Transient Global Amnesia (TGA): Is It Really Benign? A Pilot Study on Blood Biomarkers
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Inability to undergo cerebral MRI (cMRI) or electroencephalography (EEG)
- Presence of acute intracranial damage, such as brain hemorrhage, traumatic brain injury, ischemic stroke, or transient ischemic attack within the preceding 3 months
- Prolonged cardiopulmonary resuscitation lasting longer than 2 min within the past 12 months
- Neurosurgical operation within the last 6 months
- Progressive neurological disorders, such as neoplasm or dementia
- Major surgery, biopsy of a parenchymal organ, or significant trauma within the past 2 months
- Lack of willingness or ability to participate in the follow-up examinations.
4.1. sNfL and sGFAP
4.2. cMRI and EEG Assessment
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quinette, P.; Guillery-Girard, B.; Dayan, J.; de la Sayette, V.; Marquis, S.; Viader, F.; Desgranges, B.; Eustache, F. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain 2006, 129, 1640–1658. [Google Scholar] [CrossRef] [PubMed]
- Arena, J.E.; Rabinstein, A.A. Transient global amnesia. Mayo Clin. Proc. 2015, 90, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Koski, K.J.; Marttila, R.J. Transient global amnesia: Incidence in an urban population. Acta Neurol. Scand. 1990, 81, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.R.; Smith, J.; Wade, R.R.; Cherukuru, N.; Ursani, A.; Dobruskina, Y.; Crist, T.; Busch, R.F.; Dhanani, R.M.; Dreyer, N. Transient global amnesia: Current perspectives. Neuropsychiatr. Dis. Treat. 2017, 13, 2691–2703. [Google Scholar] [CrossRef]
- Hodges, J.R.; Warlow, C.P. Syndromes of transient amnesia: Towards a classification. A study of 153 cases. J. Neurol. Neurosurg. Psychiatry 1990, 53, 834–843. [Google Scholar] [CrossRef]
- Miller, J.W.; Petersen, R.C.; Metter, E.J.; Millikan, C.H.; Yanagihara, T. Transient global amnesia: Clinical characteristics and prognosis. Neurology 1987, 37, 733–737. [Google Scholar] [CrossRef]
- Zorzon, M.; Antonutti, L.; Mase, G.; Biasutti, E.; Vitrani, B.; Cazzato, G. Transient global amnesia and transient ischemic attack. Natural history, vascular risk factors, and associated conditions. Stroke 1995, 26, 1536–1542. [Google Scholar] [CrossRef]
- Arena, J.E.; Brown, R.D.; Mandrekar, J.; Rabinstein, A.A. Long-Term Outcome in Patients With Transient Global Amnesia: A Population-Based Study. Mayo Clin. Proc. 2017, 92, 399–405. [Google Scholar] [CrossRef]
- Pantoni, L.; Bertini, E.; Lamassa, M.; Pracucci, G.; Inzitari, D. Clinical features, risk factors, and prognosis in transient global amnesia: A follow-up study. Eur. J. Neurol. 2005, 12, 350–356. [Google Scholar] [CrossRef]
- Mangla, A.; Navi, B.B.; Layton, K.; Kamel, H. Transient global amnesia and the risk of ischemic stroke. Stroke 2014, 45, 389–393. [Google Scholar] [CrossRef]
- Romoli, M.; Tuna, M.A.; McGurgan, I.; Li, L.; Giannandrea, D.; Eusebi, P.; Tordo Caprioli, F.; Lotti, A.; Salvadori, N.; Sarchielli, P.; et al. Long-Term Risk of Stroke After Transient Global Amnesia in Two Prospective Cohorts. Stroke 2019, 50, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, C.; Caponnetto, C.; Conti, M.; Dagnino, N.; Del Sette, M.; Primavera, A. Prognosis of transient global amnesia: A long-term follow-up study. Eur. Neurol. 1992, 32, 52–57. [Google Scholar] [CrossRef]
- Bartsch, T.; Alfke, K.; Stingele, R.; Rohr, A.; Freitag-Wolf, S.; Jansen, O.; Deuschl, G. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain 2006, 129, 2874–2884. [Google Scholar] [CrossRef]
- Mariotto, S.; Sechi, E.; Ferrari, S. Serum neurofilament light chain studies in neurological disorders, hints for interpretation. J. Neurol. Sci. 2020, 416, 116986. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Aurell, A.; Rosengren, L.E.; Karlsson, B.; Olsson, J.E.; Zbornikova, V.; Haglid, K.G. Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke 1991, 22, 1254–1258. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as biomarkers in neurological disorders—Towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef]
- Sander, D.; Bartsch, T.; Connolly, F.; Enzinger, C.; Fischer, U.; Nellessen, N.; Poppert, H.; Szabo, K.; Topka, H. Guideline “Transient Global Amnesia (TGA)” of the German Society of Neurology (Deutsche Gesellschaft fur Neurologie): S1-guideline. Neurol. Res. Pract. 2023, 5, 15, Erratum in Neurol. Res. Pract. 2023, 5, 64. [Google Scholar] [CrossRef]
- LB, C. Transient global amnesia. In Handbook of Clinical Neurology; North-Holland Publishing Company: Amsterdam, The Netherlands, 1985; Volume 1, pp. 205–218. [Google Scholar]
- Bartsch, T.; Deuschl, G. Transient global amnesia: Functional anatomy and clinical implications. Lancet Neurol. 2010, 9, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Traenka, C.; Disanto, G.; Seiffge, D.J.; Gensicke, H.; Hert, L.; Grond-Ginsbach, C.; Peters, N.; Regeniter, A.; Kloss, M.; De Marchis, G.M.; et al. Serum Neurofilament Light Chain Levels Are Associated with Clinical Characteristics and Outcome in Patients with Cervical Artery Dissection. Cerebrovasc. Dis. 2015, 40, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Calderon, F.; Portelius, E.; Zetterberg, H.; Blennow, K.; Rosengren, L.E.; Hoglund, K. Neurofilament changes in serum and cerebrospinal fluid after acute ischemic stroke. Neurosci. Lett. 2019, 698, 58–63. [Google Scholar] [CrossRef]
- Tiedt, S.; Duering, M.; Barro, C.; Kaya, A.G.; Boeck, J.; Bode, F.J.; Klein, M.; Dorn, F.; Gesierich, B.; Kellert, L.; et al. Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology 2018, 91, e1338–e1347. [Google Scholar] [CrossRef]
- Ahn, J.W.; Hwang, J.; Lee, M.; Kim, J.H.; Cho, H.J.; Lee, H.W.; Eun, M.Y. Serum neurofilament light chain levels are correlated with the infarct volume in patients with acute ischemic stroke. Medicine 2022, 101, e30849. [Google Scholar] [CrossRef]
- Gundersen, J.K.; Gonzalez-Ortiz, F.; Karikari, T.; Kirsebom, B.E.; Mertes, K.; Zetterberg, H.; Kvartsberg, H.; Ronning, O.M.; Gisladottir, B.; Blennow, K.; et al. Neuronal plasma biomarkers in acute ischemic stroke. J. Cereb. Blood Flow. Metab. 2025, 45, 77–84. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Katan, M.; Barro, C.; Fladt, J.; Traenka, C.; Seiffge, D.J.; Hert, L.; Gensicke, H.; Disanto, G.; Sutter, R.; et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur. J. Neurol. 2018, 25, 562–568. [Google Scholar] [CrossRef]
- Akel, S.; Asztely, F.; Banote, R.K.; Axelsson, M.; Zetterberg, H.; Zelano, J. Neurofilament light, glial fibrillary acidic protein, and tau in a regional epilepsy cohort: High plasma levels are rare but related to seizures. Epilepsia 2023, 64, 2690–2700. [Google Scholar] [CrossRef]
- Dargvainiene, J.; Sahaf, S.; Franzenburg, J.; Matthies, I.; Leypoldt, F.; Wandinger, K.P.; Baysal, L.; Markewitz, R.; Kuhlenbaumer, G.; Margraf, N.G. Neurofilament light (NfL) concentrations in patients with epilepsy with recurrent isolated seizures: Insights from a clinical cohort study. Seizure 2024, 121, 91–94. [Google Scholar] [CrossRef]
- Giovannini, G.; Bedin, R.; Ferraro, D.; Vaudano, A.E.; Mandrioli, J.; Meletti, S. Serum neurofilament light as biomarker of seizure-related neuronal injury in status epilepticus. Epilepsia 2022, 63, e23–e29. [Google Scholar] [CrossRef]
- Hviid, C.V.B.; Madsen, A.T.; Winther-Larsen, A. Biological variation of serum neurofilament light chain. Clin. Chem. Lab. Med. 2022, 60, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Brum, W.S.; Ashton, N.J.; Simren, J.; di Molfetta, G.; Karikari, T.K.; Benedet, A.L.; Zimmer, E.R.; Lantero-Rodriguez, J.; Montoliu-Gaya, L.; Jeromin, A.; et al. Biological variation estimates of Alzheimer’s disease plasma biomarkers in healthy individuals. Alzheimer’s Dement. 2024, 20, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Geng, J. Glial fibrillary acidic protein as a prognostic marker of acute ischemic stroke. Hum. Exp. Toxicol. 2018, 37, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Gattringer, T.; Enzinger, C.; Pinter, D.; Fandler-Hofler, S.; Kneihsl, M.; Haidegger, M.; Eppinger, S.; Demjaha, R.; Buchmann, A.; Jerkovic, A.; et al. Serum glial fibrillary acidic protein is sensitive to acute but not chronic tissue damage in cerebral small vessel disease. J. Neurol. 2023, 270, 320–327. [Google Scholar] [CrossRef]
- Thaele, A.; Barba, L.; Abu-Rumeileh, S.; Foschi, M.; Otto, M. Neurofilament light chain and glial fibrillary acidic protein as diagnostic and prognostic biomarkers in epileptic seizures and epilepsy: A systematic review. Epilepsy Behav. 2025, 165, 110321. [Google Scholar] [CrossRef]
- Dobson, H.; Al Maawali, S.; Malpas, C.; Santillo, A.F.; Kang, M.; Todaro, M.; Watson, R.; Yassi, N.; Blennow, K.; Zetterberg, H.; et al. Elevated plasma neurofilament light and glial fibrillary acidic protein in epilepsy versus nonepileptic seizures and nonepileptic disorders. Epilepsia 2024, 65, 2751–2763. [Google Scholar] [CrossRef]
- Margraf, N.G.; Dargvainiene, J.; Theel, E.; Leypoldt, F.; Lieb, W.; Franke, A.; Berger, K.; Kuhle, J.; Kuhlenbaeumer, G. Neurofilament light (NfL) as biomarker in serum and CSF in status epilepticus. J. Neurol. 2023, 270, 2128–2138. [Google Scholar] [CrossRef]
- Nass, R.D.; Akgun, K.; Dague, K.O.; Elger, C.E.; Reichmann, H.; Ziemssen, T.; Surges, R. CSF and Serum Biomarkers of Cerebral Damage in Autoimmune Epilepsy. Front. Neurol. 2021, 12, 647428. [Google Scholar] [CrossRef]
- Katisko, K.; Cajanus, A.; Jaaskelainen, O.; Kontkanen, A.; Hartikainen, P.; Korhonen, V.E.; Helisalmi, S.; Haapasalo, A.; Koivumaa-Honkanen, H.; Herukka, S.K.; et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J. Neurol. 2020, 267, 162–167. [Google Scholar] [CrossRef]
- Eratne, D.; Kang, M.; Malpas, C.; Simpson-Yap, S.; Lewis, C.; Dang, C.; Grewal, J.; Coe, A.; Dobson, H.; Keem, M.; et al. Plasma neurofilament light in behavioural variant frontotemporal dementia compared to mood and psychotic disorders. Aust. N. Z. J. Psychiatry 2024, 58, 70–81. [Google Scholar] [CrossRef]
- Bavato, F.; Barro, C.; Schnider, L.K.; Simren, J.; Zetterberg, H.; Seifritz, E.; Quednow, B.B. Introducing neurofilament light chain measure in psychiatry: Current evidence, opportunities, and pitfalls. Mol. Psychiatry 2024, 29, 2543–2559. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.Y.; Eratne, D.; Wannan, C.; Santillo, A.F.; Velakoulis, D.; Pantelis, C.; Cropley, V. Plasma neurofilament light chain is not elevated in people with first-episode psychosis or those at ultra-high risk for psychosis. Schizophr. Res. 2024, 267, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Amorim, D.; Rivera-Baltanas, T.; Del Carmen Vallejo-Curto, M.; Rodriguez-Jamardo, C.; de Las Heras, E.; Barreiro-Villar, C.; Blanco-Formoso, M.; Fernandez-Palleiro, P.; Alvarez-Ariza, M.; Lopez, M.; et al. Plasma beta-III tubulin, neurofilament light chain and glial fibrillary acidic protein are associated with neurodegeneration and progression in schizophrenia. Sci. Rep. 2020, 10, 14271. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Chen, D.W.; Li, H.Y.; Zhu, C.; Shen, Y.Y.; Peng, Z.Y.; Li, L.; Bu, X.L.; Zeng, G.H.; Zhang, M.; et al. The Association of Serum Neurofilament Light Chain and Acute Ischaemic Stroke Is Influenced by Effective Revascularization. Dis. Markers 2022, 2022, 5236080. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, O.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Benkert, P.; Maleska Maceski, A.; Schaedelin, S.; Oechtering, J.; Zadic, A.; Vilchez Gomez, J.F.; Melie-Garcia, L.; Cagol, A.; Galbusera, R.; Subramaniam, S.; et al. Serum Glial Fibrillary Acidic Protein and Neurofilament Light Chain Levels Reflect Different Mechanisms of Disease Progression under B-Cell Depleting Treatment in Multiple Sclerosis. Ann. Neurol. 2024, 97, 104–115. [Google Scholar] [CrossRef]
| Demographic Features | n = 20 1 |
|---|---|
| Age at diagnosis of TGA | 66 (61, 75) |
| Sex (Woman) | 14 (70%) |
| TGA duration (h) | 6.0 (3.0, 9.5) |
| BMI | 25.9 (23.5, 27.4) |
| Comorbidities | |
| Arterial hypertension | 11 (55%) |
| Hypercholesteremia | 3 (15%) |
| Ischemic stroke in history | 2 (10%) |
| Years since first stroke | 5 (4, 6) |
| Atrial fibrillation | 1 (5%) |
| Diabetes | 1 (5%) |
| Smoking | 2 (11%) |
| Medication | |
| Antithrombotic treatment (ASA all) | 4 (20%) |
| TGA in the past medical history | 4 (20%) |
| Years since first TGA | 11 (7, 13) |
| Neurological diseases in past medical history (other than TGA) | |
| Migraine | 2 (10%) |
| Vestibular neuritis | 1 (5%) |
| Other | 1 (5%) |
| None | 16 (80%) |
| Vital signs at admission | |
| Systolic pressure (mmHg) | 170 (155, 192) |
| Diastolic pressure (mmHg) | 91 (88, 100) |
| Ear temperature (°C) | 36.1 (36.0, 36.6) |
| Heart frequency (bpm) | 81 (69, 90) |
| Laboratory values | |
| ESR (mm/h) | 6.0 (4.0, 7.0) |
| CRP (mg/dL) | 0.2 (0.1, 0.2) |
| HDL-C (mg/dL) | 60 (50, 69) |
| LDL-C (mg/dL) | 133 (107, 177) |
| hs-Troponin T (pg/mL) | 8.5 (7.0, 11.8) |
| NT-proBNP (pg/mL) | 95 (61, 169) |
| HbA1C (%) | 5.4 (5.3, 5.4) |
| Triggers | |
| Emotional or stressful event | 8 (42%) |
| Exercise of physical exertion | 4 (21%) |
| Data not available | 4 (21%) |
| Shower or bathtub | 1 (5%) |
| None | 2 (11%) |
| EEG findings | |
| Unilateral temporal slowing | 3 (15%) |
| Normal | 17 (85%) |
| EEG—days after TGA onset | 1 (1, 2) |
| MRI—days after TGA onset | 1 (0, 1) |
| MRI findings | |
| DWI hippocampal lesion | 5 (25%) |
| DWI locations | |
| Left | 1 (20%) |
| Left (CA1-Region), 3 mm | 1 (20%) |
| Left temporal rostral (subiculum), 2 mm | 1 (20%) |
| Right | 1 (20%) |
| Right posterior | 1 (20%) |
| Additional MRI findings | |
| Leukoaraiosis first grade | 4 (21%) |
| Leukoaraiosis second grade | 3 (16%) |
| Leukoaraiosis third grade | 1 (5%) |
| Incidental corpus callosum hyperintensity | 1 (5%) |
| DVA frontal right | 1 (5%) |
| Mild atrophy | 1 (5%) |
| MTA score 3 | 1 (5%) |
| Other | 1 (5%) |
| None | 5 (26%) |
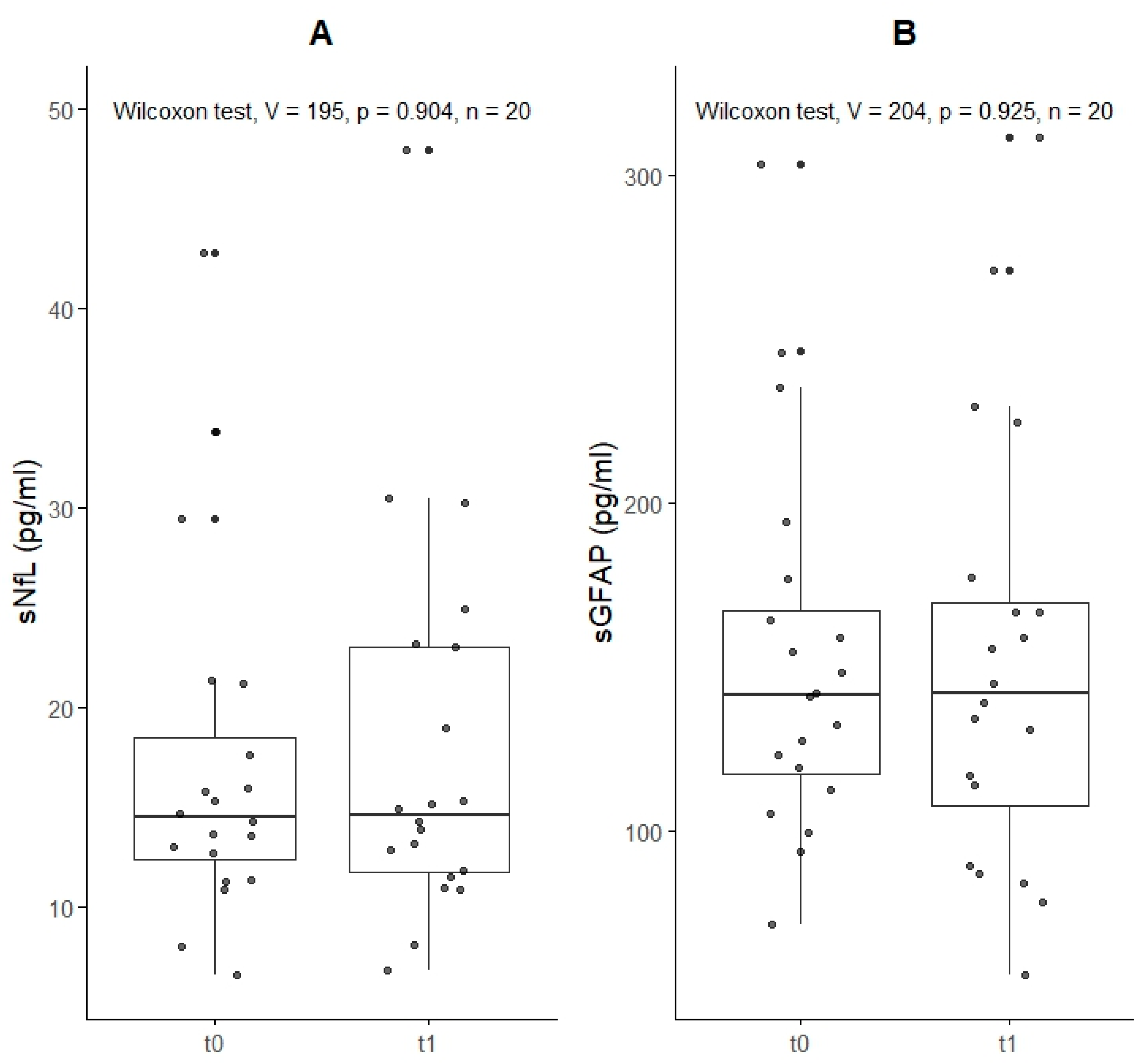
| Summary of Biomarkers at Baseline (t0) and 6 Weeks (t1) | ||||||
|---|---|---|---|---|---|---|
| Type | sNfL | sGFAP | ||||
| Baseline, n = 20 1 | 6 Weeks, n = 20 1 | p-Value 2 | Baseline, n = 20 1 | 6 Weeks, n = 20 1 | p-Value 2 | |
| Raw values, median (IQR) (pg/mL) | 14 (12, 19) | 15 (12, 23) | >0.9 | 142 (118, 167) | 142 (108, 170) | >0.9 |
| Percentile | 68 (46, 88) | 73 (27, 88) | 0.8 | 61 (33, 78) | 67 (40, 77) | 0.8 |
| Z-score | 0.45 (−0.09, 1.19) | 0.60 (−0.61, 1.19) | 0.8 | 0.27 (−0.45, 0.76) | 0.44 (−0.27, 0.75) | 0.8 |
| Characteristic | DWI—Positive, n = 5 1 | DWI—Negative, n = 15 1 | p-Value 2 |
|---|---|---|---|
| Age at TGA diagnosis | 66 (63, 74) | 66 (61, 75) | >0.9 |
| TGA duration (h) | 6.0 (4.0, 7.0) | 5.5 (3.0, 10.0) | 0.9 |
| Gender (Man) | 0 (0%) | 6 (40%) | 0.3 |
| BMI | 28.7 (27.0, 30.5) | 25.4 (23.0, 26.5) | 0.12 |
| Comorbidities | |||
| Smoking | 0 (0%) | 2 (14%) | >0.9 |
| Atrial Fibrillation | 0 (0%) | 1 (7%) | >0.9 |
| Arterial Hypertension | 3 (60%) | 8 (53%) | >0.9 |
| Diabetes | 0 (0%) | 1 (7%) | >0.9 |
| LDL-C Hypercholesteremia | 2 (40%) | 1 (7%) | 0.14 |
| Ischemic stroke in history | 1 (20%) | 1 (7%) | 0.4 |
| Years since first stroke | 7 (7, 7) | 3 (3, 3) | >0.9 |
| Neurological diseases in history (other than TGA) | 0.10 | ||
| None | 3 (60%) | 13 (87%) | |
| Migraine | 0 (0%) | 2 (13%) | |
| Other | 1 (20%) | 0 (0%) | |
| Vestibular neuritis | 1 (20%) | 0 (0%) | |
| Medication | |||
| Antihrombotic therapy (all ASA) | 3 (60%) | 1 (7%) | 0.032 |
| TGA in history | 1 (20%) | 3 (20%) | >0.9 |
| Years since first TGA | 11 (11, 11) | 8 (6, 11) | >0.9 |
| Vital Signs at admission | |||
| Systolic pressure at admission (mmHg) | 172 (148, 201) | 170 (158, 187) | >0.9 |
| Diastolic pressure at admission (mmHg) | 93 (89, 99) | 91 (88, 99) | >0.9 |
| Ear temperature at admission (°C) | 36.05 (35.93, 36.23) | 36.20 (36, 36.65) | 0.4 |
| Heart frequency (per minute) | 83 (80, 85) | 80 (68, 91) | >0.9 |
| Laboratory Values | |||
| BSG | 6 (6, 6) | 5 (3.50, 6.50) | >0.9 |
| CRP | 0.10 (0.10, 0.10) | 0.20 (0.10, 0.20) | 0.083 |
| HDL-C | 56 (51, 62) | 64 (50, 73) | 0.7 |
| LDL-C (mg/dL) | 142 (129, 204) | 124 (101, 173) | 0.5 |
| hs-Troponin T | 10.0 (6.0, 16.5) | 8.0 (7.0, 11.5) | 0.9 |
| pro—BNP | 169 (132, 207) | 79 (41, 126) | 0.3 |
| HbA1C | 5.40 (5.40, 5.40) | 5.30 (5.25, 5.90) | 0.8 |
| Triggers | >0.9 | ||
| Emotional or stressful event | 2 (50%) | 6 (40%) | |
| Exercise of physical exertion | 1 (25%) | 3 (20%) | |
| Shower or bathtube | 0 (0%) | 1 (7%) | |
| Data not available | 1 (25%) | 3 (20%) | |
| None | 0 (0%) | 2 (13%) | |
| EEG findings | >0.9 | ||
| none | 45(100%) | 12 (80%) | |
| temporal slowing | 0 | 3 (20%) | |
| EEG—days after TGA | 2 (1, 2) | 1 (0, 1) | 0.094 |
| Additional MRI findings | >0.9 | ||
| none | 3 (60%) | 4 (27%) | |
| leukoaraiosis first grade | 1 (20%) | 3 (20%) | |
| leukoaraiosis second grade | 1 (20%) | 2 (13%) | |
| leukoaraiosis third grade | 0 (0%) | 1 (7%) | |
| Incidental corpus calosum hyperintensity | 0 (0%) | 1 (7%) | |
| DVA frontal right | 0 (0%) | 1 (7%) | |
| mild atrophy | 0 (0%) | 1 (7%) | |
| MTA score 3 | 0 (0%) | 1 (7%) | |
| other | 0 (0%) | 1 (7%) | |
| MRI—days after TGA | 1 (1, 2) | 1 (0, 1) | 0.4 |
| Characteristic | sGFAP DWI—Negative Baseline | sGFAP DWI—Negative 6 Weeks | sNfL DWI—Negative Baseline | sNfL DWI—Negative 6 Weeks | sGFAP DWI—Positive Baseline | sGFAP DWI—Positive 6 Weeks | sNfL DWI—Positive Baseline | sNfL DWI—Positive 6 Weeks |
|---|---|---|---|---|---|---|---|---|
| n = | 15 | 15 | 15 | 15 | 5 | 5 | 5 | 5 |
| Raw value, median (IQR) (pg/mL) | 149 (116, 171) | 139 (116, 167) | 14 (12, 17) | 14 (12, 17) | 128 (123, 133) | 145 (87, 225) | 15 (13, 21) | 23 (15, 23) |
| Percentile | 61 (33, 79) | 58 (34, 77) | 68 (45, 88) | 70 (31, 88) | 56 (53, 65) | 71 (70, 76) | 65 (50, 95) | 83 (28, 89) |
| Z-score | 0.28 (−0.45, 0.79) | 0.20 (−0.43, 0.73) | 0.47 (−0.14, 1.15) | 0.52 (−0.52, 1.15) | 0.15 (0.08, 0.39) | 0.55 (0.52, 0.71) | 0.39 (0.00, 1.60) | 0.95 (−0.58, 1.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, F.; Moser, T.; Unterhofer, M.; Khalil, M.; Demjaha, R.; Tafrali, C.; Martinez-Serrat, M.; Kuhle, J.; Leppert, D.; Benkert, P.; et al. Transient Global Amnesia (TGA): Is It Really Benign? A Pilot Study on Blood Biomarkers. Int. J. Mol. Sci. 2025, 26, 2629. https://doi.org/10.3390/ijms26062629
Rossini F, Moser T, Unterhofer M, Khalil M, Demjaha R, Tafrali C, Martinez-Serrat M, Kuhle J, Leppert D, Benkert P, et al. Transient Global Amnesia (TGA): Is It Really Benign? A Pilot Study on Blood Biomarkers. International Journal of Molecular Sciences. 2025; 26(6):2629. https://doi.org/10.3390/ijms26062629
Chicago/Turabian StyleRossini, Fabio, Tobias Moser, Michael Unterhofer, Michael Khalil, Rina Demjaha, Cansu Tafrali, Maria Martinez-Serrat, Jens Kuhle, David Leppert, Pascal Benkert, and et al. 2025. "Transient Global Amnesia (TGA): Is It Really Benign? A Pilot Study on Blood Biomarkers" International Journal of Molecular Sciences 26, no. 6: 2629. https://doi.org/10.3390/ijms26062629
APA StyleRossini, F., Moser, T., Unterhofer, M., Khalil, M., Demjaha, R., Tafrali, C., Martinez-Serrat, M., Kuhle, J., Leppert, D., Benkert, P., Pfaff, J. A. R., Trinka, E., & Pikija, S. (2025). Transient Global Amnesia (TGA): Is It Really Benign? A Pilot Study on Blood Biomarkers. International Journal of Molecular Sciences, 26(6), 2629. https://doi.org/10.3390/ijms26062629





