Transcriptomic Alterations Induced by Tetrahydrocannabinol in SIV/HIV Infection: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
3. Results
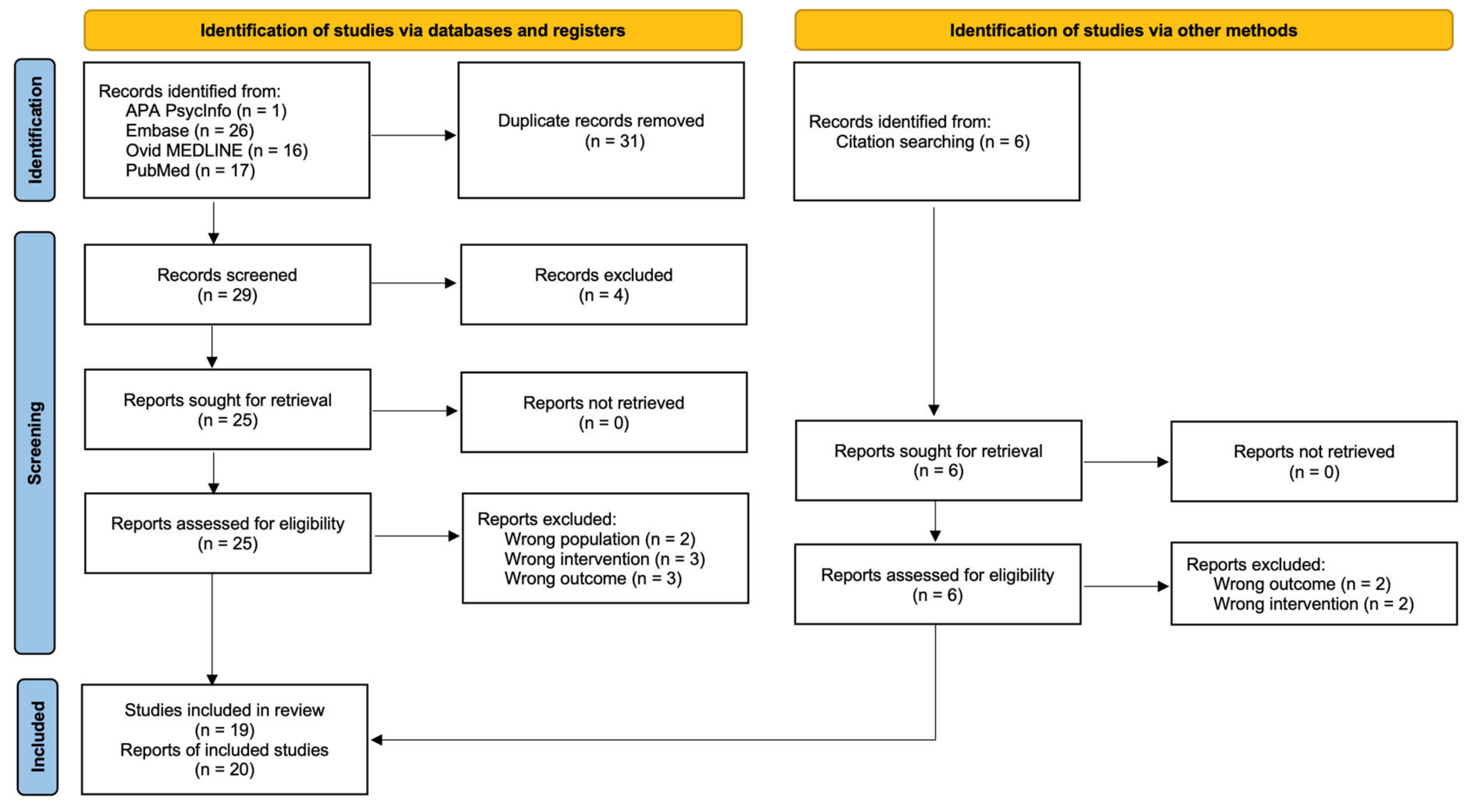
3.1. Study Selection
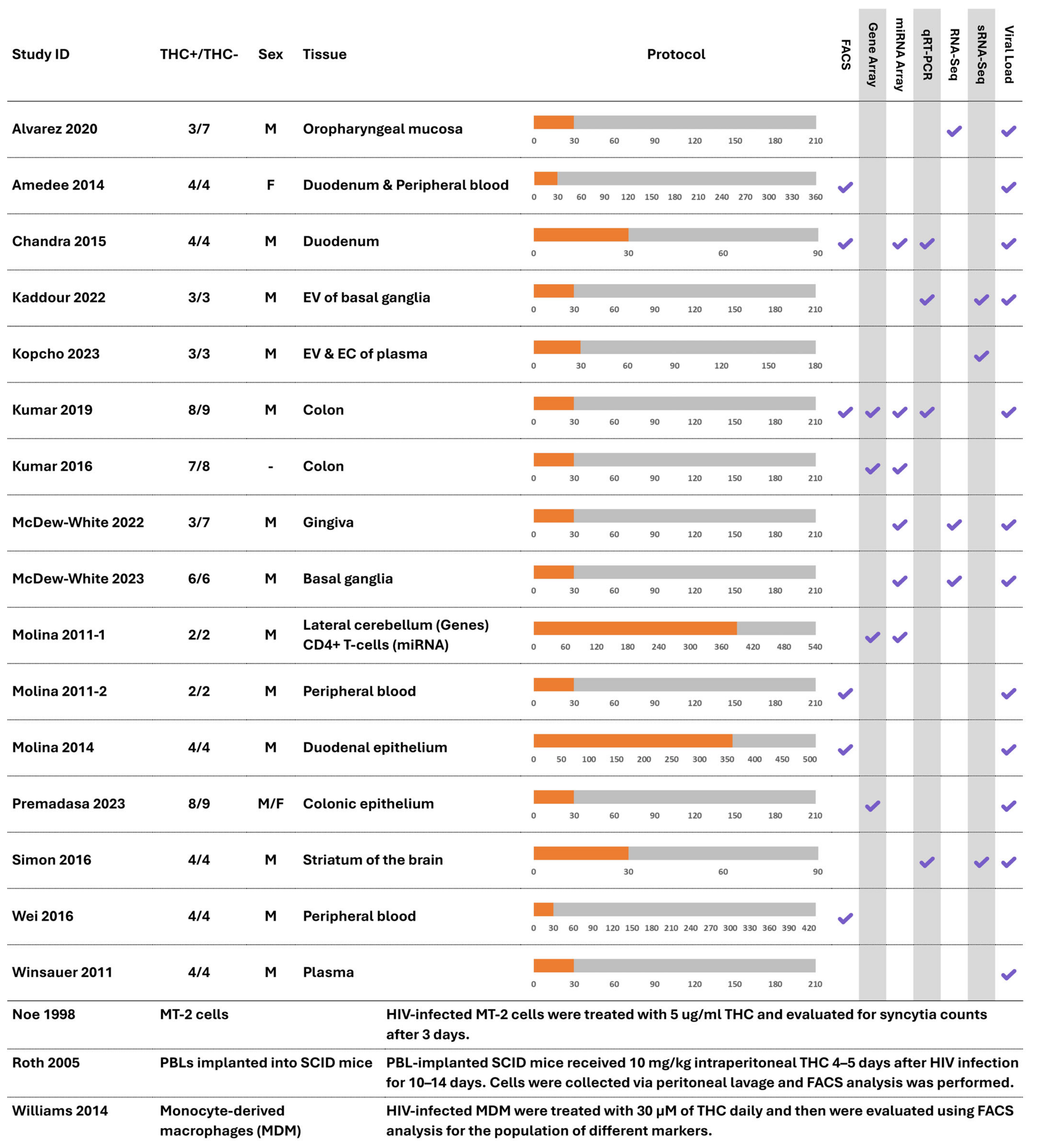
3.2. Study Characteristics
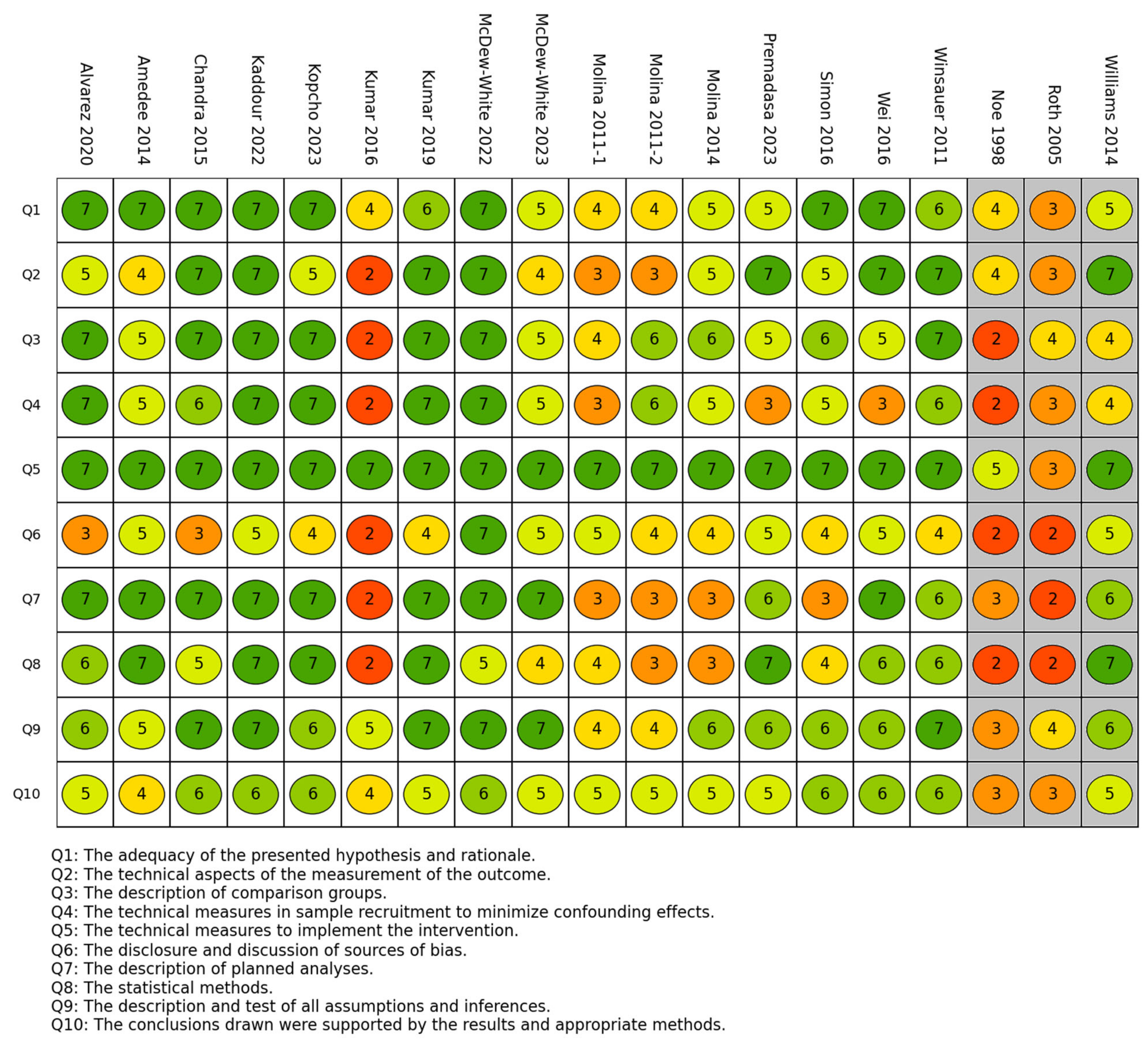
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies and Syntheses
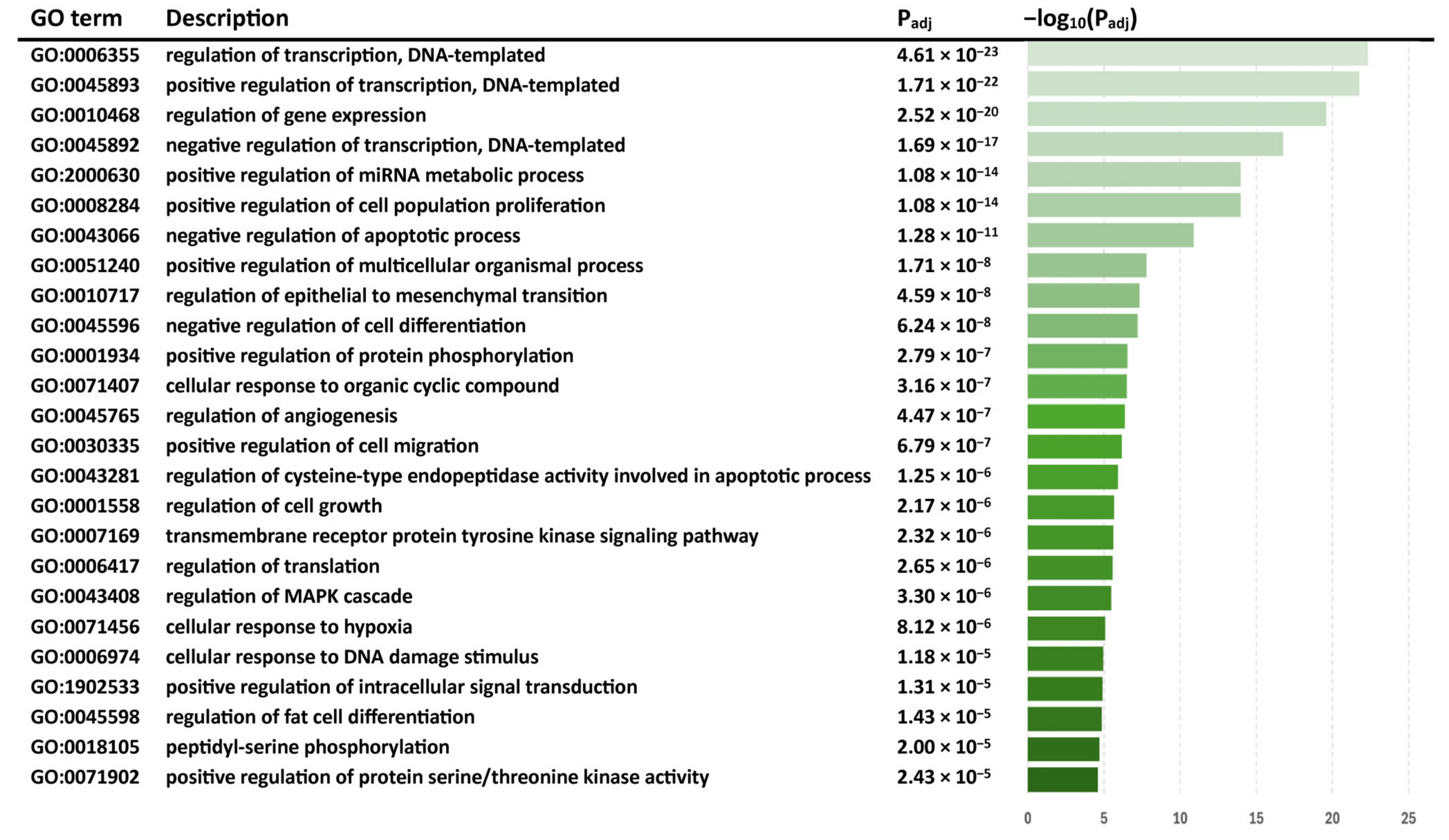
3.4.1. THC Alters Gene Expression in SIV-Infected Macaques
3.4.2. THC Alters miRNA Expression in SIV-Infected Macaques
3.4.3. The Impact of THC on Immune Cell Markers and Viral Load in SIV
3.4.4. Studies on HIV-Infected Human Cells
4. Discussion
4.1. Interpretation of Results
4.2. Limitations in the Evidence
4.3. Limitations in Review Processes
4.4. Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geneva Joint United Nations Programme on HIV AIDS. UNAIDS Data 2021; UNAIDS: Geneva, Switzerland, 2021. [Google Scholar]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Klatt, N.R.; Silvestri, G.; Hirsch, V. Nonpathogenic simian immunodeficiency virus infections. Cold Spring Harb. Perspect. Med. 2012, 2, a007153. [Google Scholar] [CrossRef]
- Manches, O.; Bhardwaj, N. Resolution of immune activation defines nonpathogenic SIV infection. J. Clin. Investig. 2009, 119, 3512–3515. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Paiardini, M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood 2011, 118, 847–854. [Google Scholar] [CrossRef]
- Pandrea, I.; Apetrei, C. Where the wild things are: Pathogenesis of SIV infection in African nonhuman primate hosts. Curr. HIV/AIDS Rep. 2010, 7, 28–36. [Google Scholar] [CrossRef]
- Watson, C.W.; Campbell, L.M.; Sun-Suslow, N.; Hong, S.; Umlauf, A.; Ellis, R.J.; Iudicello, J.E.; Letendre, S.; Marcotte, T.D.; Heaton, R.K.; et al. Daily Cannabis Use is Associated With Lower CNS Inflammation in People With HIV. J. Int. Neuropsychol. Soc. 2021, 27, 661–672. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration (SAMHSA). National Survey on Drug Use and Health (NSDUH) 2022; Substance Abuse and Mental Health Services Administration (SAMHSA): North Bethesda, MD, USA, 2022.
- Benuvia, T. Syndros (Dronabinol). U.S. Food and Drug Administration. 1985; Revised on May 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205525s003lbl.pdf (accessed on 20 February 2025).
- Unimed Pharmaceuticals. Marinol (Dronabinol). U.S. Food and Drug Administration. 1985; Revised on August 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 20 February 2025).
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Basu, S.; Maresz, K.; Bifulco, M.; Dittel, B.N. What we know and do not know about the cannabinoid receptor 2 (CB2). Semin. Immunol. 2014, 26, 369–379. [Google Scholar] [CrossRef]
- Eisenstein, T.K.; Meissler, J.J.; Wilson, Q.; Gaughan, J.P.; Adler, M.W. Anandamide and Δ9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J. Neuroimmunol. 2007, 189, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.D.; Baldwin, G.C.; Tashkin, D.P. Effects of delta-9-tetrahydrocannabinol on human immune function and host defense. Chem. Phys. Lipids 2002, 121, 229–239. [Google Scholar] [CrossRef]
- Srivastava, M.D.; Srivastava, B.I.; Brouhard, B. Δ9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 1998, 40, 179–185. [Google Scholar] [CrossRef]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Δ9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.F.K.; Miranda, K.; Wilson, K.; Singh, N.P.; Cai, G.; Putluri, N.; Nagarkatti, P.; Nagarkatti, M. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int. J. Mol. Sci. 2020, 21, 6244. [Google Scholar] [CrossRef]
- Lyman, W.; Sonett, J.; Brosnan, C.; Elkin, R.; Bornstein, M. Δ9-Tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1989, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Kok, C.; Smutny, S.; Pongratz, G. Impact of Δ9-Tetrahydrocannabinol on Rheumatoid Arthritis Synovial Fibroblasts Alone and in Co-Culture with Peripheral Blood Mononuclear Cells. Biomedicines 2022, 10, 1118. [Google Scholar] [CrossRef]
- Bhatt, H.K.; Song, D.; Musgrave, G.; Rao, P.S.S. Cannabinoid-induced changes in the immune system: The role of microRNAs. Int. Immunopharmacol. 2021, 98, 107832. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanus, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.; Katz, I.; Porat-Katz, B.S.; Shoenfeld, Y. Medical cannabis: Another piece in the mosaic of autoimmunity? Clin. Pharmacol. Ther. 2017, 101, 230–238. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Jenabian, M.A. Cannabinoids and inflammation: Implications for people living with HIV. AIDS 2019, 33, 2273–2288. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect. Dis. 2010, 10, 226. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.M.; Lifson, J.D. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv. Pharmacol. 2000, 49, 437–477. [Google Scholar]
- Sohani, Z.N.; Meyre, D.; de Souza, R.J.; Joseph, P.G.; Gandhi, M.; Dennis, B.B.; Norman, G.; Anand, S.S. Assessing the quality of published genetic association studies in meta-analyses: The quality of genetic studies (Q-Genie) tool. BMC Genet. 2015, 16, 50. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Ganie, S.A.; Debnath, A.B.; Gumi, A.M.; Mondal, T.K. Comprehensive survey and evolutionary analysis of genome-wide miRNA genes from ten diploid Oryza species. BMC Genom. 2017, 18, 711. [Google Scholar] [CrossRef]
- Sadanandam, A.; Wullschleger, S.; Lyssiotis, C.A.; Grotzinger, C.; Barbi, S.; Bersani, S.; Korner, J.; Wafy, I.; Mafficini, A.; Lawlor, R.T.; et al. A Cross-Species Analysis in Pancreatic Neuroendocrine Tumors Reveals Molecular Subtypes with Distinctive Clinical, Metastatic, Developmental, and Metabolic Characteristics. Cancer Discov. 2015, 5, 1296–1313. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Bosnjak, M.; Skunca, N.; Smuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Alvarez, X.; Sestak, K.; Byrareddy, S.N.; Mohan, M. Long Term Delta-9-tetrahydrocannabinol Administration Inhibits Proinflammatory Responses in Minor Salivary Glands of Chronically Simian Immunodeficieny Virus Infected Rhesus Macaques. Viruses 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Amedee, A.M.; Nichols, W.A.; LeCapitaine, N.J.; Stouwe, C.V.; Birke, L.L.; Lacour, N.; Winsauer, P.J.; Molina, P.E. Chronic Δ9-tetrahydrocannabinol administration may not attenuate simian immunodeficiency virus disease progression in female rhesus macaques. AIDS Res. Hum. Retroviruses 2014, 30, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Chandra, L.C.; Kumar, V.; Torben, W.; Vande Stouwe, C.; Winsauer, P.; Amedee, A.; Molina, P.E.; Mohan, M. Chronic administration of Δ9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J. Virol. 2015, 89, 1168–1181. [Google Scholar] [CrossRef]
- Kaddour, H.; McDew-White, M.; Madeira, M.M.; Tranquille, M.A.; Tsirka, S.E.; Mohan, M.; Okeoma, C.M. Chronic delta-9-tetrahydrocannabinol (THC) treatment counteracts SIV-induced modulation of proinflammatory microRNA cargo in basal ganglia-derived extracellular vesicles. J. Neuroinflamm. 2022, 19, 225. [Google Scholar] [CrossRef]
- Kopcho, S.; McDew-White, M.; Naushad, W.; Mohan, M.; Okeoma, C.M. Alterations in Abundance and Compartmentalization of miRNAs in Blood Plasma Extracellular Vesicles and Extracellular Condensates during HIV/SIV Infection and Its Modulation by Antiretroviral Therapy (ART) and Delta-9-Tetrahydrocannabinol (Δ9-THC). Viruses 2023, 15, 623. [Google Scholar] [CrossRef]
- Kumar, V.; Torben, W.; Stouwe, C.V.; Molina, P.E.; Mohan, M. delta-9-tetrahydrocannabinol mediated suppression of intestinal inflammation in chronic SIV-infected rhesus macaques involves collective targeting of matrix metalloproteinase-8 (MMP8) by miR-181a, miR-193b and miR-374-5p. J. Med. Primatol. 2016, 45, 253. [Google Scholar]
- Kumar, V.; Torben, W.; Mansfield, J.; Alvarez, X.; Vande Stouwe, C.; Li, J.; Byrareddy, S.N.; Didier, P.J.; Pahar, B.; Molina, P.E.; et al. Cannabinoid Attenuation of Intestinal Inflammation in Chronic SIV-Infected Rhesus Macaques Involves T Cell Modulation and Differential Expression of Micro-RNAs and Pro-inflammatory Genes. Front. Immunol. 2019, 10, 914. [Google Scholar] [CrossRef]
- McDew-White, M.; Lee, E.; Alvarez, X.; Sestak, K.; Ling, B.J.; Byrareddy, S.N.; Okeoma, C.M.; Mohan, M. Cannabinoid control of gingival immune activation in chronically SIV-infected rhesus macaques involves modulation of the indoleamine-2,3-dioxygenase-1 pathway and salivary microbiome. eBioMedicine 2022, 75, 103769. [Google Scholar] [CrossRef]
- McDew-White, M.; Lee, E.; Premadasa, L.S.; Alvarez, X.; Okeoma, C.M.; Mohan, M. Cannabinoids modulate the microbiota-gut-brain axis in HIV/SIV infection by reducing neuroinflammation and dysbiosis while concurrently elevating endocannabinoid and indole-3-propionate levels. J. Neuroinflamm. 2023, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Amedee, A.; LeCapitaine, N.J.; Zabaleta, J.; Mohan, M.; Winsauer, P.; Vande Stouwe, C. Cannabinoid neuroimmune modulation of SIV disease. J. Neuroimmune Pharmacol. 2011, 6, 516–527. [Google Scholar] [CrossRef]
- Molina, P.E.; Winsauer, P.; Zhang, P.; Walker, E.; Birke, L.; Amedee, A.; Stouwe, C.V.; Troxclair, D.; McGoey, R.; Varner, K.; et al. Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS Res. Hum. Retroviruses 2011, 27, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Amedee, A.M.; LeCapitaine, N.J.; Zabaleta, J.; Mohan, M.; Winsauer, P.J.; Vande Stouwe, C.; McGoey, R.R.; Auten, M.W.; LaMotte, L.; et al. Modulation of gut-specific mechanisms by chronic Δ9-tetrahydrocannabinol administration in male rhesus macaques infected with simian immunodeficiency virus: A systems biology analysis. AIDS Res. Hum. Retroviruses 2014, 30, 567–578. [Google Scholar] [PubMed]
- Premadasa, L.S.; Lee, E.; McDew-White, M.; Alvarez, X.; Jayakumar, S.; Ling, B.; Okeoma, C.M.; Byrareddy, S.N.; Kulkarni, S.; Mohan, M. Cannabinoid enhancement of lncRNA MMP25-AS1/MMP25 interaction reduces neutrophil infiltration and intestinal epithelial injury in HIV/SIV infection. JCI Insight 2023, 8, e167903. [Google Scholar] [CrossRef]
- Premadasa, L.S.; McDew-White, M.; Romero, L.; Gondo, B.; Drawec, J.A.; Ling, B.; Okeoma, C.M.; Mohan, M. Epigenetic modulation of NLRP6 inflammasome sensor as a therapeutic modality to reduce necroptosis-driven gastrointestinal mucosal dysfunction in HIV/SIV infection. bioRxiv 2024, 18, 18. [Google Scholar]
- Simon, L.; Song, K.; Vande Stouwe, C.; Hollenbach, A.; Amedee, A.; Mohan, M.; Winsauer, P.; Molina, P. Delta9-Tetrahydrocannabinol (Δ9-THC) Promotes Neuroimmune-Modulatory MicroRNA Profile in Striatum of Simian Immunodeficiency Virus (SIV)-Infected Macaques. J. Neuroimmune Pharmacol. 2016, 11, 192–213. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, L.; Cong, Z.; Wu, X.; Wang, H.; Qin, C.; Molina, P.; Chen, Z. Chronic Δ9-Tetrahydrocannabinol Administration Reduces IgE+B Cells but Unlikely Enhances Pathogenic SIVmac251 Infection in Male Rhesus Macaques of Chinese Origin. J. Neuroimmune Pharmacol. 2016, 11, 584–591. [Google Scholar] [CrossRef]
- Winsauer, P.J.; Molina, P.E.; Amedee, A.M.; Filipeanu, C.M.; McGoey, R.R.; Troxclair, D.A.; Walker, E.M.; Birke, L.L.; Stouwe, C.V.; Howard, J.M.; et al. Tolerance to chronic delta-9-tetrahydrocannabinol (Δ9-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp. Clin. Psychopharmacol. 2011, 19, 154–172. [Google Scholar] [CrossRef]
- Noe, S.N.; Nyland, S.B.; Ugen, K.; Friedman, H.; Klein, T.W. Cannabinoid receptor agonists enhance syncytia formation in MT-2 cells infected with cell free HIV-1MN. Adv. Exp. Med. Biol. 1998, 437, 223–229. [Google Scholar]
- Roth, M.D.; Tashkin, D.P.; Whittaker, K.M.; Choi, R.; Baldwin, G.C. Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci. 2005, 77, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Appelberg, S.; Goldberger, B.A.; Klein, T.W.; Sleasman, J.W.; Goodenow, M.M. Δ9-Tetrahydrocannabinol treatment during human monocyte differentiation reduces macrophage susceptibility to HIV-1 infection. J. Neuroimmune Pharmacol. 2014, 9, 369–379. [Google Scholar] [CrossRef]
- Anderson, M.G.; Clements, J.E. Two strains of SIVmac show differential transactivation mediated by sequences in the promoter. Virology 1992, 191, 559–568. [Google Scholar] [CrossRef]
- Daniel, M.D.; Letvin, N.L.; King, N.W.; Kannagi, M.; Sehgal, P.K.; Hunt, R.D.; Kanki, P.J.; Essex, M.; Desrosiers, R.C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 1985, 228, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Lance, A.; Mani, R.; Seegers, S.L.; Avalos, B.R.; Druhan, L.J. CSF3R Splicing Regulates Granulopoiesis Via Splice Variant Specific Responses to G-CSF. Blood 2021, 138, 3130. [Google Scholar] [CrossRef]
- Ramasundara, M.; Leach, S.T.; Lemberg, D.A.; Day, A.S. Defensins and inflammation: The role of defensins in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009, 24, 202–208. [Google Scholar] [CrossRef]
- Bayani, J.; Diamandis, E.P. The physiology and pathobiology of human kallikrein-related peptidase 6 (KLK6). Clin. Chem. Lab. Med. 2012, 50, 211–233. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Tamiya, T.; Kashiwagi, I.; Takahashi, R.; Yasukawa, H.; Yoshimura, A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: Regulation of T-cell inflammation by SOCS1 and SOCS3. Arter. Thromb. Vasc. Biol. 2011, 31, 980–985. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Carow, B.; Rottenberg, M.E. SOCS3, a Major Regulator of Infection and Inflammation. Front. Immunol. 2014, 5, 58. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Gao, Y.; Qi, D.; Zhao, L.; Zhao, L.; Liu, C.; Tao, T.; Zhou, C.; Sun, X.; et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Neill, D.R.; Malak, H.A.; Spelmink, L.; Khandaker, S.; Dalla Libera Marchiori, G.; Dearing, E.; Kirby, A.; Yang, M.; Achour, A.; et al. Pneumolysin binds to the mannose receptor C type 1 (MRC-1) leading to anti-inflammatory responses and enhanced pneumococcal survival. Nat. Microbiol. 2019, 4, 62–70. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bertaggia, E.; Scabia, G.; Dalise, S.; Lo Verso, F.; Santini, F.; Vitti, P.; Chisari, C.; Sandri, M.; Maffei, M. Haptoglobin is required to prevent oxidative stress and muscle atrophy. PLoS ONE 2014, 9, e100745. [Google Scholar] [CrossRef]
- Horikawa, S.; Ishii, Y.; Hamashima, T.; Yamamoto, S.; Mori, H.; Fujimori, T.; Shen, J.; Inoue, R.; Nishizono, H.; Itoh, H.; et al. PDGFRalpha plays a crucial role in connective tissue remodeling. Sci. Rep. 2015, 5, 17948. [Google Scholar] [CrossRef]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Choi Kyung, H.; Shin Chang, H.; Lee Woo, J.; Ji, H.; Kim Hyeon, H. Dual-strand tumor suppressor miR-193b-3p and -5p inhibit malignant phenotypes of lung cancer by suppressing their common targets. Biosci. Rep. 2019, 39, BSR20190634. [Google Scholar]
- Waseem, M.; Gujrati, H.; Wang, B.-D. Tumor suppressive miR-99b-5p as an epigenomic regulator mediating mTOR/AR/SMARCD1 signaling axis in aggressive prostate cancer. Front. Oncol. 2023, 13, 1184186. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Barbera Betancourt, A.; Lyons, P.A.; Zhang, Z.; Suo, C.; Lee, J.C.; McKinney, E.F.; Modis, L.K.; Ellson, C.; Smith, K.G.C. miR-374a-5p regulates inflammatory genes and monocyte function in patients with inflammatory bowel disease. J. Exp. Med. 2022, 219, e20211366. [Google Scholar] [CrossRef]
- Devara, D.; Choudhary, Y.; Kumar, S. Role of MicroRNA-502-3p in Human Diseases. Pharmaceuticals 2023, 16, 532. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back. to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef]
- Yoo, A.S.; Staahl, B.T.; Chen, L.; Crabtree, G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 2009, 460, 642–646. [Google Scholar] [CrossRef]
- De Filippo, K.; Rankin, S.M. CXCR 4, the master regulator of neutrophil trafficking in homeostasis and disease. Eur. J. Clin. Investig. 2018, 48, e12949. [Google Scholar] [CrossRef]
- Bagri, A.; Gurney, T.; He, X.; Zou, Y.R.; Littman, D.R.; Tessier-Lavigne, M.; Pleasure, S.J. The chemokine SDF1 regulates migration of dentate granule cells. Development 2002, 129, 4249–4260. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, R.; Haigwood, N.L.; Persidsky, Y.; Ho, W.Z. SIV infection of rhesus macaques of Chinese origin: A suitable model for HIV infection in humans. Retrovirology 2013, 10, 89. [Google Scholar] [CrossRef]
- Trichel, A.M.; Rajakumar, P.A.; Murphey-Corb, M. Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J. Med. Primatol. 2002, 31, 171–178. [Google Scholar] [CrossRef]
- Miyoshi, I.; Kubonishi, I.; Yoshimoto, S.; Akagi, T.; Ohtsuki, Y.; Shiraishi, Y.; Nagata, K.; Hinuma, Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 1981, 294, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Carnahan, J.; Greenberg, M.E. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994, 263, 1618–1623. [Google Scholar] [CrossRef]
- Lipsky, R.H.; Marini, A.M. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann. N. Y. Acad. Sci. 2007, 1122, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Raborn, E.S.; Jamerson, M.; Marciano-Cabral, F.; Cabral, G.A. Cannabinoid inhibits HIV-1 Tat-stimulated adhesion of human monocyte-like cells to extracellular matrix proteins. Life Sci. 2014, 104, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Henriquez, J.E.; Rizzo, M.D.; Schulz, M.A.; Crawford, R.B.; Gulick, P.; Kaminski, N.E. Δ9-Tetrahydrocannabinol Suppresses Secretion of IFNalpha by Plasmacytoid Dendritic Cells From Healthy and HIV-Infected Individuals. J. Acquir. Immune Defic. Syndr. 2017, 75, 588–596. [Google Scholar] [CrossRef]
- Lyu, Y.; Kopcho, S.; Mohan, M.; Okeoma, C.M. Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues. Cells 2020, 9, 2243. [Google Scholar] [CrossRef]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fatty Acids. 2002, 66, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Demuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Jha, N.K.; Ojha, S. Pharmacological Properties, Therapeutic Potential and Molecular Mechanisms of JWH133, a CB2 Receptor-Selective Agonist. Front. Pharmacol. 2021, 12, 702675. [Google Scholar] [CrossRef]
- Agudelo, M.; Figueroa, G.; Yndart, A.; Casteleiro, G.; Munoz, K.; Samikkannu, T.; Atluri, V.; Nair, M.P. Alcohol and Cannabinoids Differentially Affect HIV Infection and Function of Human Monocyte-Derived Dendritic Cells (MDDC). Front. Microbiol. 2015, 6, 1452. [Google Scholar] [CrossRef]
- Fan, H.; Derynck, R. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999, 18, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Ciesielski, C.J.; Hunt, A.E.; Horwood, N.J.; Beech, J.T.; Hayes, L.A.; Denys, A.; Feldmann, M.; Brennan, F.M.; Foxwell, B.M. A novel mechanism for TNF-alpha regulation by p38 MAPK: Involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J. Immunol. 2004, 173, 6928–6937. [Google Scholar] [CrossRef] [PubMed]
- Canault, M.; Duerschmied, D.; Brill, A.; Patten, I.S.; Bergmeier, W.; Wagner, D.D. p38 MAPK Inhibition Prevents TACE-Mediated Receptor Shedding and Improves the Hemostatic Function of Stored Platelets. Blood 2008, 112, 990. [Google Scholar] [CrossRef]
- Starr, A.; Jordan-Sciutto, K.L.; Mironets, E. Confound, Cause, or Cure: The Effect of Cannabinoids on HIV-Associated Neurological Sequelae. Viruses 2021, 13, 1242. [Google Scholar] [CrossRef]
- Henriquez, J.E.; Rizzo, M.D.; Crawford, R.B.; Gulick, P.; Kaminski, N.E. Interferon-alpha-Mediated Activation of T Cells from Healthy and HIV-Infected Individuals Is Suppressed by Δ9-Tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2018, 367, 49–58. [Google Scholar] [CrossRef]
- Roberts, B.Z.; Minassian, A.; Halberstadt, A.L.; He, Y.V.; Chatha, M.; Geyer, M.A.; Grant, I.; Young, J.W. HIV Transgenic Rats Demonstrate Impaired Sensorimotor Gating But Are Insensitive to Cannabinoid (Δ9-Tetrahydrocannabinol)-Induced Deficits. Int. J. Neuropsychopharmacol. 2021, 24, 894–906. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Introduction: The Clans and Families of Cysteine Peptidases. In Handbook of Proteolytic Enzymes, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volumes 1–2, pp. 1743–1773. [Google Scholar]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Okutani, D.; Lodyga, M.; Han, B.; Liu, M. Src protein tyrosine kinase family and acute inflammatory responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L129–L141. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.J.; Bothwell, A.L.M. Canonical and Non-Canonical Wnt Signaling in Immune Cells. Trends Immunol. 2018, 39, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Chantry, D.; Turner, M.; Abney, E.; Feldmann, M. Modulation of cytokine production by transforming growth factor-beta. J. Immunol. 1989, 142, 4295–4300. [Google Scholar] [CrossRef]
- Silva, L.B.; dos Santos Neto, A.P.; Maia, S.M.A.S.; dos Santos Guimarães, C.; Quidute, I.L.; Carvalho, A.d.A.T.; Júnior, S.A.; Leão, J.C. The Role of TNF-α as a Proinflammatory Cytokine in Pathological Processes. Open Dent. J. 2019, 13, 332–338. [Google Scholar] [CrossRef]
- Janssens, R.; Struyf, S.; Proost, P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 2018, 15, 299–311. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Bai, X.; Li, Y.; Ni, C.; Zhao, X.; Zhang, D. ITGA3 Is Associated With Immune Cell Infiltration and Serves as a Favorable Prognostic Biomarker for Breast Cancer. Front. Oncol. 2021, 11, 658547. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, C.E.; Breivogel, C.S.; Gamage, T.F.; Gay, E.A.; Thomas, B.F.; Craft, R.M.; Wiley, J.L. Sex, THC, and hormones: Effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol. Depend. 2019, 194, 20–27. [Google Scholar] [CrossRef]
- Fattore, L.; Fratta, W. How important are sex differences in cannabinoid action? Br. J. Pharmacol. 2010, 160, 544–548. [Google Scholar] [CrossRef]
- Fogel, J.S.; Kelly, T.H.; Westgate, P.M.; Lile, J.A. Sex differences in the subjective effects of oral Δ9-THC in cannabis users. Pharmacol. Biochem. Behav. 2017, 152, 44–51. [Google Scholar] [CrossRef]
- Gaffal, E.; Cron, M.; Glodde, N.; Tuting, T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 2013, 68, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Zaunders, J.J.; Munier, M.L.; Kaufmann, D.E.; Ip, S.; Grey, P.; Smith, D.; Ramacciotti, T.; Quan, D.; Finlayson, R.; Kaldor, J.; et al. Early proliferation of CCR5+ CD38+++ antigen-specific CD4+ Th1 effector cells during primary HIV-1 infection. Blood 2005, 106, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Lelievre, J.D.; Donkova-Petrini, V.; Aouba, A.; Melica, G.; Balbo, M.; Weiss, L.; Levy, Y. HIV-specific regulatory T cells are associated with higher CD4 cell counts in primary infection. AIDS 2008, 22, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Ament, S.A.; Campbell, R.R.; Lobo, M.K.; Receveur, J.P.; Agrawal, K.; Borjabad, A.; Byrareddy, S.N.; Chang, L.; Clarke, D.; Emani, P.; et al. The single-cell opioid responses in the context of HIV (SCORCH) consortium. Mol. Psychiatry 2024, 29, 3950–3961. [Google Scholar] [CrossRef]
- Yndart Arias, A.; Kolishetti, N.; Vashist, A.; Madepalli, L.; Llaguno, L.; Nair, M. Anti-inflammatory effects of CBD in human microglial cell line infected with HIV-1. Sci. Rep. 2023, 13, 7376. [Google Scholar] [CrossRef]

| Study ID | Target Tissue | Finding |
|---|---|---|
| Alvarez 2020 [37] | Oropharyngeal mucosa | ↓ Pro-inflammatory genes: SAMHD1, PELI3, KLK6, and ICAM1 ↓ Anti-inflammatory regulatory genes: SOCS3, NR1D1, and IL4R |
| Chandra 2015 [39] | Duodenum | ~ Pro-inflammatory cytokine genes: TNF, IL1B, CCL2, CXCL11, and IFNG |
| Kumar 2016 [42] | Colon | ↓ Pro-inflammatory genes: DEFA4, DEFA5, DEFA6, and MMP8 |
| Kumar 2019 [43] | Colon | ↑ Tight junction protein genes: CLDN3 and OCLN ↑ PROM1 (an intestinal epithelial regeneration-associated gene) ↑ MUC13 (an inhibitor of intestinal inflammation and epithelial apoptosis) |
| McDew-White 2022 [44] | Gingiva | ↓ Pro-inflammatory genes: ADA2, ALOX5AP, CSF3R, IL21R, CXCL10, CXCR6, GADD4B, KLK6, KLRD1, LTB, MAMU-DRB1, SEMA7A, SLA, TIMP1, and VCAM1 ↓ Anti-inflammatory regulatory genes: IDO1, MRC1, STAB1, and TNFAIP6 ↑ Oral epithelial barrier regulating genes: CASP14, CDH13, DSC3, ITGA3, ITGA6, KRT10, and LAMB4 ↑ Active anti-inflammatory genes: PARD3B, TRIM35, and TGFB2 ↑ Tissue protection and repair genes: HP and PDGFC |
| McDew-White 2023 [45] | Basal ganglia | ↑ Neuropeptide signaling pathway genes: GLRA2, TAC3, CARTPT, and GRP ↓ Cytoskeleton organization genes: KRT4, KRT5, KRT6A, KRT15, KRT16, KRT23, KRT24, KRT76, KRT78, and KRT80 |
| Molina 2011-1 [46] | Lateral cerebellum tissue | ↓ IKBKG and CARM1 (pro-inflammatory genes) |
| Premadasa 2023 [49] | Colonic epithelium | ↓CCL15 (associated with neutrophil infiltration) ↑ TIMP2 (a tissue protection and repair gene) |
| Simon 2016 [51] | Striatum of the brain | ↑BDNF (an important modulator gene of neuronal survival) ↓ TNF (a pro-inflammatory cytokine gene) |
| Term ID | GO BP Terms | P | Padj | Overlap | OR |
|---|---|---|---|---|---|
| GO:0045109 | intermediate filament organization | 2.43 × 10−10 | 4.82 × 10−7 | 12/68 | 15.1 |
| GO:0014066 | regulation of phosphatidylinositol 3-kinase signaling | 7.24 × 10−7 | 4.78 × 10−4 | 10/90 | 8.8 |
| GO:0098742 | cell–cell adhesion via plasma-membrane adhesion molecules | 1.42 × 10−6 | 7.07 × 10−4 | 13/172 | 5.8 |
| GO:1901701 | cellular response to oxygen-containing compound | 2.19 × 10−6 | 8.70 × 10−4 | 20/406 | 3.7 |
| GO:0060429 | epithelium development | 2.64 × 10−6 | 8.73 × 10−4 | 12/154 | 5.9 |
| GO:0071677 | positive regulation of mononuclear cell migration | 4.78 × 10−6 | 1.05 × 10−3 | 6/31 | 16.6 |
| GO:0090026 | positive regulation of monocyte chemotaxis | 4.54 × 10−6 | 1.05 × 10−3 | 5/18 | 26.6 |
| GO:1902533 | positive regulation of intracellular signal transduction | 9.23 × 10−6 | 1.66 × 10−3 | 22/525 | 3.1 |
| GO:0071222 | cellular response to lipopolysaccharide | 1.34 × 10−5 | 2.21 × 10−3 | 10/124 | 6.1 |
| GO:0010605 | negative regulation of macromolecule metabolic process | 1.82 × 10−5 | 2.58 × 10−3 | 12/186 | 4.8 |
| GO:0019221 | cytokine-mediated signaling pathway | 2.50 × 10−5 | 3.02 × 10−3 | 14/257 | 4.1 |
| GO:0016525 | negative regulation of angiogenesis | 3.53 × 10−5 | 3.51 × 10−3 | 8/86 | 7.1 |
| GO:0051241 | negative regulation of multicellular organismal process | 3.48 × 10−5 | 3.51 × 10−3 | 13/231 | 4.2 |
| GO:0043405 | regulation of MAP kinase activity | 4.29 × 10−5 | 4.05 × 10−3 | 9/114 | 6.0 |
| GO:0050727 | regulation of inflammatory response | 5.16 × 10−5 | 4.27 × 10−3 | 13/240 | 4.0 |
| GO:0043588 | skin development | 5.70 × 10−5 | 4.45 × 10−3 | 7/68 | 8.0 |
| GO:0061626 | pharyngeal arch artery morphogenesis | 5.84 × 10−5 | 4.45 × 10−3 | 3/6 | 68.7 |
| GO:0010604 | positive regulation of macromolecule metabolic process | 8.96 × 10−5 | 6.58 × 10−3 | 16/364 | 3.2 |
| GO:0046425 | regulation of receptor signaling pathway via JAK-STAT | 1.14 × 10−4 | 7.81 × 10−3 | 6/53 | 8.8 |
| GO:0048660 | regulation of smooth muscle cell proliferation | 1.27 × 10−4 | 7.81 × 10−3 | 6/54 | 8.7 |
| GO:0042417 | dopamine metabolic process | 1.41 × 10−4 | 8.00 × 10−3 | 4/19 | 18.4 |
| GO:0043280 | positive regulation of cysteine endopeptidase in apoptosis | 1.90 × 10−4 | 9.92 × 10−3 | 8/109 | 5.5 |
| GO:2001235 | positive regulation of apoptotic signaling pathway | 2.74 × 10−4 | 1.27 × 10−2 | 6/62 | 7.4 |
| GO:0045670 | regulation of osteoclast differentiation | 3.39 × 10−4 | 1.40 × 10−2 | 5/42 | 9.3 |
| GO:0001934 | positive regulation of protein phosphorylation | 4.30 × 10−4 | 1.55 × 10−2 | 15/377 | 2.9 |
| GO:0006865 | amino acid transport | 5.21 × 10−4 | 1.77 × 10−2 | 5/46 | 8.4 |
| GO:0033275 | actin-myosin filament sliding | 6.02 × 10−4 | 1.84 × 10−2 | 3/12 | 22.9 |
| GO:0045429 | positive regulation of nitric oxide biosynthetic process | 5.84 × 10−4 | 1.84 × 10−2 | 4/27 | 12.0 |
| GO:0051260 | protein homo-oligomerization | 5.94 × 10−4 | 1.84 × 10−2 | 8/129 | 4.6 |
| GO:1903428 | positive regulation of reactive oxygen species biosynthesis | 6.02 × 10−4 | 1.84 × 10−2 | 3/12 | 22.9 |
| GO:0071248 | cellular response to metal ion | 8.02 × 10−4 | 2.18 × 10−2 | 8/135 | 4.4 |
| GO:0046942 | carboxylic acid transport | 1.00 × 10−3 | 2.46 × 10−2 | 5/53 | 7.2 |
| GO:0051091 | positive regulation of DNA-binding transcription factor activity | 9.73 × 10−4 | 2.46 × 10−2 | 11/246 | 3.3 |
| GO:0030198 | extracellular matrix organization | 1.10 × 10−3 | 2.67 × 10−2 | 9/176 | 3.7 |
| GO:0002719 | negative regulation of cytokine production in immunity | 1.46 × 10−3 | 3.27 × 10−2 | 3/16 | 15.8 |
| GO:1904646 | cellular response to amyloid-beta | 1.42 × 10−3 | 3.27 × 10−2 | 4/34 | 9.2 |
| GO:0001755 | neural crest cell migration | 1.77 × 10−3 | 3.69 × 10−2 | 4/36 | 8.6 |
| GO:0050772 | positive regulation of axonogenesis | 2.16 × 10−3 | 4.05 × 10−2 | 4/38 | 8.1 |
| GO:0042509 | regulation of tyrosine phosphorylation of STAT protein | 2.51 × 10−3 | 4.49 × 10−2 | 5/65 | 5.7 |
| GO:0032880 | regulation of protein localization | 2.89 × 10−3 | 4.95 × 10−2 | 6/97 | 4.6 |
| GO:0051045 | negative regulation of membrane protein proteolysis | 3.02 × 10−3 | 4.99 × 10−2 | 2/6 | 34.2 |
| Study ID | Target Tissue | Results |
|---|---|---|
| Chandra 2015 [39] | Duodenum | ↑ Immunomodulatory miRNAs: miR-149, miR-24, and miR-99 |
| Kaddour 2022 [40] | Extracellular vesicles of basal ganglia | ↑ Neuroinflammation regulatory miRNAs: mml-let-7a-5p and mml-let-7c-5p |
| Kopcho 2023 [41] | Extracellular vesicles and condensates from plasma | ↓ miR-335-5p (a regulator of the PI3K-Akt pathway) ↓ miR-139-5p (a regulator of the MAPK signaling pathway) |
| Kumar 2016 [42] | Colon | ↑ Immunomodulatory miRNAs: miR-193b-5p and miR-374a-5p |
| Kumar 2019 [43] | Colon | ↓ Pro-inflammatory miRNAs: miR-21, miR-141, and miR-222 |
| McDew-White 2022 [44] | Gingiva | ↓ Pro-inflammatory miRNAs: miR-142-3p, miR-223, miR-146a, and miR-34c |
| McDew-White 2023 [45] | Basal ganglia | ↑ miR-218-5p (a neuroinflammation regulatory miRNA) |
| Molina 2011-1 [46] | CD4+ cells | ↑ miRNAs associated with the regulation of T-cell activation: miR-142-3p, miR-142-5p, and miR-150 |
| Simon 2016 [51] | Striatum of the brain | ↑ miR-105-5p (associated with nervous system development) ↑ miR-767-5p (an immunomodulatory miRNA) |
| Cell Type | Duodenum | Peripheral Blood | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amedee 2014 [38] | Chandra 2015 [39] | Kumar 2019 [43] | Molina 2014 [47] | Amedee 2014 [38] | Kumar 2019 [43] | Molina 2011-2 [47] | Molina 2014 [47] | Wei 2016 [52] | |
| B-cell | - | - | - | - | - | - | - | - | ~ |
| B-cell IgE+ | - | - | - | - | - | - | - | - | ~ |
| Macrophage (CD3−CD14−) | - | - | ↑ | - | - | - | - | - | - |
| T-cell CD4+ | ~ | ↑ | ~ | ~ | - | ~ | ~ | ~ | ~ |
| T-cell CD4+ (apoptosis marker) | - | - | - | - | - | - | ~ | - | - |
| T-cell CD4+ (CCR5+ total) | ~ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD4+ (central) | ↑ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD4+ (CXCR4+ total) | ~ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD4+ (effector) | ↑ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD4+ (programmed death marker) | - | - | ↓ | - | - | - | - | - | - |
| T-cell CD4+ (proliferation marker) | - | - | ~ | - | - | - | ~ | - | ~ |
| T-cell CD8+ | ~ | ~ | ~ | ~ | - | ~ | ~ | ~ | ~ |
| T-cell CD8+ (activation marker) | - | - | ↓ | - | - | - | - | - | - |
| T-cell CD8+ (apoptosis marker) | - | - | - | - | - | - | ~ | - | - |
| T-cell CD8+ (CCR5+) | ~ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD8+ (Central) | ~ | - | - | ↑ | ↓ | - | - | ~ | - |
| T-cell CD8+ (CXCR4+) | ~ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD8+ (effector) | ~ | - | - | ~ | ~ | - | - | ~ | - |
| T-cell CD8+ (programmed death marker) | - | - | ↓ | - | - | - | - | - | - |
| T-cell CD8+ (proliferation marker) | - | - | ~ | - | - | - | ~ | - | ~ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valizadeh, A.; Veenhuis, R.T.; Bradley, B.A.; Xu, K. Transcriptomic Alterations Induced by Tetrahydrocannabinol in SIV/HIV Infection: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2598. https://doi.org/10.3390/ijms26062598
Valizadeh A, Veenhuis RT, Bradley BA, Xu K. Transcriptomic Alterations Induced by Tetrahydrocannabinol in SIV/HIV Infection: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(6):2598. https://doi.org/10.3390/ijms26062598
Chicago/Turabian StyleValizadeh, Amir, Rebecca T. Veenhuis, Brooklyn A. Bradley, and Ke Xu. 2025. "Transcriptomic Alterations Induced by Tetrahydrocannabinol in SIV/HIV Infection: A Systematic Review" International Journal of Molecular Sciences 26, no. 6: 2598. https://doi.org/10.3390/ijms26062598
APA StyleValizadeh, A., Veenhuis, R. T., Bradley, B. A., & Xu, K. (2025). Transcriptomic Alterations Induced by Tetrahydrocannabinol in SIV/HIV Infection: A Systematic Review. International Journal of Molecular Sciences, 26(6), 2598. https://doi.org/10.3390/ijms26062598







