sciences. 2025; 26(6):2649. https://doi.org/10.3390/ijms26062649
Chicago/Turabian StyleKook, Taewon, Mi-Young Lee, Tae Hwan Kwak, Dongtak Jeong, Doo Sun Sim, Myung Ho Jeong, Youngkeun Ahn, Hyun Kook, Woo Jin Park, and Seung Pil Jang. 2025. "Intratracheal Delivery of a Phospholamban Decoy Peptide Attenuates Cardiac Damage Following Myocardial Infarction" International Journal of Molecular Sciences 26, no. 6: 2649. https://doi.org/10.3390/ijms26062649
APA StyleKook, T., Lee, M.-Y., Kwak, T. H., Jeong, D., Sim, D. S., Jeong, M. H., Ahn, Y., Kook, H., Park, W. J., & Jang, S. P. (2025). Intratracheal Delivery of a Phospholamban Decoy Peptide Attenuates Cardiac Damage Following Myocardial Infarction. International Journal of Molecular Sciences, 26(6), 2649. https://doi.org/10.3390/ijms26062649





