Functionalized Nanomaterials in Cancer Treatment: A Review
Abstract
1. Introduction
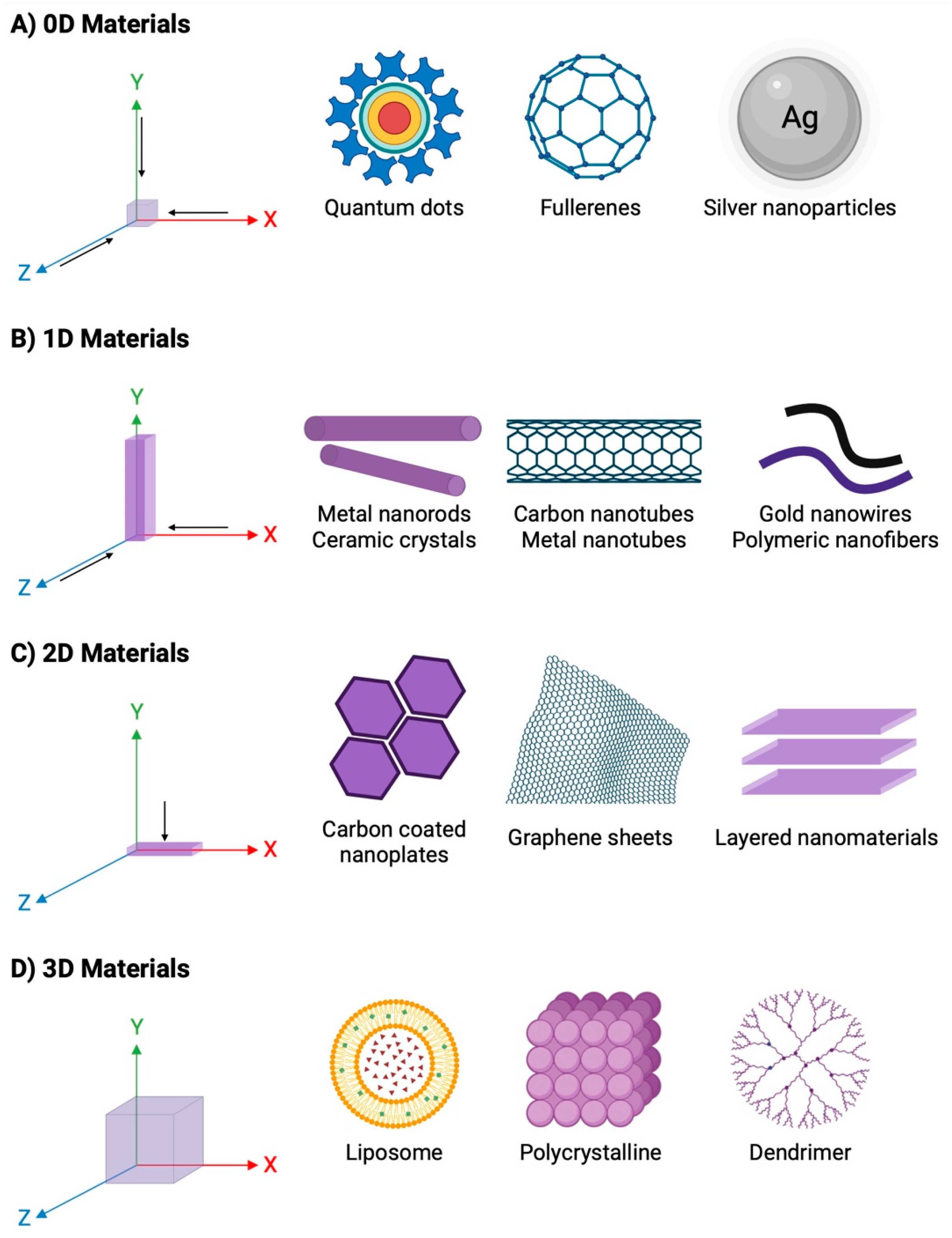
2. Nanomaterials
3. Therapeutic Role of Nanomaterial in Cancer
3.1. Metallic Nanoparticles
3.1.1. Gold
3.1.2. Platinum
3.1.3. Silver
3.2. Organic Nanoparticles
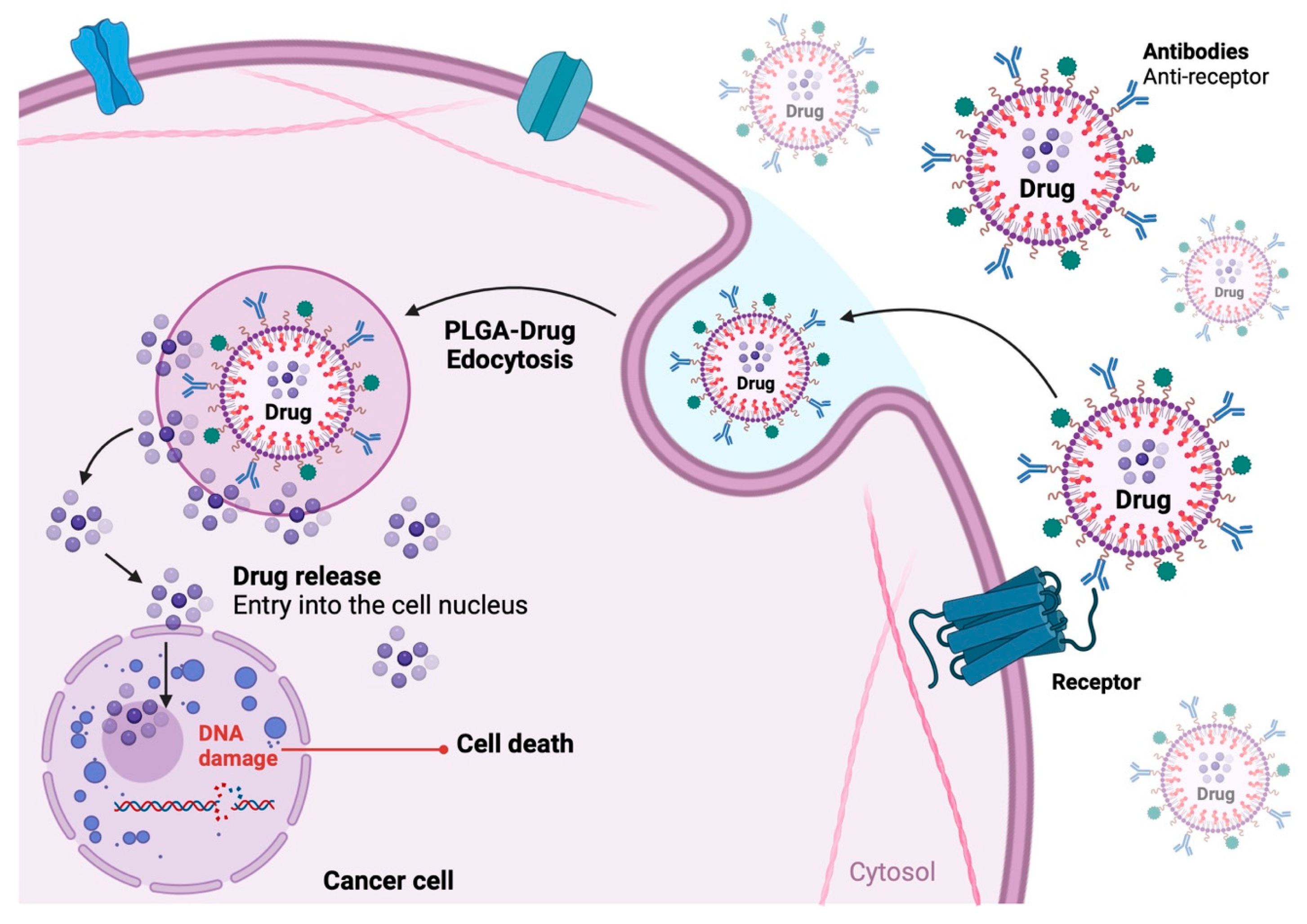
3.2.1. PLGA-Based Nanoparticles
3.2.2. Lipid Nanoparticles
3.2.3. Metal–Organic Frameworks
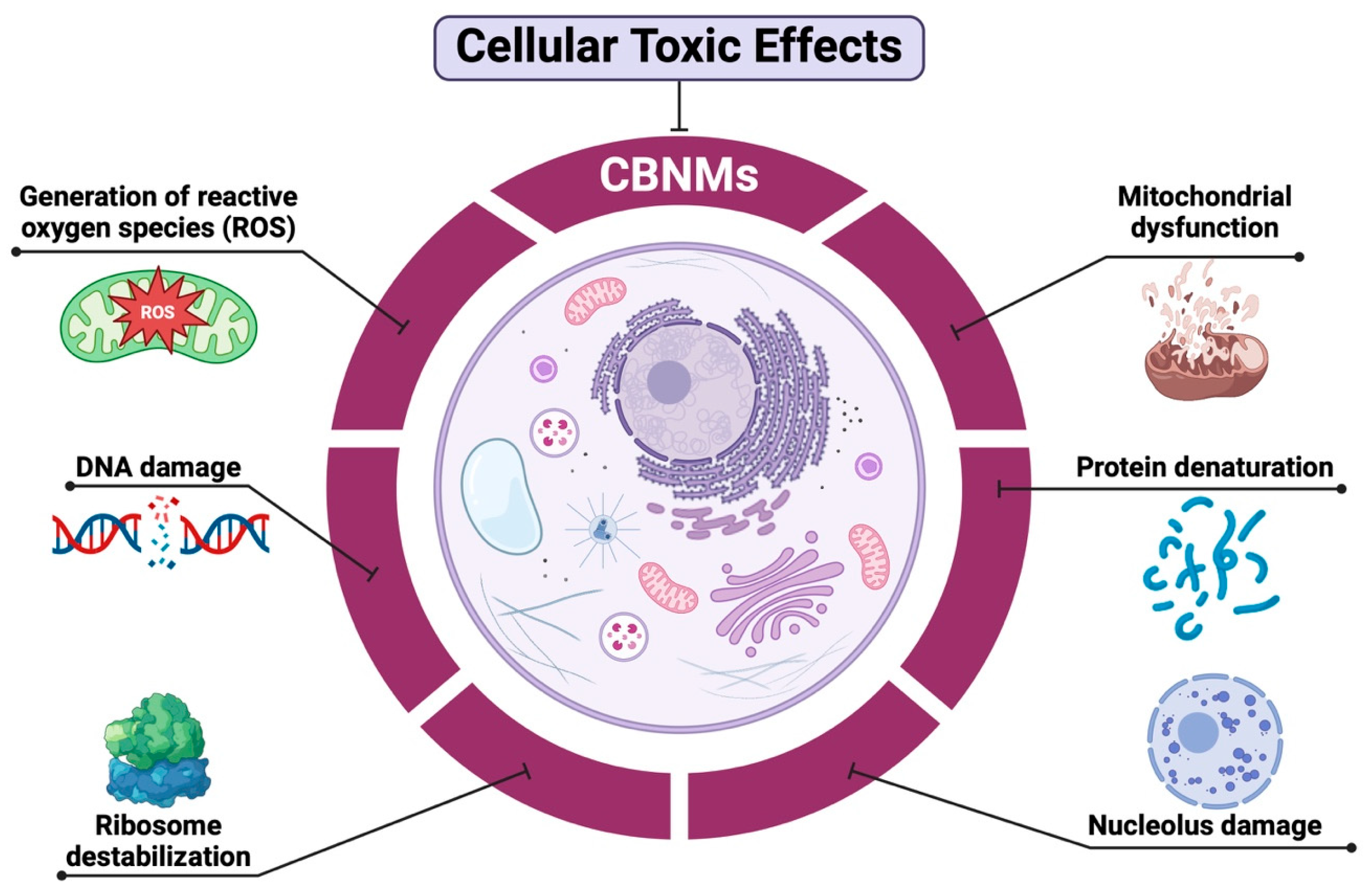
3.3. Carbon-Based Nanomaterials
| Nanoparticle Type | Functionalization Material | Type of Cancer | Mechanism of Action | Combined Radiotherapy | Reference |
|---|---|---|---|---|---|
| AuNPs | 2-thiouracil | Breast cancer cells (MDA-MB-231) | Antiproliferative activity of 2-TU and PTT effect | No | [45] |
| AuNPs | HER-2 and dasatinib | Breast cancer cells (BT-474 and MCF-7) | IIncrease in the activity of dasantinib | Yes | [46] |
| AuNPs | Trastuzumab | Gastric cancer cells (MKN7 and NCI-N87) and in vitro and vivo; melanoma cells (G361) | Autophagy | No | [47] |
| AuNPs | Anti-HER2 | melanoma cells (G361) | Apoptosis and cell cycle arrest | No | [48] |
| PtNPs | PEG-coated and doxorubicin | lung cancer cells (A549) in vitro and in vivo | Apoptosis and cell cycle arrest | No | [53] |
| PtNPs | Doxorubicin-conjugated PtNPs in octopod form | Breast cancer cells (MCF-7 and MDA-MB-231) | Mitochondrial dysfunction and activation of caspases-3 and -9 (Apoptosis) | No | [54] |
| PtNPs | PEG-coated and doxorubicin | Breast cancer cells (MCF-7/ADR) | Combination of chemotherapy and phototherapy | No | [55] |
| PtNPs | Chitosan | Breast cancer cells (MDA-MB-231; MCF7) | Apoptosis | No | [57,58] |
| PtNPs | Lactic-co-glycolic acid, PEG, anti-EGFR | Breast cancer cells (MDA-MB-231) | Oxidative state of PtNPs | No | [59] |
| PtNPs | Hyaluronic acid | Breast cancer cells (MDA-MB-231) in vitro and in vivo | Photothermal therapy | No | [60] |
| AgNPs | Glucose | Prostate cancer cells (DU-145, PC-3 and LNCaP) | Oxidative damage, DNA fragmentation, apoptosis and cell cycle arrest | No | [65] |
| AgNPs | Polyvinyl alcohol, PGE, polyvinylpyrrolidone and conjugated with doxorubicin | Breast cancer cells (MCF-7) | Oxidative stress and lipid peroxidation | No | [67] |
| AgNPs | Chitosan | Breast cancer cells (MCF-7) | DNA damage, mitochondrial damage and apoptosis | No | [68,69] |
| AgNPs | PGE, folic acid and conjugated with doxorubicin | Adenocarcinoma; lymphocytic leukemia | Generation of reactive oxygen species | No | [71] |
| AgNPs | Anti-EGFR | Nasopharyngeal carcinoma | Apoptosis | Yes | [72] |
| AgNPs | IgG | Pancreatic cancer cell (Panc-1) | Apoptosis | No | [73] |
| Nanoparticle Type | Functionalization Material | Type of Cancer | Mechanism of Action | Reference |
|---|---|---|---|---|
| PEG-PLGA | anti-CD133; methioninase; pemetrexed | Gastric cancer cells (CD133+ SGC7901 and MKN45) | Apoptosis a and inhibition of DNA synthesis | [81] |
| PLGA | anti-CD133; oxaliplatin | Colorectal cancer (Caco-2) | Inhibition of the DNA synthesis | [82] |
| Lipid-PLGA | Anti-CD44; salinomycin | CD44+ prostate cancer cells (DU145 and 22RV1) | - | [83] |
| PLGA | Anti-EGFR; paclitaxel | Triple-negative breast cancer (MDA-MB-468 TNBC) and in vivo | Condensed chromatin, fragmented nucleus and formation of apoptotic bodies | [85] |
| PLGA | Antibodies against Frizzled7; doxorubicin | Triple-negative breast cancer (MDA-MB-231) | Apoptosis and/or necrosis | [86] |
| LPNPs | methotrexate | Human lung cancer cell (A549) | Apoptosis | [89] |
| LPNPs | mitoxantrone | Breast cancer (MCF-7) | - | [90] |
| LPNPs | Folic acid; doxorubicin | Brain cancer (U87 MG) | - | [91] |
| LPNPs | Doxorubicin; siRNA | Burkitt lymphoma (Raji) | Apoptosis | [93] |
| mPEG-PCL-DDAB | Lycopen; insulin-like growth factor 1 receptor siRNA | Breast cancer (MCF-7) | Apoptosis and arrested cell cycle | [94] |
| LPHNPs | Curcumin; cabazitaxel | Prostate cancer (LNCaP and PC3) and in vivo | - | [96] |
| LPHNPs | EGF; 5-fluorouracil; sulforaphane | Colon carcinoma (HCT-15) | Apoptosis | [99] |
| LPHNPs | Polypeptide GE11; salinomycin | Osteosarcoma (U2OS) and in vivo | Suppress the migration and proliferation | [100] |
| Polidopamine nanoparticles | Cetuximab; 5-fluorouracil; irinotecan; leucovorin | Colon carcinoma (HTC116 and HT29) | - | [101] |
| MOFs | Di-peptide (WQPDTAHHWA-TL); paclitaxel | Prostate cancer (Lncap) | Apoptosis | [107] |
| Iron-based MOFs | Graphene oxide; luteolin and matrine | Colon cancer (RKO) | ROS, upregulated caspase-3 and caspase-9 and inhibition in the migration | [108] |
| Copper-based MOFs | Heparin; doxorubicin | Cancer breast (MCF-7) | Nuclei fragmentation and chromatin condensation | [109] |
| nMOFs | Hyaluronic acid; doxorubicin | HepG2 cells, Hela cells, U87MG cells, and 4T1 cells and in vivo | Apoptosis | [110] |
| Nanoparticle Type | Functionalization Material | Type of Cancer | Mechanism of Action | Combined Radiotherapy | Reference |
|---|---|---|---|---|---|
| Multi-walled carbon nanotubes | m-tetrahydroxyphenylch-lorine | Ovarian cancer (SKOV3) | Oxidative stress; apoptosis and mitochondrial damage | PDT; PTT | [117] |
| Carbon nanotubes | Arginylglycylaspartic acid; camptothecin | Melanoma and breast cancer (A375 and MCF7) | Increase in expression of caspase-3, NF-kB and Bax | No | [124] |
| Single-walled carbon nanotubes | Arginylglycylaspartic acid; camptothecin; chitosan; docetaxel | Lung cancer, breast cancer (A549 cells and MCF-7) and in vivo | - | No | [125] |
| Carbon nanotubes | Epidermal growth factor; chitosan and etoposide | Lung cancer (A549) | Apoptosis and necrosis | No | [127] |
| Single-walled carbon nanotubes | Cetumixab; 7-ethyl-10-hydroxycamptothecin | Colorectal cancer (HCT116, HT29 and SW620) | Apoptosis and necrosis | No | [128] |
| Ghapene oxide | PEG; cisplatin; doxorubicin | Squamous cell carcinoma and breast cancer (CAL-27) and MCF-7) | Apoptosis and necrosis | No | [133] |
| Ghapene oxide | PEG-polycaprolactone; doxorubicin | Lung cancer and skin cancer (A549 and B16) | Necrosis | No | [136] |
| Graphene | PEG; oxidized sodium alginate; paclitaxel | Paclitaxel-resistant gastric carcinoma cell (HGC-27/PTX) | Apoptosis, oxidative stress | PTT | [138] |
| Graphene | Trastuzumab | Osteosarcoma (MG63, HOS, 143B) | Oxidative stress, necroptosis | No | [139] |
| Graphene oxide | Antibodies against vascular endothelial growth factor; HAS; paclitaxel | Adrenocortical carcinoma (SW-13) and in vivo | Apoptosis | PTT | [140] |
| Graphene oxide | Integrin αvβ3 antibody; pyropheo-phorbide-a; PEG | Glioblastoma and breast cancer (U87-MG, MCF-7 cells. | Apoptosis | PDT | [141] |
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. American Cancer Society Releases Latest Global Cancer Statistics; Cancer Cases Expected to Rise to 35 Million Worldwide by 2050. News Release. 30 April 2024. Available online: https://pressroom.cancer.org/GlobalCancerStatistics2024 (accessed on 8 April 2024).
- Kiri, S.; Ryba, T. Cancer, metastasis, and the epigenome. Mol. Cancer 2024, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Khanizadeh, A.; Ghaemi, A.; Pourmadadi, M.; Javadi, S.; Rahdar, A.; Yazdian, F.; Ghazy, E. Advancing Cancer Therapy: Unveiling the Cutting-Edge Potential of Carmustine Nano Carriers for Targeted Treatment. J. Drug Deliv. Sci. Technol. 2024, 99, 105943. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M. Cell Cycle Checkpoints and Their Inactivation in Human Cancer. Cell Prolif. 2000, 33, 261–274. [Google Scholar] [CrossRef]
- Mee, T.; Kirkby, N.F.; Defourny, N.N.; Kirkby, K.J.; Burnet, N.G. The Use of Radiotherapy, Surgery, and Chemotherapy in the Curative Treatment of Cancer: Results from the FORTY (Favourable Outcomes from Radiotherapy) Project. Br. J. Radiol. 2023, 96, 20230334. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and Beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical Cancer Immunotherapy: Current Progress and Prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- He, S.; Gou, X.; Zhang, S.; Zhang, X.; Huang, H.; Wang, W.; Yi, L.; Zhang, R.; Duan, Z.; Zhou, P.; et al. Nanodelivery Systems as a Novel Strategy to Overcome Treatment Failure of Cancer. Small Methods 2024, 8, e2301127. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology: A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer Treatment Therapies: Traditional to Modern Approaches to Combat Cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Lepeltier, E.; Rijo, P.; Rizzolio, F.; Popovtzer, R.; Petrikaite, V.; Assaraf, Y.G.; Passirani, C. Nanomedicine to Target Multidrug Resistant Tumors. Drug Resist. Updat. 2020, 52, 100704. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Lin, C.M.; Liu, Q.; Huang, L. Nanoformulations for Combination or Cascade Anticancer Therapy. Adv. Drug Deliv. Rev. 2017, 115, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Zaimy, M.A.; Saffarzadeh, N.; Mohammadi, A.; Pourghadamyari, H.; Izadi, P.; Sarli, A.; Moghaddam, L.K.; Paschepari, S.R.; Azizi, H.; Torkamandi, S.; et al. New Methods in the Diagnosis of Cancer and Gene Therapy of Cancer Based on Nanoparticles. Cancer Gene Ther. 2017, 24, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, W.; Huang, P.; Zeng, X.; Mei, L. Smart Materials for Drug Delivery and Cancer Therapy. VIEW 2021, 2, 20200042. [Google Scholar] [CrossRef]
- Bleeker, E.A.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.; et al. Considerations on the EU Definition of a Nanomaterial: Science to Support Policy Making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef]
- Pizarro Barraza, F.; Dhandayuthapani, T.; Ananthakumar, R.; Manikandan, V.S.; Shanmuga Sundar, D.; Carolina Venegas, A.; Pedro Sotomayor, S.; Juan Campos, N.; Mauricio, J.M.; Arun, T. Unlocking the Potential: Mining Tailings as a Source of Sustainable Nanomaterials. Renew. Sustain. Energy Rev. 2024, 202, 114665. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Kebede, M.A.; Imae, T. Low-Dimensional Nanomaterials. In Advanced Supramolecular Nanoarchitectonics; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 3–16. [Google Scholar] [CrossRef]
- Asghar, N.; Hussain, A.; Nguyen, D.A.; Ali, S.; Hussain, I.; Junejo, A.; Ali, A. Advancement in Nanomaterials for Environmental Pollutants Remediation: A Systematic Review on Bibliometric Analysis, Material Types, Synthesis Pathways, and Related Mechanisms. J. Nanobiotechnol. 2024, 22, 26. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Select 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Schaming, D.; Remita, H. Nanotechnology: From the Ancient Time to Nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, P.; Mishra, V. Recent Advances on Classification, Properties, Synthesis, and Characterization of Nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; Srivastava, N., Srivastava, M., Mishra, P.K., Gupta, V.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and Inorganic Nanomaterials: Fabrication, Properties, and Applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications, and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Rahman, M.; Hoque, M.A.; Rahman, G.; Gafur, M.; Khan, R.A.; Hossain, M.K. Study on the Mechanical, Electrical and Optical Properties of Metal-Oxide Nanoparticles Dispersed Unsaturated Polyester Resin Nanocomposites. Res. Phys. 2019, 13, 102264. [Google Scholar] [CrossRef]
- Astefanei, A.; Núñez, O.; Galceran, M.T. Characterisation and Determination of Fullerenes: A Critical Review. Anal. Chim. Acta 2015, 882, 1–21. [Google Scholar] [CrossRef]
- Park, S.J.; Deshmukh, M.A.; Kang, B.C.; Jeon, J.Y.; Chen, C.; Ha, T.J. A Review of Advanced Electronic Applications Based on Carbon Nanomaterials. ECS J. Solid State Sci. Technol. 2020, 9, 071002. [Google Scholar] [CrossRef]
- Choudhary, F.; Mudgal, P.; Parvez, A.; Sharma, P.; Farooqi, H. A Review on Synthesis, Properties and Prospective Applications of Carbon Nanomaterials. Nano-Struct. Nano-Objects 2024, 38, 101186. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting Cancer Cells with Nanotherapeutics and Nanodiagnostics: Current Status and Future Perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by Noble Metal Nanoparticles: Controlled Synthesis for the Optimization and Understanding of Activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef]
- Basova, T.; Vikulova, E.; Dorovskikh, S.; Hassan, A.K.; Morozova, N. The Use of Noble Metal Coatings and Nanoparticles for the Modification of Medical Implant Materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Villalobos Gutiérrez, P.T.; Muñoz Carrillo, J.L.; Sandoval Salazar, C.; Viveros Paredes, J.M.; Gutiérrez Coronado, O. Functionalized Metal Nanoparticles in Cancer Therapy. Pharmaceutics 2023, 15, 1932. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Gutiérrez de la Rosa, S.Y.; Muñiz Díaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, Ó. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
- Mohapatra, A.; Sathiyamoorthy, P.; Park, I.-K. Metallic Nanoparticle-Mediated Immune Cell Regulation and Advanced Cancer Immunotherapy. Pharmaceutics 2021, 13, 1867. [Google Scholar] [CrossRef]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold Nanoparticles as Novel Agents for Cancer Therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef]
- Boussoufi, F.; Navarro Gallón, S.M.; Chang, R.; Webster, T.J. Synthesis and Study of Cell-Penetrating Peptide-Modified Gold Nanoparticles. Int. J. Nanomed. 2018, 13, 6199–6205. [Google Scholar] [CrossRef]
- Sakore, P.; Bhattacharya, S.; Belemkar, S.; Prajapati, B.G.; Elossaily, G.M. The Theranostic Potential of Green Nanotechnology-Enabled Gold Nanoparticles in Cancer: A Paradigm Shift on Diagnosis and Treatment Approaches. Results Chem. 2024, 7, 101264. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Molecular Pharmaceutics of Gold Nanoparticles for Cancer Therapy. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Lorenzana-Vázquez, G.; Pavel, I.; Meléndez, E. Gold Nanoparticles Functionalized with 2-Thiouracil for Antiproliferative and Photothermal Therapies in Breast Cancer Cells. Molecules 2023, 28, 4453. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, M.; Varshosaz, J.; Rostami, M.; Haghiralsadat, F.; Akbari, V.; Khorshid, P. Anti HER-2 Aptamer Functionalized Gold Nanoparticles of Dasatinib for Targeted Chemo-Radiotherapy in Breast Cancer Cells. Biomater. Adv. 2023, 154, 213591. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Kuroda, S.; Kanaya, N.; Morihiro, T.; Aoyama, K.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; et al. HER2-Targeted Gold Nanoparticles Potentially Overcome Resistance to Trastuzumab in Gastric Cancer. Nanomedicine 2018, 14, 1919–1929. [Google Scholar] [CrossRef]
- Jeon, H.J.; Choi, B.B.R.; Park, K.H.; Hwang, D.S.; Kim, U.K.; Kim, G.C. Induction of Melanoma Cell-Selective Apoptosis Using Anti-HER2 Antibody-Conjugated Gold Nanoparticles. Yonsei Med. J. 2019, 60, 509–516. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A Review on Metal Complexes and Its Anti-Cancer Activities: Recent Updates from In Vivo Studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Yue, L.; Jianming, G.; Xue, G.; Huanyu, Z.; Keru, M.; Yuan, S.; Baihui, C.; Yubo, D.; Tianyu, C.; Dongxu, Y.; et al. Inhibitory Effects of Platinum Nanoparticles Coated with Polyethylene Glycol and Conjugated with Rutin on the MCF-7 Breast Cancer Cell Line. Arab. J. Chem. 2023, 17, 105567. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kotcherlakota, R.; Haque, S.; Bhattacharya, D.; Kumar, J.M.; Chakravarty, S.; Patra, C.R. Improved Delivery of Doxorubicin Using Rationally Designed PEGylated Platinum Nanoparticles for the Treatment of Melanoma. Mater. Sci. Eng. C 2020, 108, 110375. [Google Scholar] [CrossRef]
- Patel, P.; Umapathy, D.; Manivannan, S.; Nadar, V.M.; Venkatesan, R.; Joseph Arokiyam, V.A.; Pappu, S.; Ponnuchamy, K. A Doxorubicin-Platinum Conjugate System: Impacts on PI3K/AKT Actuation and Apoptosis in Breast Cancer Cells. RSC Adv. 2021, 11, 4818–4828. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous Platinum Nanoparticle-Based Nanoplatforms for Combined Chemo-Photothermal Breast Cancer Therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, E.; Ponnuchamy, K.; Muthusamy, G.; Varatharajan, N.; Sabapathi, D.; Selvaraj, A. Chitosan-Stabilized Platinum Nanoparticles Induce Apoptotic Cell Death in Breast Cancer Cells. Appl. Nanosci. 2023, 13, 3867–3873. [Google Scholar] [CrossRef]
- Felicia Aswathy, W.; Jose, J.; Anila, E.I. Chitosan Stabilized Platinum Nanoparticles: Synthesis, Characterization, and Cytotoxic Impacts on Human Breast Cancer Cells. Mater. Chem. Phys. 2024, 326, 129864. [Google Scholar] [CrossRef]
- López Ruiz, A.; Villaseco Arribas, E.; McEnnis, K. Poly(Lactic-Co-Glycolic Acid) Encapsulated Platinum Nanoparticles for Cancer Treatment. Mater. Adv. 2022, 3, 2858–2870. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Zhao, X.; Zhou, Z.; Wang, Y.; Cheng, Y.; Zhang, Q. Hyaluronic Acid-Encapsulated Platinum Nanoparticles for Targeted Photothermal Therapy of Breast Cancer. J. Biomed. Nanotechnol. 2017, 13, 1457–1467. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Machado, V.; Dias, F.; Figueiredo, P.; Palmeira, C.; Martins, G.; Fernandes, R.; Malheiro, A.R.; Mikkonen, K.S.; Teixeira, A.L.; et al. Glucose-Functionalized Silver Nanoparticles as a Potential New Therapy Agent Targeting Hormone-Resistant Prostate Cancer Cells. Int. J. Nanomed. 2022, 17, 4321–4337. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.; Reddy, P.; Jeon, H.-J.; Ahn, C.W. Role of Capping Agents in Controlling Silver Nanoparticles Size, Antibacterial Activity and Potential Application as Optical Hydrogen Peroxide Sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Ziko, L.; Siam, R.; Mamdouh, W. Core-Shell Silver/Polymeric Nanoparticles-Based Combinatorial Therapy Against Breast Cancer In Vitro. Sci. Rep. 2016, 6, 30729. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.; Abdelfattah, A.; Younis, M.; Aldalaan, S.; Tawfeek, H. Chitosan-Capped Silver Nanoparticles with Potent and Selective Intrinsic Activity Against Breast Cancer Cells. Nanotechnol. Rev. 2023, 12, 20220546. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Malaikozhundan, B.; Thangaraj, M.; Perumal, E.; Murugan, T.; Velusamy, P.; Anbu, P.; Vaseeharan, B. Chitosan-Coated Silver Nanoparticles Promoted Antibacterial, Antibiofilm, Wound-Healing of Murine Macrophages and Antiproliferation of Human Breast Cancer MCF7 Cells. Polym. Test. 2020, 90, 106675. [Google Scholar] [CrossRef]
- Di Corato, R.; Palumberi, D.; Marotta, R.; Scotto, M.; Carregal-Romero, S.; Rivera Gil, P.; Parak, W.J.; Pellegrino, T. Magnetic Nanobeads Decorated with Silver Nanoparticles as Cytotoxic Agents and Photothermal Probes. Small 2012, 8, 2731–2742. [Google Scholar] [CrossRef]
- Srinivasan, S.; Bhardwaj, V.; Nagasetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Multifunctional Surface-Enhanced Raman Spectroscopy-Detectable Silver Nanoparticles Combined Photodynamic Therapy and pH-Triggered Chemotherapy. J. Biomed. Nanotechnol. 2016, 12, 2202–2219. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y.; Lu, H.; Zhao, D. Silver Nanoparticles Coupled to Anti-EGFR Antibodies Sensitize Nasopharyngeal Carcinoma Cells to Irradiation. Mol. Med. Rep. 2017, 16, 9005–9010. [Google Scholar] [CrossRef]
- Nedelcu, A.; Mocan, T.; Sabau, L.I.; Matea, C.T.; Tabaran, F.; Pop, T.; Delcea, C.; Mosteanu, O.; Mocan, L. In Vitro Photothermal Therapy of Pancreatic Cancer Mediated by Immunoglobulin G-Functionalized Silver Nanoparticles. Sci. Rep. 2024, 14, 14417. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in Tumor Immunotherapy: New Strategies and Challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing Poly(Lactic-Co-Glycolic Acid) Particles for Biomedical Applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Sah, E.; Sah, H. Recent Trends in Preparation of Poly(Lactide-Co-Glycolide) Nanoparticles by Mixing Polymeric Organic Solution with Antisolvent. J. Nanomater. 2015, 2015, 794601. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-Co-Glycolide Nanoparticles for Controlled Delivery of Anticancer Agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, H.T.; Yang, W.F.; Li, Y.F.; Liu, C. Evaluation of METase-Pemetrexed-Loaded PEG-PLGA Nanoparticles Modified with Anti-CD133-ScFV for Treatment of Gastric Carcinoma. Biosci. Rep. 2018, 38, BSR20171001. [Google Scholar] [CrossRef]
- Zumaya, A.L.V.; Rimpelová, S.; Štějdířová, M.; Ulbrich, P.; Vilčáková, J.; Hassouna, F. Antibody conjugated PLGA nanocarriers and superparamagnetic nanoparticles for targeted delivery of oxaliplatin to cells from colorectal carcinoma. Int. J. Mol. Sci. 2022, 23, 1200. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Liu, Y. Enhanced Targeting of Prostate Cancer-Initiating Cells by Salinomycin-Encapsulated Lipid-PLGA Nanoparticles Linked with CD44 Antibodies. Oncol. Lett. 2019, 17, 4024–4033. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple-Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V.; Krishnan, S.; Palanimuthu, V.R.; Sankarankutty, S.; Kalaimani, J.K.; Karupiah, S.; Kit, N.S.; Hock, T.T. Anti-EGFR Anchored Paclitaxel-Loaded PLGA Nanoparticles for the Treatment of Triple-Negative Breast Cancer: In Vitro and In Vivo Anticancer Activities. PLoS ONE 2018, 13, e0206109. [Google Scholar] [CrossRef] [PubMed]
- Hoover, E.C.; Ruggiero, O.M.; Swingler, R.N.; Day, E.S. FZD7-Targeted Nanoparticles to Enhance Doxorubicin Treatment of Triple-Negative Breast Cancer. ACS Omega 2024, 9, 14323–14335. [Google Scholar] [CrossRef] [PubMed]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef]
- Sivadasan, D.; Ramakrishnan, K.; Mahendran, J.; Ranganathan, H.; Karuppaiah, A.; Rahman, H. Solid Lipid Nanoparticles: Applications and Prospects in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6199. [Google Scholar] [CrossRef]
- Chen, T.; Pan, F.; Luo, G.; Jiang, K.; Wang, H.; Ding, T.; Li, W.; Zhan, C.; Wei, X. Morphology-Driven Protein Corona Manipulation for Preferential Delivery of Lipid Nanodiscs. Nano Today 2022, 46, 101609. [Google Scholar] [CrossRef]
- Patra, S.; Dey, J.; Kar, S.; Chakraborty, A.; Tawate, M. Methotrexate-Loaded Surface-Modified Solid Lipid Nanoparticles Targeting Cancer Expressing COX-2 Enzyme. Langmuir 2024, 40, 14811–14822. [Google Scholar] [CrossRef]
- Granja, A.; Lima-Sousa, R.; Alves, C.G.; de Melo-Diogo, D.; Pinheiro, M.; Sousa, C.T.; Correia, I.J.; Reis, S. Mitoxantrone-Loaded Lipid Nanoparticles for Breast Cancer Therapy—Quality-by-Design Approach and Efficacy Assessment in 2D and 3D In Vitro Cancer Models. Int. J. Pharm. 2021, 607, 121044. [Google Scholar] [CrossRef]
- Jain, P.; Pandey, V.; Soni, V. Bioconjugate-Loaded Solid Lipid Nanoparticles for Enhanced Anticancer Drug Delivery to Brain Cancer Cells: An In Vitro Evaluation. Indian J. Med. Res. 2022, 156, 139–148. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-León, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid Nanoparticles for siRNA Delivery in Cancer Treatment. J. Control. Release 2023, 361, 130–146. [Google Scholar] [CrossRef]
- Butowska, K.; Han, X.; Gong, N.; El-Mayta, R.; Haley, R.M.; Xue, L.; Zhong, W.; Guo, W.; Wang, K.; Mitchell, M.J. Doxorubicin-Conjugated siRNA Lipid Nanoparticles for Combination Cancer Therapy. Acta Pharm. Sin. B 2023, 13, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Mennati, A.; Rostamizadeh, K.; Manjili, H.K.; Fathi, M.; Danafar, H. Co-Delivery of siRNA and Lycopene Encapsulated Hybrid Lipid Nanoparticles for Dual Silencing of Insulin-Like Growth Factor 1 Receptor in MCF-7 Breast Cancer Cell Line. Int. J. Biol. Macromol. 2022, 200, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid polymer hybrid nanoparticles as potent vehicles for drug delivery in cancer therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Zhu, C.; Xiang, C. Anti-Prostate Cancer Therapy: Aptamer-Functionalized, Curcumin and Cabazitaxel Co-Delivered, Tumor Targeted Lipid-Polymer Hybrid Nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Ahmed, N.; ur Rehman, A.; Khan, G.M. Lipid Polymer Hybrid Carrier Systems for Cancer Targeting: A Review. Int. J. Polym. Mater. Polym. Biomater. 2017, 67, 86–100. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid Polymer Hybrid Nanoparticles: A Custom-Tailored Next-Generation Approach for Cancer Therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.; Alrobaian, M.; Afzal, O.; Kazmi, I.; Almalki, W.H.; Altamimi, A.S.A.; Al-Abbasi, F.A.; Alharbi, K.S.; Altowayan, W.M.; et al. EGF-Functionalized Lipid-Polymer Hybrid Nanoparticles of 5-Fluorouracil and Sulforaphane with Enhanced Bioavailability and Anticancer Activity Against Colon Carcinoma. Biotechnol. Appl. Biochem. 2022, 69, 2205–2221. [Google Scholar] [CrossRef]
- Du, L.; Xu, Y.; Han, B.; Wang, Y.; Zeng, Q.; Shao, M.; Yu, Z. EGFR-Targeting Peptide Conjugated Polymer-Lipid Hybrid Nanoparticles for Delivery of Salinomycin to Osteosarcoma. J. Cancer Res. Ther. 2023, 19, 1544–1551. [Google Scholar] [CrossRef]
- Djermane, R.; Nieto, C.; Vega, M.A.; Del Valle, E.M.M. EGFR-Targeting Polydopamine Nanoparticles Co-Loaded with 5-Fluorouracil, Irinotecan, and Leucovorin to Potentially Enhance Metastatic Colorectal Cancer Therapy. Sci. Rep. 2024, 14, 29265. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, C.; Kumar, R.; Kalra, N.; Saini, A.; Sharma, S.; Singh, A. Concomitant Role of Metal Clusters and Ligands in the Synthesis and Control of Porosity in Metal-Organic Frameworks: A Literature Review. Results Chem. 2023, 5, 101206. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; El-Morsy, M.T.; Faried, H.; Diab, A.H.; Shehab, S.; Saleh, A.M.; Ali, G.A.M. Metal-Organic Frameworks in Drug Delivery: Engineering Versatile Platforms for Therapeutic Applications. RSC Adv. 2024, 14, 30201–30229. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Metal-Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules 2020, 25, 1598. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, M.; Parisse, V.; Guglielmi, P.; Giannini, G.; Secci, D. Metal-Organic Frameworks (MOFs) as Biomolecule Drug Delivery Systems for Anticancer Purposes. Eur. J. Med. Chem. 2022, 244, 114801. [Google Scholar] [CrossRef]
- Zhao, H.; Gong, L.; Wu, H.; Liu, C.; Liu, Y.; Xiao, C.; Liu, C.; Chen, L.; Jin, M.; Gao, Z.; et al. Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect Against Prostate Cancer Cells. Pharmaceutics 2023, 15, 1874. [Google Scholar] [CrossRef]
- Shen, J.J.; Xue, S.J.; Mei, Z.H.; Li, T.T.; Li, H.F.; Zhuang, X.F.; Pan, L.M. Synthesis, Characterization, and Efficacy Evaluation of a pH-Responsive Fe-MOF@GO Composite Drug Delivery System for Treating Colorectal Cancer. Heliyon 2024, 10, e28066. [Google Scholar] [CrossRef]
- Darvishi, S.; Hosseinzadeh, H.; Kazeminava, F.; Mahoutforoush, A.; Tajik, M.; Rasoulzadehzali, M.; Mohammadi, R.; Sadjadi, S.; Javanbakht, S. Heparin-Functionalized Cu-Based Metal-Organic Framework: An Efficient Active and Passive Targeting Nanocarrier for Anticancer Doxorubicin Drug Delivery. Int. J. Biol. Macromol. 2024, 282, 136648. [Google Scholar] [CrossRef]
- Dong, J.; Ding, J.; Luo, S.; Li, R.; Wang, Y.; Xiao, B.; Pei, Y.; Chen, X.; Sun, W.; Pei, Z. Remodeling Tumor Microenvironment Using Prodrug nMOFs for Synergistic Cancer Therapy. J. Nanobiotechnol. 2025, 23, 123. [Google Scholar] [CrossRef]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Carbon Nanoparticles and Their Biomedical Applications. Appl. Sci. 2022, 12, 7865. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Phototherapy Combined with Carbon Nanomaterials (1D and 2D) and Their Applications in Cancer Therapy. Materials 2020, 13, 4830. [Google Scholar] [CrossRef]
- Amaral, S.I.; Costa-Almeida, R.; Goncalves, I.C.; Magalhaes, F.D.; Pinto, A.M. Carbon Nanomaterials for Phototherapy of Cancer and Microbial Infections. Carbon 2022, 190, 194–244. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulate Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Marangon, I.; Ménard-Moyon, C.; Silva, A.K.; Bianco, A.; Luciani, N.; Gazeau, F. Synergic Mechanisms of Photothermal and Photodynamic Therapies Mediated by Photosensitizer/Carbon Nanotube Complexes. Carbon 2016, 97, 110–123. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent Advances in Bioactive 1D and 2D Carbon Nanomaterials for Biomedical Applications. Nanomedicine 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Abousalman-Rezvani, Z.; Eskandari, P.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Functionalization of Carbon Nanotubes by Combination of Controlled Radical Polymerization and “Grafting to” Method. Adv. Colloid Interface Sci. 2020, 278, 102126. [Google Scholar] [CrossRef]
- Eskandari, P.; Abousalman-Rezvani, Z.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Polymer-Functionalization of Carbon Nanotubes by in Situ Conventional and Controlled Radical Polymerizations. Adv. Colloid Interface Sci. 2021, 294, 102471. [Google Scholar] [CrossRef]
- Eskandari, P.; Abousalman-Rezvani, Z.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Mardani, H. Polymer Grafting on Graphene Layers by Controlled Radical Polymerization. Adv. Colloid Interface Sci. 2019, 273, 102021. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on Functionalized Carbon Nanotubes for Cancer Theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef]
- Polo, E.; Nitka, T.T.; Neubert, E.; Erpenbeck, L.; Vuković, L.; Kruss, S. Control of Integrin Affinity by Confining RGD Peptides on Fluorescent Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 17693–17703. [Google Scholar] [CrossRef]
- Koh, B.; Park, S.B.; Yoon, E.; Yoo, H.M.; Lee, D.; Heo, J.N.; Ahn, S. αVβ3-Targeted Delivery of Camptothecin-Encapsulated Carbon Nanotube-Cyclic RGD in 2D and 3D Cancer Cell Culture. J. Pharm. Sci. 2019, 108, 3704–3712. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.X.; Huang, H.Y.; Chen, L.Q.; Cui, J.H.; Liu, Y.; Jin, H.; Lee, B.J.; Cao, Q.R. Effective Deactivation of A549 Tumor Cells in Vitro and in Vivo by RGD-Decorated Chitosan-Functionalized Single-Walled Carbon Nanotube Loading Docetaxel. Int. J. Pharm. 2018, 543, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, X.X.; Zhou, Q.; Zhang, F.Y.; Wang, Q.L.; Liu, Y.Q.; Zou, Y.; Tao, Q.; Ji, X.M.; Yu, S.Q. EGF-Functionalized Single-Walled Carbon Nanotubes for Targeting Delivery of Etoposide. Nanotechnology 2012, 23, 045104. [Google Scholar] [CrossRef]
- Lee, P.C.; Chiou, Y.C.; Wong, J.M.; Peng, C.L.; Shieh, M.J. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials 2013, 34, 8756–8765. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Tu, Q.; Pang, L.; Chen, Y.; Zhang, Y.; Zhang, R.; Lu, B.; Wang, J. Effects of surface charges of graphene oxide on neuronal outgrowth and branching. Analyst 2014, 139, 105–115. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Sattari, S.; Adeli, M.; Beyranvand, S.; Nemati, M. Functionalized graphene platforms for anticancer drug delivery. Int. J. Nanomed. 2021, 16, 5955–5980. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef]
- Tanida, S.; Mizoshita, T.; Ozeki, K.; Tsukamoto, H.; Kamiya, T.; Kataoka, H.; Sakamuro, D.; Joh, T. Mechanisms of cisplatin-induced apoptosis and of cisplatin sensitivity: Potential of BIN1 to act as a potent predictor of cisplatin sensitivity in gastric cancer treatment. Int. J. Surg. Oncol. 2012, 2012, 862879. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Liu, D.Z.; Cheng, Y.; Liu, M.; Ye, W.L.; Zhang, B.L.; Liu, X.Y.; Zhou, S.Y. Dual subcellular compartment delivery of doxorubicin to overcome drug resistant and enhance antitumor activity. Sci. Rep. 2015, 5, 16125. [Google Scholar] [CrossRef]
- Ma, N.; Song, A.; Li, Z.; Luan, Y. Redox-sensitive prodrug molecules meet graphene oxide: An efficient graphene oxide-based nanovehicle toward cancer therapy. ACS Biomater. Sci. Eng. 2019, 5, 1384–1391. [Google Scholar] [CrossRef]
- Dilenko, H.; Bartoň Tománková, K.; Válková, L.; Hošíková, B.; Kolaříková, M.; Malina, L.; Bajgar, R.; Kolářová, H. Graphene-based photodynamic therapy and overcoming cancer resistance mechanisms: A comprehensive review. Int. J. Nanomed. 2024, 19, 5637–5680. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Z.; Feng, X.; Shen, G.; Huang, H.; Liang, Y.; Zhao, B.; Li, G.; Hu, Y. Graphene oxide (GO)-based nanosheets with combined chemo/photothermal/photodynamic therapy to overcome gastric cancer (GC) paclitaxel resistance by reducing mitochondria-derived adenosine-triphosphate (ATP). J. Nanobiotechnol. 2021, 19, 146. [Google Scholar] [CrossRef]
- Li, L.; Luo, C.; Song, Z.; Reyes-Vargas, E.; Clayton, F.; Huang, J.; Jensen, P.; Chen, X. Association of anti-HER2 antibody with graphene oxide for curative treatment of osteosarcoma. Nanomedicine 2018, 14, 581–593. [Google Scholar] [CrossRef]
- Deng, W.; Qiu, J.; Wang, S.; Yuan, Z.; Jia, Y.; Tan, H.; Lu, J.; Zheng, R. Development of biocompatible and VEGF-targeted paclitaxel nanodrugs on albumin and graphene oxide dual-carrier for photothermal-triggered drug delivery in vitro and in vivo. Int. J. Nanomed. 2018, 13, 439–453. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Search Results for Cancer and Nanoparticles (Phases 3 and 4). Available online: https://clinicaltrials.gov/search?cond=Cancer&intr=nanoparticles&aggFilters=phase:4%203%202. (accessed on 25 February 2025).
- ClinicalTrials.gov. A Clinical Study on the Benefits of Carbon Nanoparticles Injection Time in Patients with Thyroid Cancer. Available online: https://clinicaltrials.gov/study/NCT06791005?cond=Cancer&intr=nanoparticles&aggFilters=phase:4%203%202&rank=1. (accessed on 25 February 2025).
- ClinicalTrials.gov. Activated by Radiotherapy With or Without Cetuximab in LA-HNSCC. Available online: https://clinicaltrials.gov/study/NCT04892173?cond=Cancer&intr=nanoparticles&aggFilters=phase:4%203%202&page=5&rank=44. (accessed on 25 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez Coronado, O.; Sandoval Salazar, C.; Muñoz Carrillo, J.L.; Gutiérrez Villalobos, O.A.; Miranda Beltrán, M.d.l.L.; Soriano Hernández, A.D.; Beltrán Campos, V.; Villalobos Gutiérrez, P.T. Functionalized Nanomaterials in Cancer Treatment: A Review. Int. J. Mol. Sci. 2025, 26, 2633. https://doi.org/10.3390/ijms26062633
Gutiérrez Coronado O, Sandoval Salazar C, Muñoz Carrillo JL, Gutiérrez Villalobos OA, Miranda Beltrán MdlL, Soriano Hernández AD, Beltrán Campos V, Villalobos Gutiérrez PT. Functionalized Nanomaterials in Cancer Treatment: A Review. International Journal of Molecular Sciences. 2025; 26(6):2633. https://doi.org/10.3390/ijms26062633
Chicago/Turabian StyleGutiérrez Coronado, Oscar, Cuauhtémoc Sandoval Salazar, José Luis Muñoz Carrillo, Oscar Alexander Gutiérrez Villalobos, María de la Luz Miranda Beltrán, Alejandro David Soriano Hernández, Vicente Beltrán Campos, and Paola Trinidad Villalobos Gutiérrez. 2025. "Functionalized Nanomaterials in Cancer Treatment: A Review" International Journal of Molecular Sciences 26, no. 6: 2633. https://doi.org/10.3390/ijms26062633
APA StyleGutiérrez Coronado, O., Sandoval Salazar, C., Muñoz Carrillo, J. L., Gutiérrez Villalobos, O. A., Miranda Beltrán, M. d. l. L., Soriano Hernández, A. D., Beltrán Campos, V., & Villalobos Gutiérrez, P. T. (2025). Functionalized Nanomaterials in Cancer Treatment: A Review. International Journal of Molecular Sciences, 26(6), 2633. https://doi.org/10.3390/ijms26062633








