Phenotypic Physiological and Metabolomic Analyses Reveal Crucial Metabolic Pathways in Quinoa (Chenopodium quinoa Willd.) in Response to PEG-6000 Induced Drought Stress
Abstract
1. Introduction
2. Results
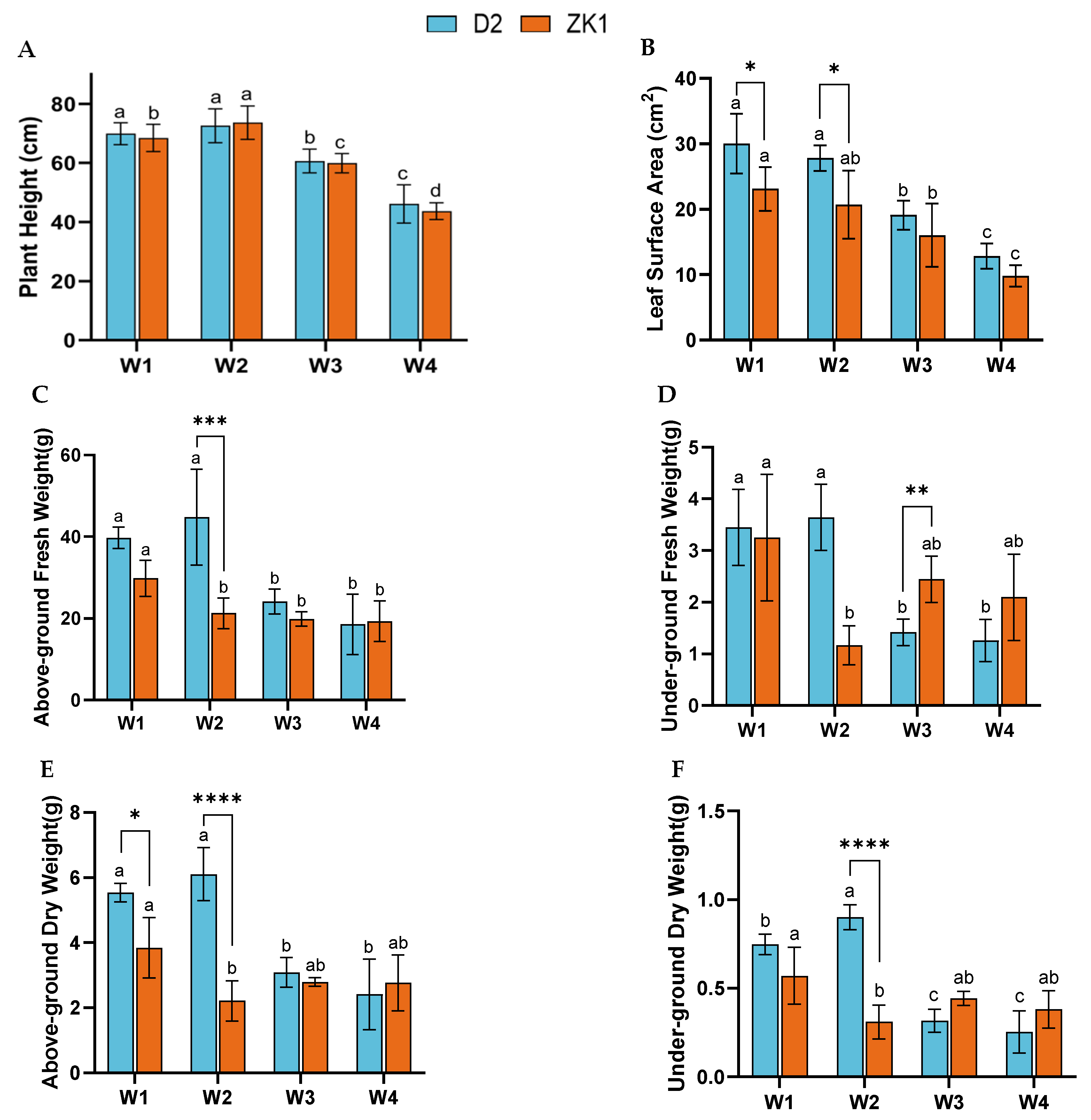
2.1. Effect of Drought Stress on Growth Characteristics
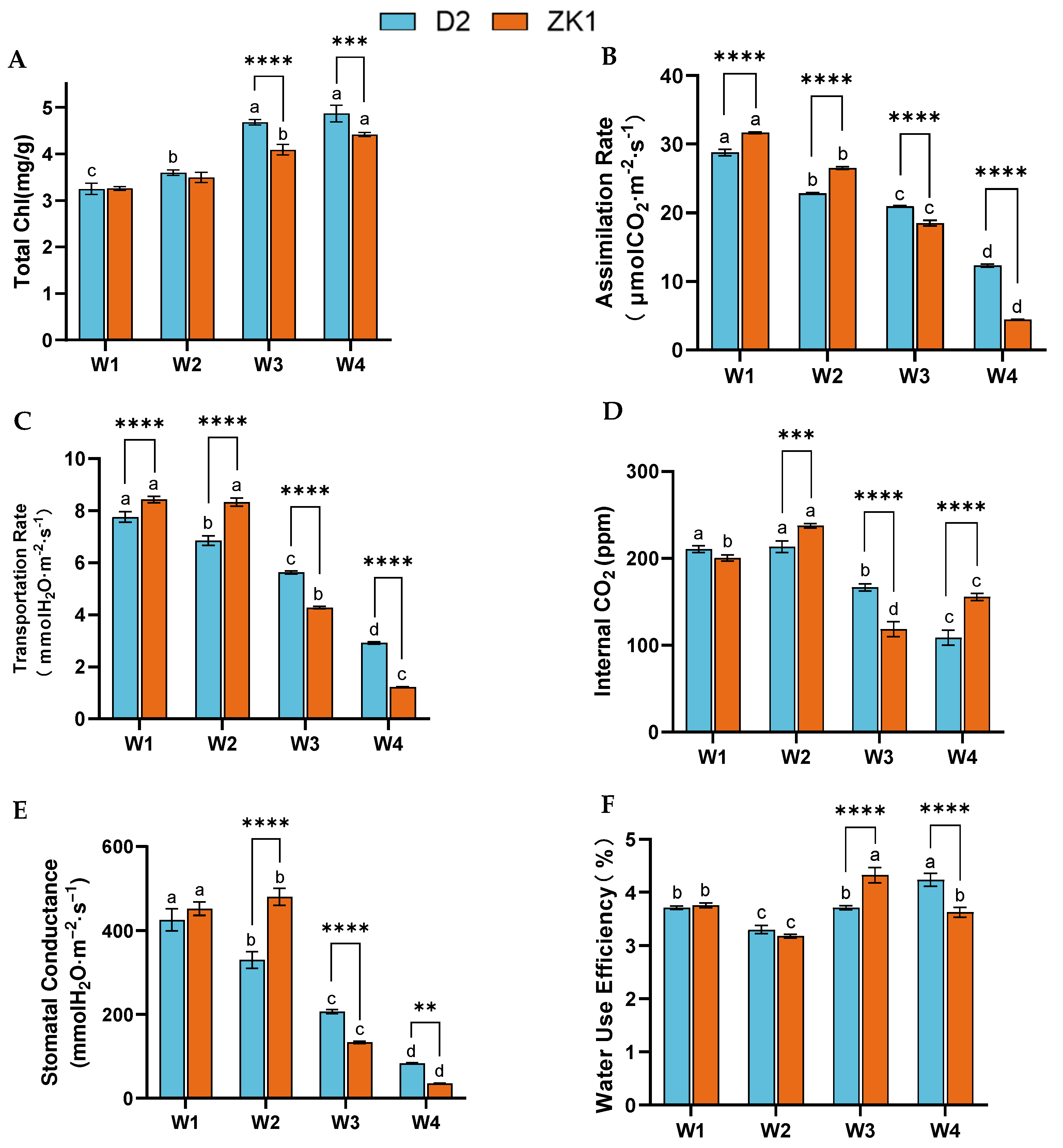
2.2. Photosynthetic Indexes Under Drought Stress
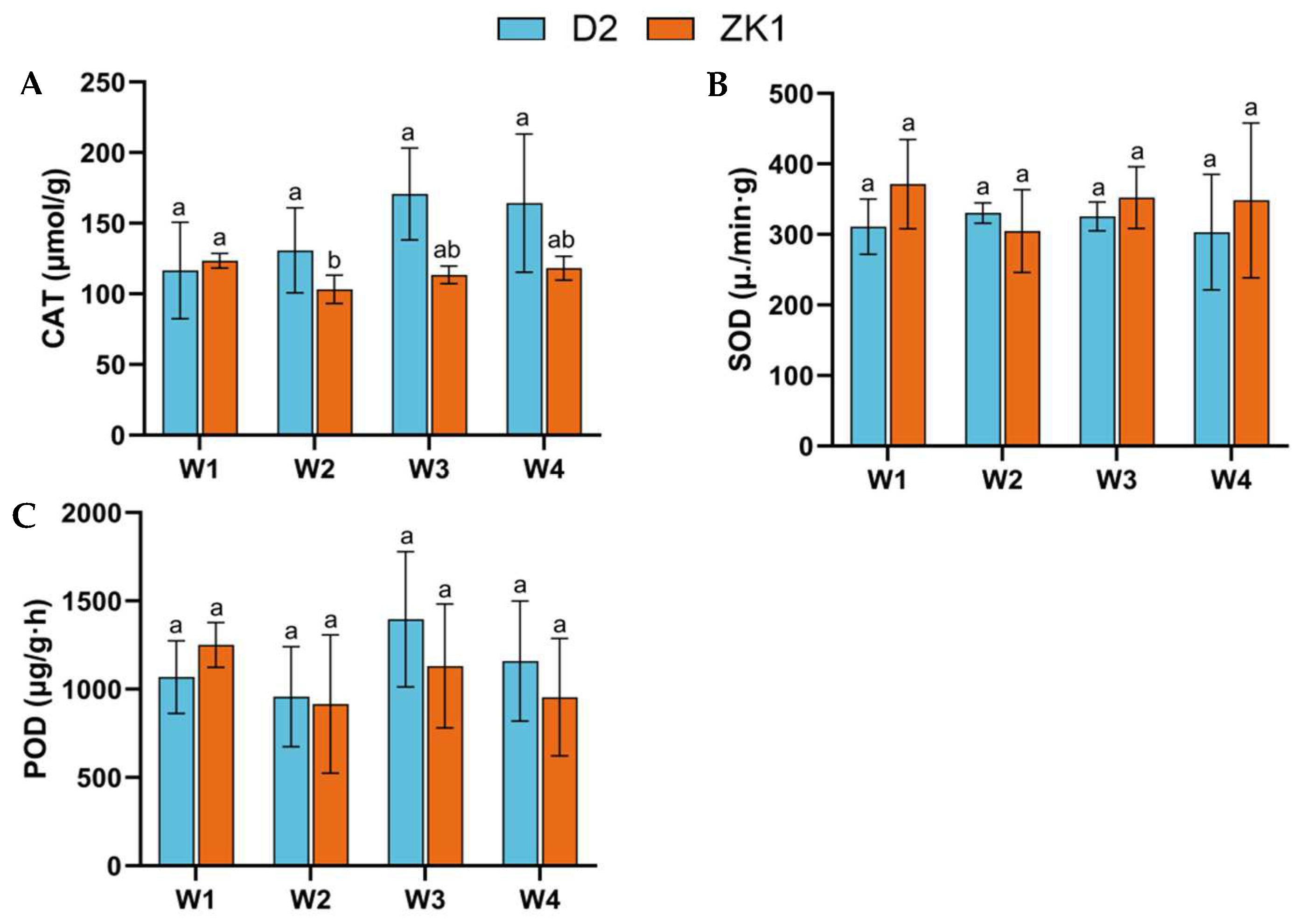
2.3. Antioxidant Enzyme Activity Under Drought Stress
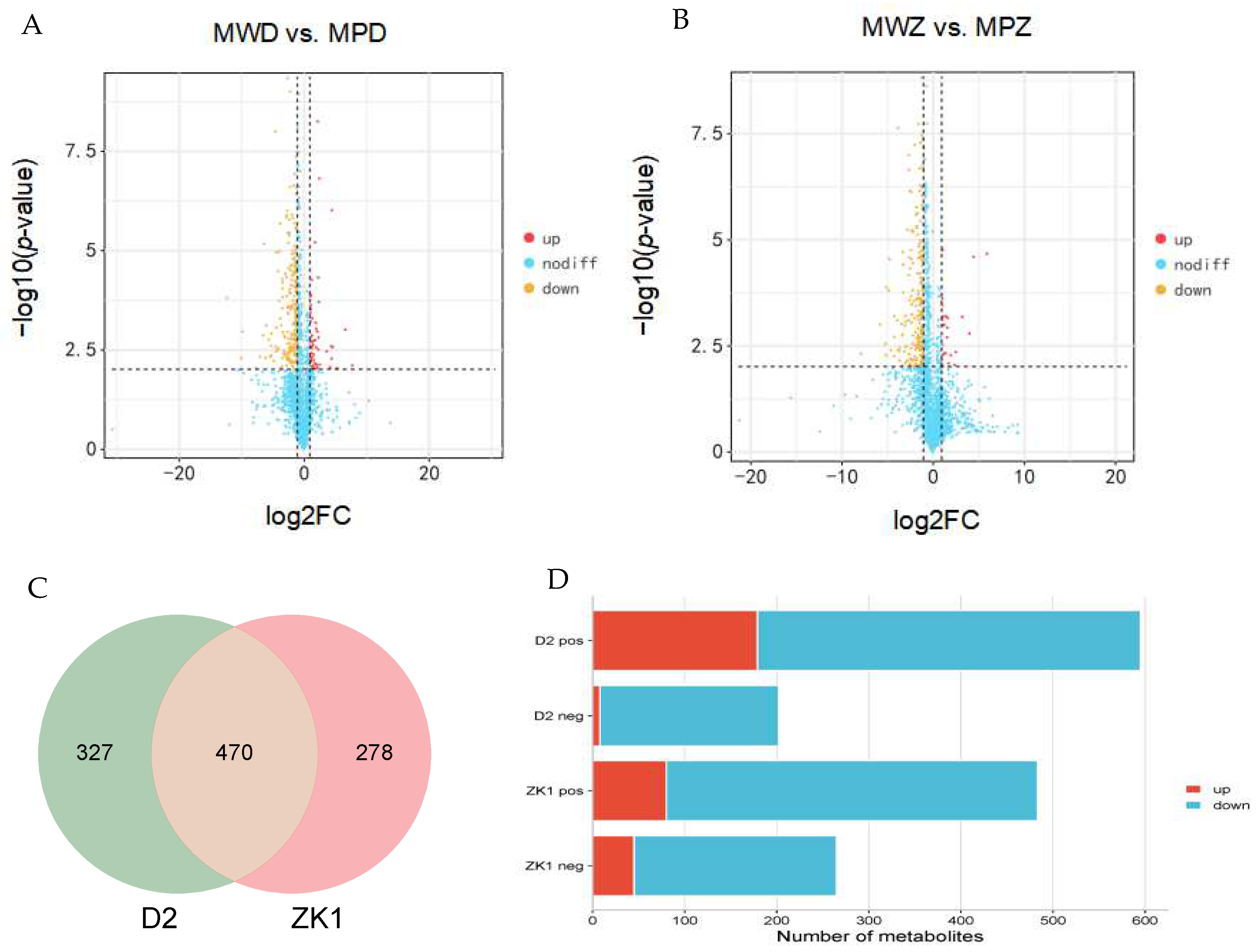
2.4. Standardization and Basic Analysis of Metabolomic Data
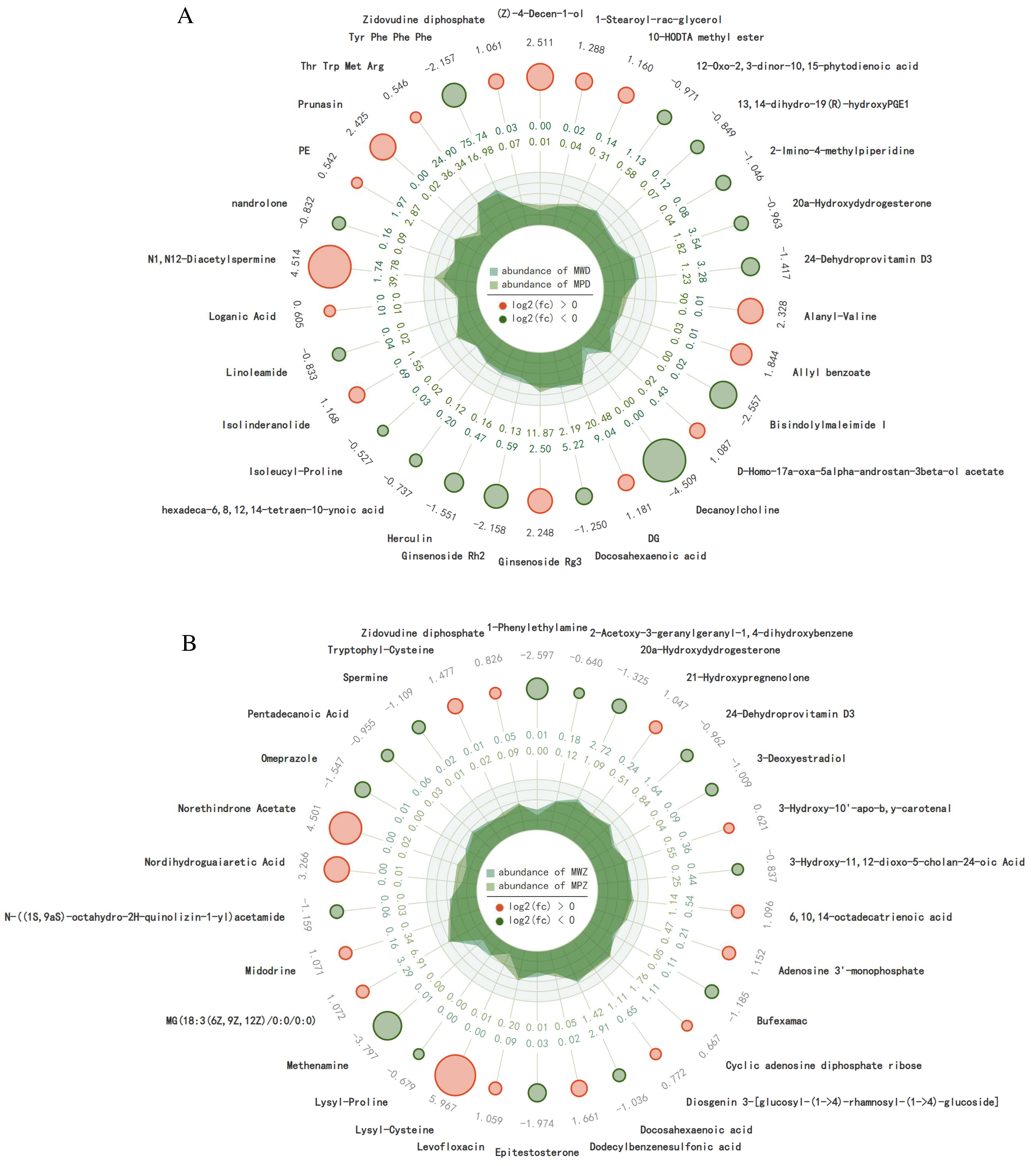
2.5. Differential Accumulation of Metabolites in Response to Drought Stress
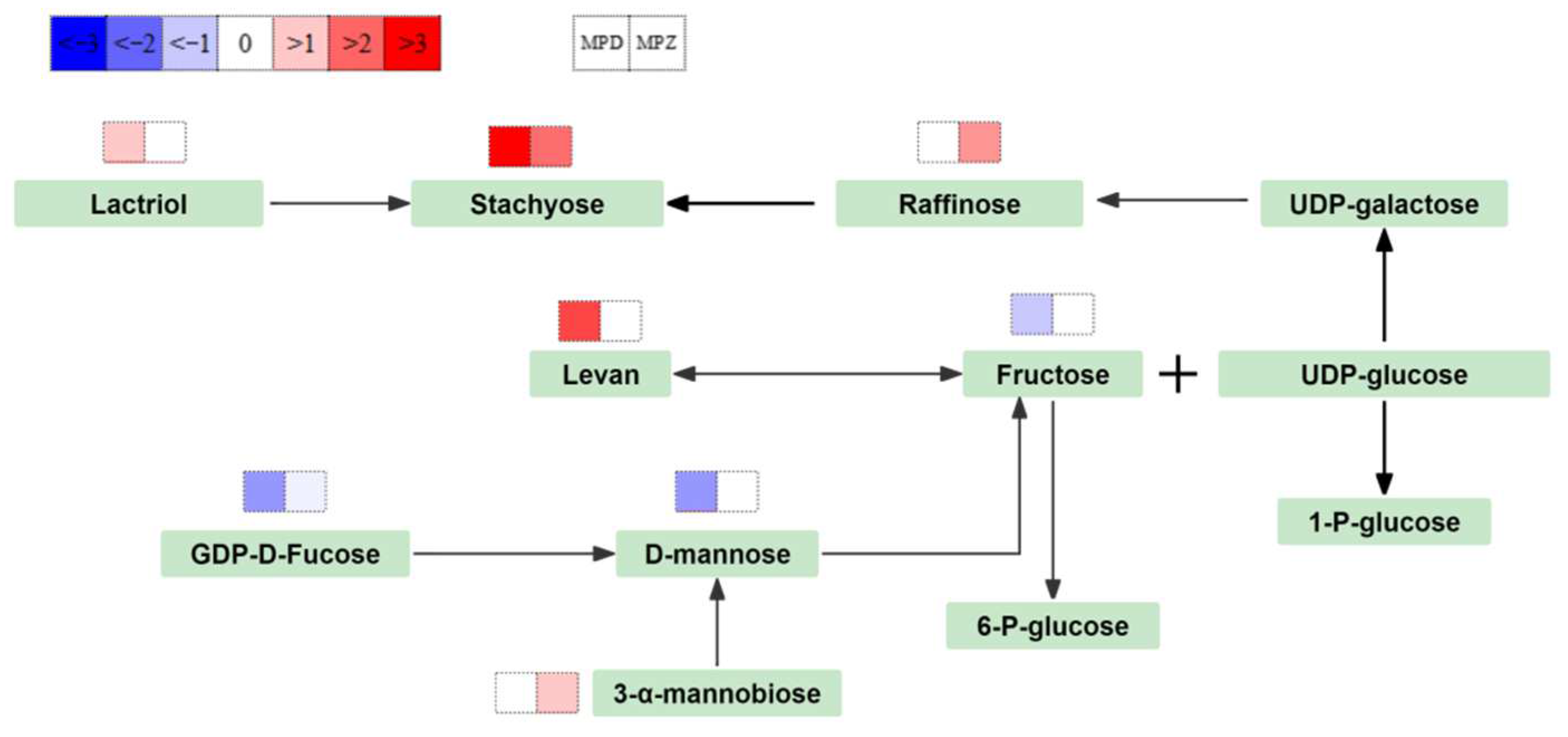
2.6. Annotation and Enrichment Analysis of Metabolites in the KEGG Database
3. Discussion
3.1. Phenotypes, Physiological, and Biochemical Indexes
3.2. Lipid Metabolism
3.3. Carbohydrate Metabolism
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Growth Analysis and Determination of Chlorophyll Pigments
4.2.1. Determination of Seedling Growth Indicators
4.2.2. Determination of Leaf Chlorophyll Content
4.3. Oxidative Stress Indicators and Proline Content Measurement
4.3.1. Determination of Leaf Photosynthetic Characteristic Indicators
4.3.2. Determination of Antioxidant Enzyme Activity
4.4. Metabolomic Analysis
4.4.1. Metabolite Extraction and Identification
4.4.2. LC-MS Data Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, T.; Feng, Y.; Liang, C.; Pan, L.; He, L.; Cai, Y. Metabolomics and transcriptomics analyses of two contrasting cherry rootstocks in response to drought stress. Biology 2021, 10, 201. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Shi, X. Hybrid sequencing reveals insight into heat sensing and signaling of bread wheat. Plant J. 2019, 19, 1015–1032. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Mao, J.; Xu, Y.; Wang, M.; Hu, Y.; Wang, S.; Liu, S.; Qiao, Z.; Cao, X. Metabolomics and physiological methods revealed the effects of drought stress on the quality of broomcorn millet during the flowering stage. Agronomy 2024, 14, 236. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.E. Biochar mitigates combined effects of drought and salinity stress in quinoa. Agronomy 2020, 10, 912. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar] [CrossRef]
- Li, Q.; Dong, B.; Qiao, Y.; Liu, M.; Zhang, J. Root growth, available soil water, and water-use efficiency of winter wheat under different irrigation regimes applied at different growth stages in North China. Agric. Water Manag. 2010, 97, 1676–1682. [Google Scholar] [CrossRef]
- Turner, N.C.; Wright, G.C.; Siddique, K.H. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 193–231. [Google Scholar] [CrossRef]
- Han, D.; Zhang, Z.; Ding, H.; Chai, L.; Liu, W.; Li, H.; Yang, G. Isolation and characterization of MbWRKY2 gene involved in enhanced drought tolerance in transgenic tobacco. J. Plant Interact. 2018, 13, 163–172. [Google Scholar] [CrossRef]
- Li, W.; Li, P.; Chen, H.; Zhong, J.; Liang, X.; Wei, Y.; Zhang, L.; Wang, H.; Han, D. Overexpression of a Fragaria vesca 1R-MYB transcription factor gene (FvMYB114) increases salt and cold tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 5261. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, Z.; Ding, H.; Wang, F.; Liu, W.; Li, H.; Yang, G. Molecular cloning and functional analysis of MbWRKY3 involved in improved drought tolerance in transformed tobacco. J. Plant Interact. 2018, 13, 329–337. [Google Scholar] [CrossRef]
- Yang, R.; Sun, Y.; Zhao, Y.; Bai, C.; Liu, Y.; Sun, J.; Wang, Z.; Yuan, F.; Wang, X.; Liu, W.; et al. Overexpression of MtNAC33 enhances biomass yield and drought tolerance in alfalfa. Plant Biotechnol. J. 2025, 1–3. [Google Scholar] [CrossRef]
- Alseekh, S.; Scossa, F.; Wen, W.; Luo, J.; Yan, J.; Beleggia, R.; Klee, H.J.; Huang, S.; Papa, R.; Fernie, A.R. Domestication of crop metabolomes: Desired and unintended consequences. Trends Plant Sci. 2021, 26, 650–661. [Google Scholar] [CrossRef]
- Adamski, J.; Suhre, K. Metabolomics platforms for genome wide association studies-linking the genome to the metabolome. Curr. Opin. Biotechnol. 2013, 24, 39–47. [Google Scholar] [CrossRef]
- Dawid, C.; Hille, K. Functional metabolomics—A useful tool to characterize stress-induced metabolome alterations opening new avenues towards tailoring food crop quality. Agronomy 2018, 8, 138. [Google Scholar] [CrossRef]
- Lanzinger, A.; Frank, T.; Reichenberger, G.; Herz, M.; Engel, K.H. Metabolite profiling of barley grain subjected to induced drought stress: Responses of free amino acids in differently adapted cultivars. J. Agric. Food Chem. 2015, 63, 4252–4261. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Wang, B.; Song, X.; Wang, X.; Wei, X. Non-targeted metabolomics analysis of metabolite changes in two quinoa genotypes under drought stress. BMC Plant Biol. 2023, 23, 503. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Cao, B.; Wei, X.; Shen, Z.; Su, N. Assessment and comparison of nutritional qualities of thirty quinoa (Chenopodium quinoa Willd.) seed varieties. Food Chem. X 2023, 24, 100808. [Google Scholar] [CrossRef]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty seeds: Nutrients, bioactives, bioavailability, and health benefits: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondiere, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on α-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd.). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Lorenzo Rodriguez, J.M.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Masoero, F.; Trevisan, M.; Lucini, L. Evaluation of phenolic profile and antioxidant capacity in gluten-free flours. Food Chem. 2017, 228, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Ul Haq, M.Z.; Ahmed, S.; Hirich, A.; Bazile, D. Challenges and perspectives for integrating quinoa into the agri-food system. Plants 2023, 12, 3361. [Google Scholar] [CrossRef]
- Aguilar, P.C.; Jacobsen, S.E. Cultivation of quinoa on the Peruvian altiplano. Food Rev. Int. 2003, 19, 31–41. [Google Scholar] [CrossRef]
- González, J.A.; Bruno, M.; Valoy, M.; Prado, F.E. Genotypic variation of gas exchange parameters and leaf stable carbon and nitrogen isotopes in ten quinoa cultivars grown under drought: Gas exchange in quinoa under drought. J. Agron. Crop Sci. 2011, 197, 81–93. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Vacher, J.J. Responses of two main Andean crops, quinoa (Chenopodium quinoa Willd.) and papa amarga (Solanum juzepczukii Buk.) to drought on the Bolivian Altiplano: Significance of local adaptation. Agric. Ecosyst. Environ. 1998, 68, 99–108. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Sci. Hortic. 2009, 122, 281–287. [Google Scholar] [CrossRef]
- Nickel, J.; Spanier, L.P.; Botelho, F.T.; Gularte, M.A.; Helbig, E. Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd. grains. Food Chem. 2016, 209, 139–143. [Google Scholar] [CrossRef]
- Cocozza, C.; Pulvento, C.; Lavini, A.; Riccardi, M.; d’Andria, R.; Tognetti, R. Effects of increasing salinity stress and decreasing water availability on ecophysiological traits of quinoa (Chenopodium quinoa Willd.) grown in a mediterranean-type agroecosystem. J. Agron. Crop Sci. 2013, 199, 229–240. [Google Scholar] [CrossRef]
- Razzaghi, F.; Ahmadi, S.H.; Adolf, V.I.; Jensen, C.R.; Jacobsen, S.E.; Andersen, M.N. Water relations and transpiration of quinoa (Chenopodium quinoa Willd.) under salinity and soil drying. J. Agron. Crop Sci. 2011, 197, 348–360. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; He, Z.; Ma, D.; Sun, W.; Xu, X.; Tian, Q. Effects of drought stress on leaf functional traits and biomass characteristics of Atriplex canescens. Plants 2024, 13, 2006. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant. 2018, 40, 25. [Google Scholar] [CrossRef]
- Ma, H.; Li, P.; Liu, X.; Li, C.; Zhang, S.; Wang, X.; Tao, X. Poly-γ-glutamic acid enhanced the drought resistance of maize by improving photosynthesis and affecting the rhizosphere microbial community. BMC Plant Biol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Lu, X.; Huang, M.; Mao, Y.; Li, C.; Yu, W.; Li, C. Carbon monoxide is involved in melatonin-enhanced drought resistance in tomato seedlings by enhancing chlorophyll synthesis pathway. BMC Plant Biol. 2024, 24, 97. [Google Scholar] [CrossRef]
- Narayan, J.A.; Chakravarthi, M.; Nerkar, G.; Manoj, V.M.; Dharshini, S.; Subramonian, N.; Premachandran, M.N.; Kumar, R.A.; Surendar, K.K.; Hemaprabha, G.; et al. Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind. Crop Prod. 2021, 159, 113035. [Google Scholar] [CrossRef]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; Fritschi, F.B. Genome-wide association mapping of soybean chlorophyll traits based on canopy spectral reflectance and leaf extracts. BMC Plant Biol. 2016, 16, 174. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and nondestructive evaluation of wheat chlorophyll under drought stress using hyperspectral imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Li, J.; Wei, S.; Yan, Y.; Yang, J.; Zhao, M.; Langdale, J.A.; Zhou, W. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Wang, C.; Song, H.; Bai, Y.; Zhang, H.; Hu, Z.; Wang, L.; Shi, K.; Zheng, X.; Yan, T.; et al. The MdMEK2–MdMPK6–MdWRKY17 pathway stabilizes chlorophyll levels by directly regulating MdSUFB in apple under drought stress. Plant J. 2021, 108, 814–828. [Google Scholar] [CrossRef]
- Hu, H.; He, B.; Ma, L.; Chen, X.; Han, P.; Luo, Y.; Liu, Y.; Fei, X.; Wei, A. Physiological and transcriptome analyses reveal the photosynthetic response to drought stress in drought-sensitive (Fengjiao) and drought-tolerant (Hanjiao) Zanthoxylum bungeanum cultivars. Front. Plant Sci. 2022, 13, 968714. [Google Scholar] [CrossRef]
- Liu, W.; Yang, F.; Xie, Z.; Wang, W.; Huang, J.; Wei, Y.; Yang, Z. Evaluation of drought tolerance and physiological response to drought stress of different varieties of quinoa seedlings. Agric. Res. Arid. Areas 2021, 39, 10–18. [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R. Influence of arbuscular mycorrhiza fungi and drought stress on fatty acids profile of sesame (Sesamum indicum L.). Field Crop. Res. 2021, 262, 108035. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, D.; Guo, C. Role of trienoic fatty acids in higher plants stress responses. Mol. Plant Breed. 2010, 8, 365–369. [Google Scholar]
- Asakura, H.; Yamakawa, T.; Tamura, T.; Ueda, R.; Taira, S.; Saito, Y.; Abe, K.; Asakura, T. Transcriptomic and metabolomic analyses provide insights into the upregulation of fatty acid and phospholipid metabolism in tomato fruit under drought stress. J. Agric. Food Chem. 2021, 69, 2894–2905. [Google Scholar] [CrossRef]
- Lu, S.; Fadlalla, T.; Tang, S.; Li, L.; Ali, U.; Li, Q.; Guo, L. Genome-wide analysis of phospholipase D gene family and profiling of phospholipids under abiotic stresses in brassica napus. Plant Cell Physiol. 2019, 60, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Woods, A.S.; Jackson, S.N. Brain tissue lipidomics: Direct probing using matrix-assisted laser desorption/ionization mass spectrometry. AAPS J. 2006, 8, E391–E395. [Google Scholar] [CrossRef] [PubMed]
- Haimi, P.; Uphoff, A.; Hermansson, M.; Somerharju, P. Software tools for analysis of mass spectrometric lipidome data. Anal. Chem. 2006, 78, 8324–8331. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Uddin, M.; Tanaka, K.; Yin, L.; Shi, Z.; Qi, Y.; Mano, J.; Matsui, K.; Shimomura, N.; Sakaki, T.; et al. Maintenance of chloroplast structure and function by overexpression of the rice MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE gene leads to enhanced salt tolerance in tobacco. Plant Physiol. 2014, 165, 1144–1155. [Google Scholar] [CrossRef]
- Wang, M. Lipidomics Under Salt Stress in Oryza. sativa L. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2020. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Khan, M.; Wang, Y.; Xiao, W.; Fang, T.; Qu, J.; Xiao, P.; Li, C.; Liu, J. Transcription factors ABF4 and ABR1 synergistically regulate amylase-mediated starch catabolism in drought tolerance. Plant Physiol. 2022, 191, 591–609. [Google Scholar] [CrossRef]
- Hashida, S.; Kawai-Yamada, M. Inter-organelle NAD metabolism underpinning light responsive NADP dynamics in plants. Front. Plant Sci. 2019, 10, 960. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate valves: Old shuttles with new perspectives. Plant Biol. 2019, 21, 21–30. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Shi, H.; Yao, J.; Liu, X.; Wang, F.; Zeng, L.; Xie, Z.; Zhu, J.K. Reciprocal regulation between nicotinamide adenine dinucleotide metabolism and abscisic acid and stress response pathways in Arabidopsis. PLoS Genet. 2020, 16, e1008892. [Google Scholar] [CrossRef]
- Ullah, S.; Kolo, Z.; Egbichi, I.; Keyster, M.; Ludidi, N. Nitric oxide influences glycine betaine content and ascorbate peroxidase activity in maize. S. Afr. J. Bot. 2016, 105, 218–225. [Google Scholar] [CrossRef]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-{1-[(Phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine–xanthine oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic “Shamouti” orange ovular callus lines1. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]


| Plant Material | Treatment | LC-MS Samples |
|---|---|---|
| D2 | Water | MWD |
| Drought | MPD | |
| ZK1 | Water | MWZ |
| Drought | MPZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Q.; Wu, Y.; Du, H.; Wang, Y.; Zhang, Y. Phenotypic Physiological and Metabolomic Analyses Reveal Crucial Metabolic Pathways in Quinoa (Chenopodium quinoa Willd.) in Response to PEG-6000 Induced Drought Stress. Int. J. Mol. Sci. 2025, 26, 2599. https://doi.org/10.3390/ijms26062599
Bao Q, Wu Y, Du H, Wang Y, Zhang Y. Phenotypic Physiological and Metabolomic Analyses Reveal Crucial Metabolic Pathways in Quinoa (Chenopodium quinoa Willd.) in Response to PEG-6000 Induced Drought Stress. International Journal of Molecular Sciences. 2025; 26(6):2599. https://doi.org/10.3390/ijms26062599
Chicago/Turabian StyleBao, Qinghan, Yang Wu, Huishi Du, Yang Wang, and Yongping Zhang. 2025. "Phenotypic Physiological and Metabolomic Analyses Reveal Crucial Metabolic Pathways in Quinoa (Chenopodium quinoa Willd.) in Response to PEG-6000 Induced Drought Stress" International Journal of Molecular Sciences 26, no. 6: 2599. https://doi.org/10.3390/ijms26062599
APA StyleBao, Q., Wu, Y., Du, H., Wang, Y., & Zhang, Y. (2025). Phenotypic Physiological and Metabolomic Analyses Reveal Crucial Metabolic Pathways in Quinoa (Chenopodium quinoa Willd.) in Response to PEG-6000 Induced Drought Stress. International Journal of Molecular Sciences, 26(6), 2599. https://doi.org/10.3390/ijms26062599






