Identification of Novel Genetic Variants and Food Intake Factors Associated with Type 2 Diabetes in South Korean Adults, Using an Illness–Death Model
Abstract
1. Introduction
2. Results
2.1. Demographic and Lifestyle Characteristics
2.2. Association Between Food Intake and T2D
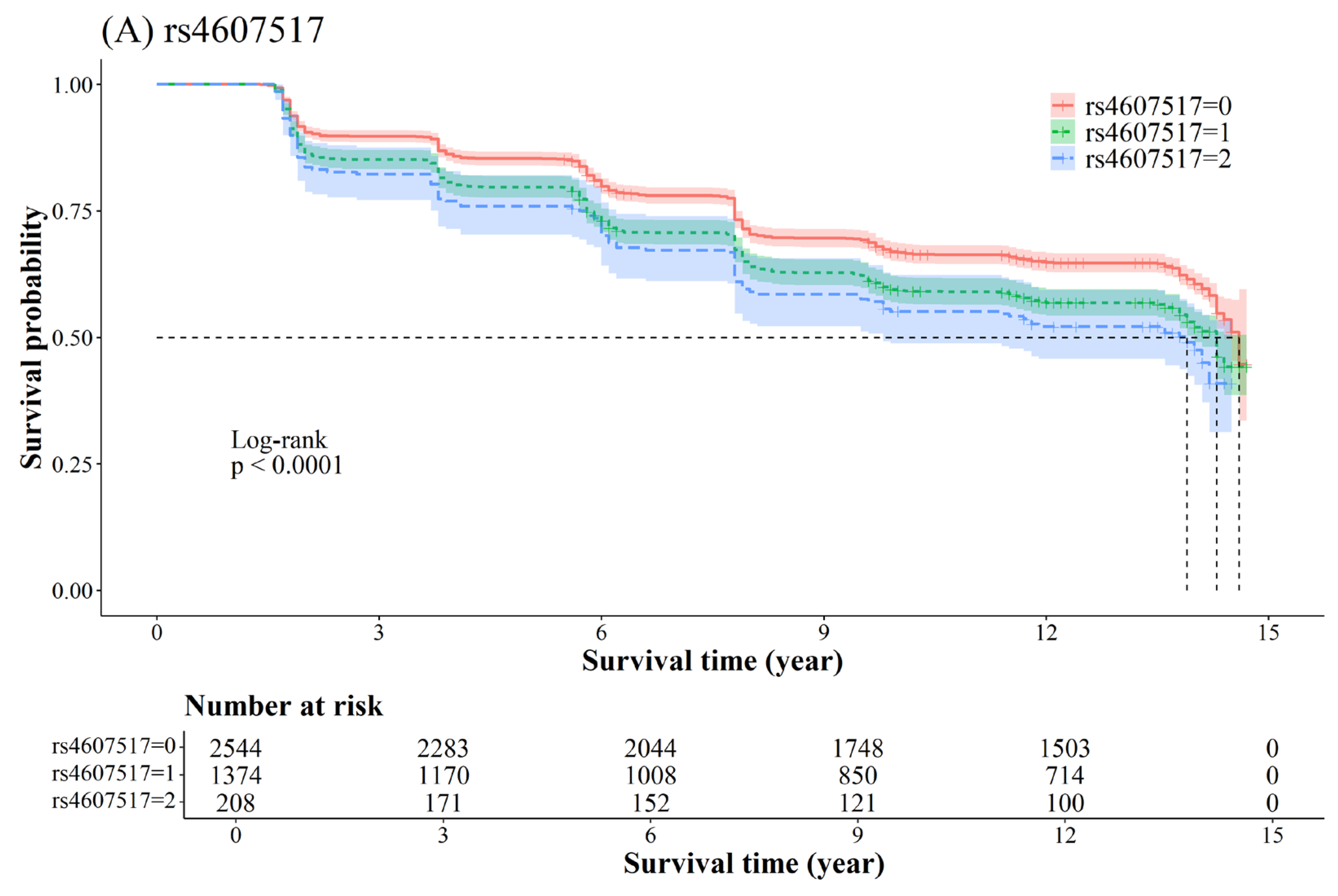
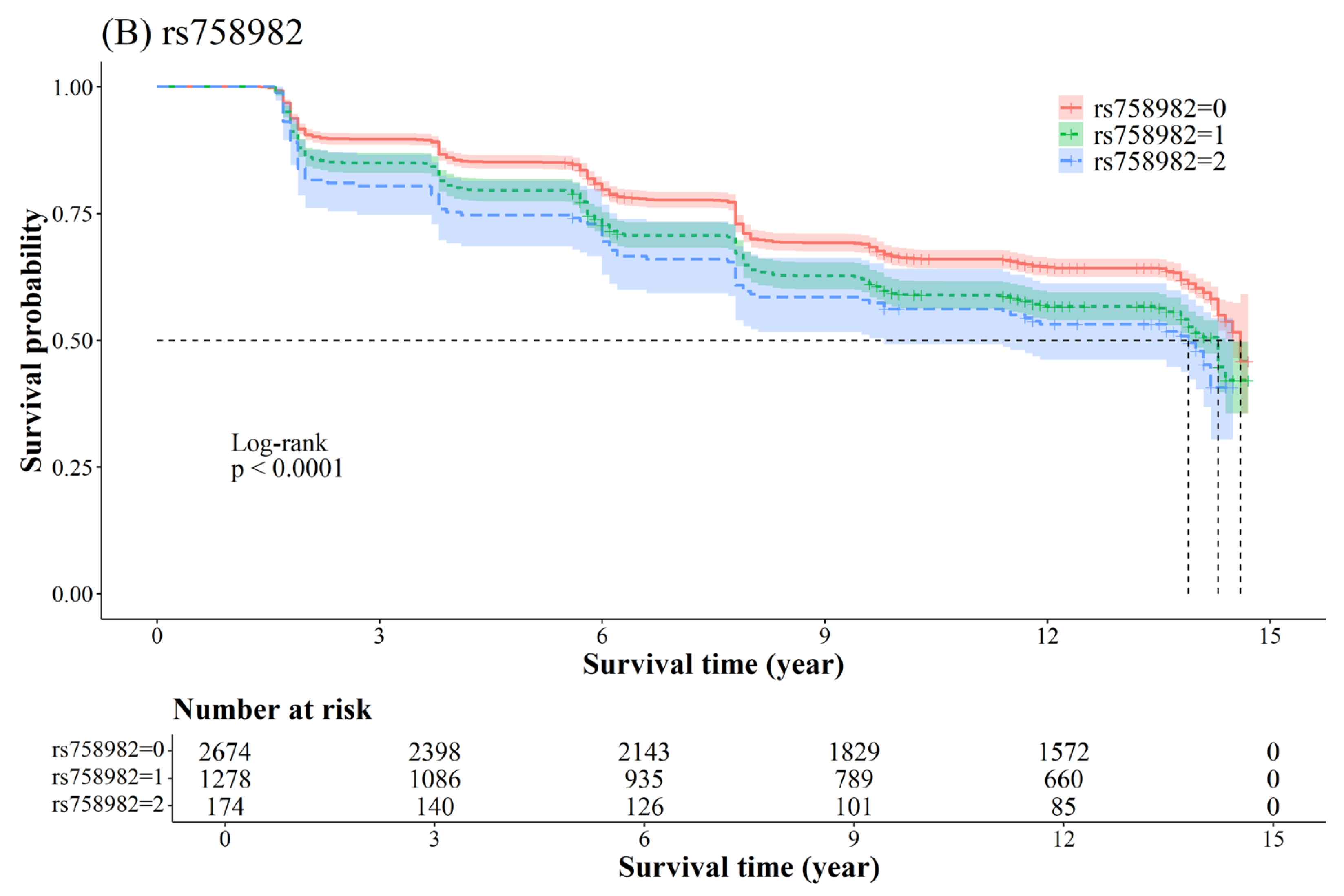
2.3. Association Between SNP and T2D
2.4. Functional Annotation
3. Discussion
3.1. Food Intake Factors
3.2. Genetic Variants
3.3. Strengths and Limitations
4. Materials and Methods
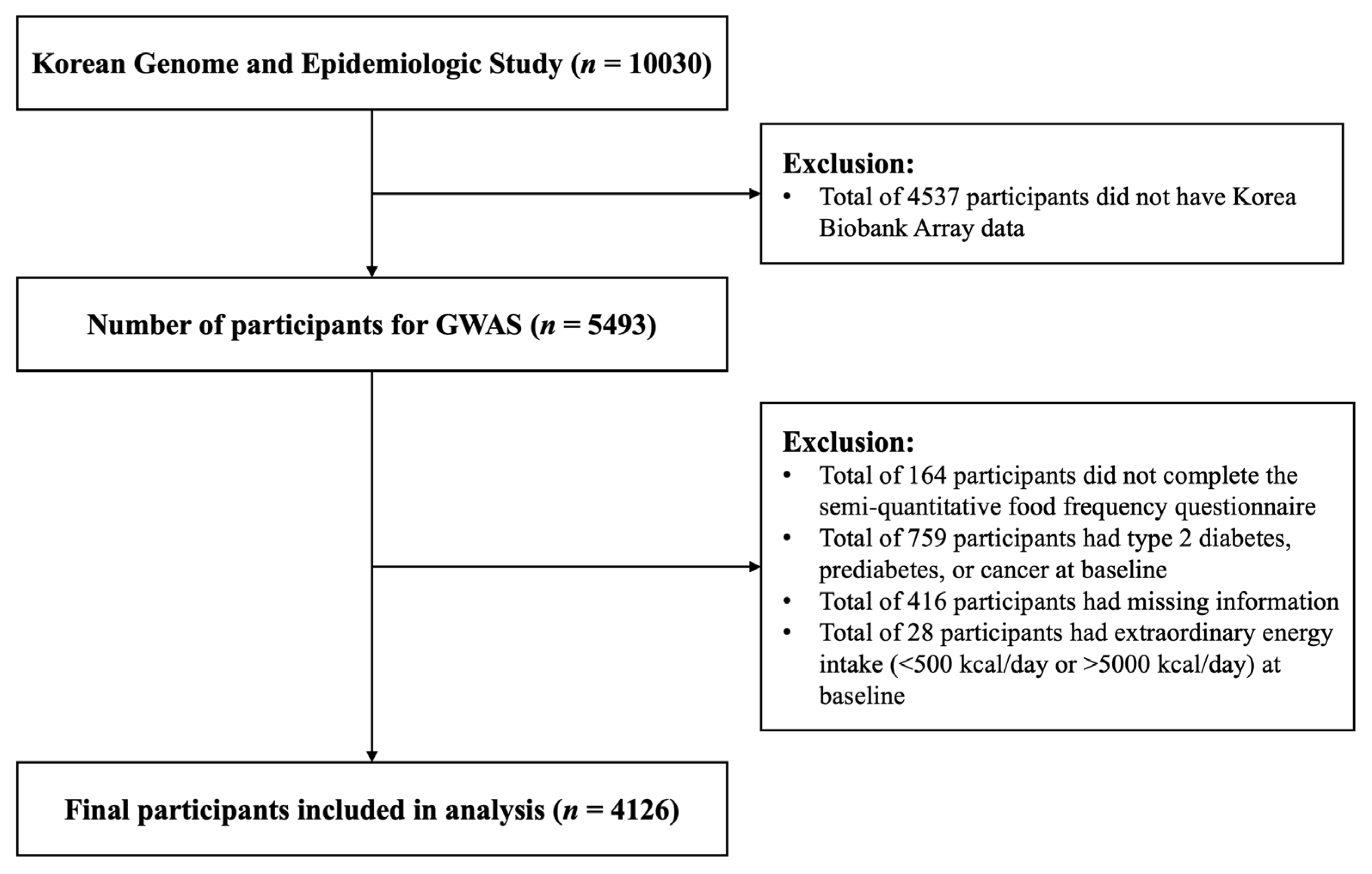
4.1. Study Population
4.2. General Characteristics and Anthropometric Measurements
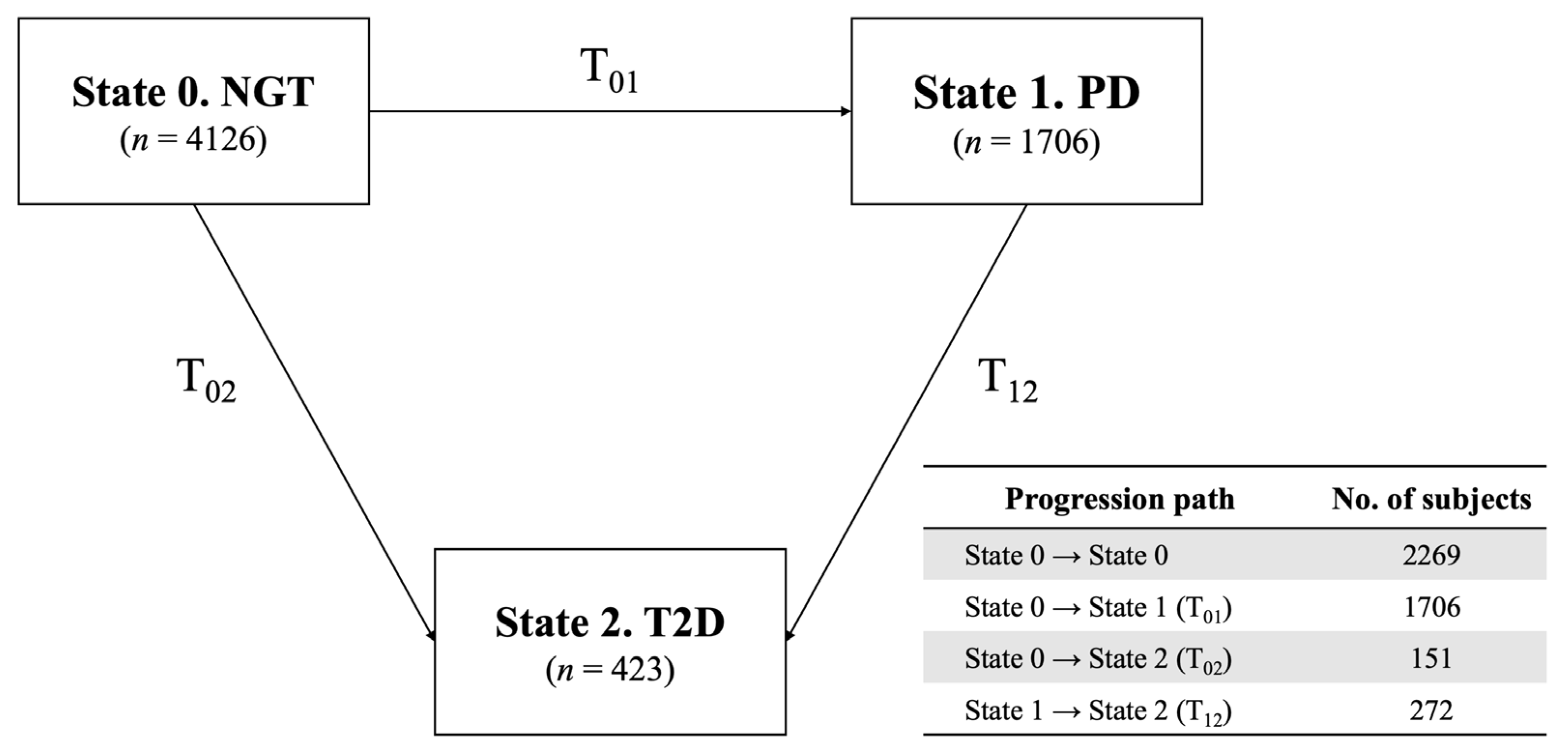
4.3. Definition of Type 2 Diabetes and Prediabetes
4.4. Assessment of Dietary Intake
4.5. Genotyping and Quality Control
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Han, K.D.; Ko, S.H.; Yang, Y.S.; Choi, J.H.; Choi, K.M.; Kwon, H.S.; Won, K.C. Diabetes Fact Sheet in Korea 2021. Diabetes Metab. J. 2022, 46, 417–426. [Google Scholar] [CrossRef]
- Federation International Diabetes. IDF Diabetes Atlas 10th. 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 10 March 2025).
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Beeri, M.S.; Bendlin, B.B. The link between type 2 diabetes and dementia: From biomarkers to treatment. Lancet Diabetes Endocrinol. 2020, 8, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Wolfe, C.D.A.; O’Connell, M.D.L.; Wang, Y. Diabetes as an Independent Risk Factor for Stroke Recurrence in Ischemic Stroke Patients: An Updated Meta-Analysis. Neuroepidemiology 2021, 55, 427–435. [Google Scholar] [CrossRef]
- Djousse, L.; Driver, J.A.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Association between modifiable lifestyle factors and residual lifetime risk of diabetes. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 17–22. [Google Scholar] [CrossRef]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Taylor, R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia 2008, 51, 1781–1789. [Google Scholar] [CrossRef]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathophysiology of prediabetes. Curr. Diab Rep. 2009, 9, 193–199. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Schunkert, H.; Konig, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–338. [Google Scholar] [CrossRef]
- Rung, J.; Cauchi, S.; Albrechtsen, A.; Shen, L.; Rocheleau, G.; Cavalcanti-Proenca, C.; Bacot, F.; Balkau, B.; Belisle, A.; Borch-Johnsen, K.; et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat. Genet. 2009, 41, 1110–1115. [Google Scholar] [CrossRef]
- DIAbetes Genetics Replication And Meta-Analysis (DIAGRAM) Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; Mexican American Type 2 Diabetes (MAT2D) Consortium; Type 2 Diabetes Genetic Exploration by Nex-Generation Sequencing in Muylti-Ethnic Samples (T2D-GENES) Consortium; Mahajan, A.; Go, M.J.; Zhang, W.; Below, J.E.; Gaulton, K.J.; et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 2014, 46, 234–244. [Google Scholar] [CrossRef]
- Go, M.J.; Lee, Y.; Park, S.; Kwak, S.H.; Kim, B.J.; Lee, J. Genetic-risk assessment of GWAS-derived susceptibility loci for type 2 diabetes in a 10 year follow-up of a population-based cohort study. J. Hum. Genet. 2016, 61, 1009–1012. [Google Scholar] [CrossRef]
- Ohn, J.H.; Kwak, S.H.; Cho, Y.M.; Lim, S.; Jang, H.C.; Park, K.S.; Cho, N.H. 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: A community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 27–34. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Standards of medical care in diabetes—2016 abridged for primary care providers. Clin. Diabetes 2016, 34, 3. [Google Scholar]
- Franks, P.W. Gene x environment interactions in type 2 diabetes. Curr. Diab Rep. 2011, 11, 552–561. [Google Scholar] [CrossRef]
- Hougaard, P. Multi-state models: A review. Lifetime Data Anal. 1999, 5, 239–264. [Google Scholar] [CrossRef]
- Tebe, C.; Martinez-Laguna, D.; Carbonell-Abella, C.; Reyes, C.; Moreno, V.; Diez-Perez, A.; Collins, G.S.; Prieto-Alhambra, D. The association between type 2 diabetes mellitus, hip fracture, and post-hip fracture mortality: A multi-state cohort analysis. Osteoporos. Int. 2019, 30, 2407–2415. [Google Scholar] [CrossRef]
- Huang, T.S.; Lin, C.L.; Lu, M.J.; Yeh, C.T.; Liang, K.H.; Sun, C.C.; Shyu, Y.C.; Chien, R.N. Diabetes, hepatocellular carcinoma, and mortality in hepatitis C-infected patients: A population-based cohort study. J. Gastroenterol. Hepatol. 2017, 32, 1355–1362. [Google Scholar] [CrossRef]
- Minooee, S.; Ramezani Tehrani, F.; Rahmati, M.; Amanollahi Soudmand, S.; Tohidi, M.; Sabet, Z.; Azizi, F. The association between serum total testosterone and progression of hyperglycemia: A 15-year prospective cohort study. Andrology 2019, 7, 148–155. [Google Scholar] [CrossRef]
- Yerramalla, M.S.; Fayosse, A.; Dugravot, A.; Tabak, A.G.; Kivimäki, M.; Singh-Manoux, A.; Sabia, S. Association of moderate and vigorous physical activity with incidence of type 2 diabetes and subsequent mortality: 27 year follow-up of the Whitehall II study. Diabetologia 2020, 63, 537–548. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; KoGES group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Moon, S.; Kim, Y.J.; Han, S.; Hwang, M.Y.; Shin, D.M.; Park, M.Y.; Lu, Y.; Yoon, K.; Jang, H.M.; Kim, Y.K.; et al. The Korea Biobank Array: Design and Identification of Coding Variants Associated with Blood Biochemical Traits. Sci. Rep. 2019, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, R.E.; Elvestad, M.; Molin, M.; Aune, D. Fruit and vegetable consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective studies. BMJ Nutr. Prev. Health 2021, 4, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, P.; Yuan, Z. Fruit and vegetable intake is inversely associated with type 2 diabetes in Chinese women: Results from the China Health and Nutrition Survey. Int. J. Food Sci. Nutr. 2021, 72, 208–218. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.M.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef]
- Safabakhsh, M.; Koohdani, F.; Bagheri, F.; Siassi, F.; Khajehnasiri, F.; Sotoudeh, G. Fruit and vegetable intake and pre-diabetes: A case-control study. Eur. J. Nutr. 2018, 57, 2953–2962. [Google Scholar] [CrossRef]
- Barouti, A.A.; Tynelius, P.; Lager, A.; Bjorklund, A. Fruit and vegetable intake and risk of prediabetes and type 2 diabetes: Results from a 20-year long prospective cohort study in Swedish men and women. Eur. J. Nutr. 2022, 61, 3175–3187. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Whincup, P.H.; Thomas, M.C.; Sattar, N. Associations Between Dietary Fiber and Inflammation, Hepatic Function, and Risk of Type 2 Diabetes in Older Men: Potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 2009, 32, 1823–1825. [Google Scholar] [CrossRef]
- Livesey, G.; Tagami, H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): Meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am. J. Clin. Nutr. 2011, 93, 143–150. [Google Scholar] [CrossRef] [PubMed]
- van Woudenbergh, G.J.; van Ballegooijen, A.J.; Kuijsten, A.; Sijbrands, E.J.; van Rooij, F.J.; Geleijnse, J.M.; Hofman, A.; Witteman, J.C.; Feskens, E.J. Eating fish and risk of type 2 diabetes: A population-based, prospective follow-up study. Diabetes Care 2009, 32, 2021–2026. [Google Scholar] [CrossRef]
- Kaushik, M.; Mozaffarian, D.; Spiegelman, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009, 90, 613–620. [Google Scholar] [CrossRef]
- Mark, A.B.; Poulsen, M.W.; Andersen, S.; Andersen, J.M.; Bak, M.J.; Ritz, C.; Holst, J.J.; Nielsen, J.; de Courten, B.; Dragsted, L.O.; et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care 2014, 37, 88–95. [Google Scholar] [CrossRef]
- Son, J.; Lee, Y.; Park, K. Effects of processed red meat consumption on the risk of type 2 diabetes and cardiovascular diseases among Korean adults: The Korean Genome and Epidemiology Study. Eur. J. Nutr. 2019, 58, 2477–2484. [Google Scholar] [CrossRef]
- Mari-Sanchis, A.; Gea, A.; Basterra-Gortari, F.J.; Martinez-Gonzalez, M.A.; Beunza, J.J.; Bes-Rastrollo, M. Meat Consumption and Risk of Developing Type 2 Diabetes in the SUN Project: A Highly Educated Middle-Class Population. PLoS ONE 2016, 11, e0157990. [Google Scholar] [CrossRef]
- Steinbrecher, A.; Erber, E.; Grandinetti, A.; Kolonel, L.N.; Maskarinec, G. Meat consumption and risk of type 2 diabetes: The Multiethnic Cohort. Public Health Nutr. 2011, 14, 568–574. [Google Scholar] [CrossRef]
- Sabate, J.; Burkholder-Cooley, N.M.; Segovia-Siapco, G.; Oda, K.; Wells, B.; Orlich, M.J.; Fraser, G.E. Unscrambling the relations of egg and meat consumption with type 2 diabetes risk. Am. J. Clin. Nutr. 2018, 108, 1121–1128. [Google Scholar] [CrossRef]
- Vang, A.; Singh, P.N.; Lee, J.W.; Haddad, E.H.; Brinegar, C.H. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: Findings from Adventist Health Studies. Ann. Nutr. Metab. 2008, 52, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Isanejad, M.; LaCroix, A.Z.; Thomson, C.A.; Tinker, L.; Larson, J.C.; Qi, Q.; Qi, L.; Cooper-DeHoff, R.M.; Phillips, L.S.; Prentice, R.L.; et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. Br. J. Nutr. 2017, 117, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Schulze, M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch. Intern. Med. 2004, 164, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.; Virtanen, S.M.; Rasanen, L.; Tuomilehto, J.; Stengard, J.; Pekkanen, J.; Nissinen, A.; Kromhout, D. Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 1995, 18, 1104–1112. [Google Scholar] [CrossRef]
- van Dam, R.M.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J.; Hu, F.B. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002, 25, 417–424. [Google Scholar] [CrossRef]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Consumption of Dairy Products in Relation to Type 2 Diabetes Mellitus in Chinese People: The Henan Rural Cohort Study and an Updated Meta-Analysis. Nutrients 2020, 12, 3827. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef]
- Zhang, J.; Lim, K.; Shin, S. Dairy product consumption and type 2 diabetes among Korean adults: A prospective cohort study based on the Health Examinees (HEXA) study. Epidemiol. Health 2022, 44, e2022019. [Google Scholar] [CrossRef]
- Jeon, J.; Jang, J.; Park, K. Effects of Consuming Calcium-Rich Foods on the Incidence of Type 2 Diabetes Mellitus. Nutrients 2018, 11, 31. [Google Scholar] [CrossRef]
- Slurink, I.A.L.; Voortman, T.; Ochoa-Rosales, C.; Ahmadizar, F.; Kavousi, M.; Kupper, N.; Smeets, T.; Soedamah-Muthu, S.S. Dairy Product Consumption in Relation to Incident Prediabetes and Longitudinal Insulin Resistance in the Rotterdam Study. Nutrients 2022, 14, 415. [Google Scholar] [CrossRef]
- Hruby, A.; Ma, J.; Rogers, G.; Meigs, J.B.; Jacques, P.F. Associations of Dairy Intake with Incident Prediabetes or Diabetes in Middle-Aged Adults Vary by Both Dairy Type and Glycemic Status. J. Nutr. 2017, 147, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Gilbert, J.A. Milk products, insulin resistance syndrome and type 2 diabetes. J. Am. Coll. Nutr. 2009, 28 (Suppl. S1), 91S–102S. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, H.; Kang, F.; Ning, G.; Ni, Q.; Wang, W.; Wang, Q. β-Cell glucokinase expression was increased in type 2 diabetes subjects with better glycemic control. J. Diabetes 2023, 15, 409–418. [Google Scholar] [CrossRef]
- Osbak, K.K.; Colclough, K.; Saint-Martin, C.; Beer, N.L.; Bellanne-Chantelot, C.; Ellard, S.; Gloyn, A.L. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009, 30, 1512–1526. [Google Scholar] [CrossRef]
- Porte, D., Jr.; Kahn, S.E. Beta-cell dysfunction and failure in type 2 diabetes: Potential mechanisms. Diabetes 2001, 50 (Suppl. S1), S160–S163. [Google Scholar] [CrossRef]
- Wang, Z.; Ramanadham, S.; Ma, Z.A.; Bao, S.; Mancuso, D.J.; Gross, R.W.; Turk, J. Group VIA phospholipase A2 forms a signaling complex with the calcium/calmodulin-dependent protein kinase IIβ expressed in pancreatic islet β-cells. J. Biol. Chem. 2005, 280, 6840–6849. [Google Scholar] [CrossRef]
- Hägglund, M.G.; Sreedharan, S.; Nilsson, V.C.; Shaik, J.H.; Almkvist, I.M.; Bäcklin, S.; Wrange, O.; Fredriksson, R. Identification of SLC38A7 (SNAT7) protein as a glutamine transporter expressed in neurons. J. Biol. Chem. 2011, 286, 20500–20511. [Google Scholar] [CrossRef]
- Hägglund, M.G.A.; Hellsten, S.V.; Bagchi, S.; Philippot, G.; Löfqvist, E.; Nilsson, V.C.O.; Almkvist, I.; Karlsson, E.; Sreedharan, S.; Tafreshiha, A.; et al. Transport of L-glutamine, L-alanine, L-arginine and L-histidine by the neuron-specific Slc38a8 (SNAT8) in CNS. J. Mol. Biol. 2015, 427 Pt B, 1495–1512. [Google Scholar] [CrossRef]
- Saha, S.; Fang, X.; Green, C.D.; Das, A. mTORC1 and SGLT2 Inhibitors-A Therapeutic Perspective for Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 15078. [Google Scholar] [CrossRef]
- Yoneyama, Y.; Inamitsu, T.; Chida, K.; Iemura, S.I.; Natsume, T.; Maeda, T.; Hakuno, F.; Takahashi, S.I. Serine Phosphorylation by mTORC1 Promotes IRS-1 Degradation through SCFβ-TRCP E3 Ubiquitin Ligase. iScience 2018, 5, 1–18. [Google Scholar] [CrossRef]
- Hsu, P.P.; Kang, S.A.; Rameseder, J.; Zhang, Y.; Ottina, K.A.; Lim, D.; Peterson, T.R.; Choi, Y.; Gray, N.S.; Yaffe, M.B.; et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 2011, 332, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Park, S.Y.; Su, J.; Bailey, K.; Ottosson-Laakso, E.; Shcherbina, L.; Oskolkov, N.; Zhang, E.; Thevenin, T.; Fadista, J.; et al. TCF7L2 is a master regulator of insulin production and processing. Hum. Mol. Genet. 2014, 23, 6419–6431. [Google Scholar] [CrossRef]
- Cauchi, S.; El Achhab, Y.; Choquet, H.; Dina, C.; Krempler, F.; Weitgasser, R.; Nejjari, C.; Patsch, W.; Chikri, M.; Meyre, D.; et al. TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: A global meta-analysis. J. Mol. Med. 2007, 85, 777–782. [Google Scholar] [CrossRef]
- Grant, S.F.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef]
- Chang, T.J.; Chiu, Y.F.; Sheu, W.H.; Shih, K.C.; Hwu, C.M.; Quertermous, T.; Jou, Y.S.; Kuo, S.S.; Chang, Y.C.; Chuang, L.M. Genetic polymorphisms of PCSK2 are associated with glucose homeostasis and progression to type 2 diabetes in a Chinese population. Sci. Rep. 2015, 5, 14380. [Google Scholar] [CrossRef]
- Leak, T.S.; Keene, K.L.; Langefeld, C.D.; Gallagher, C.J.; Mychaleckyj, J.C.; Freedman, B.I.; Bowden, D.W.; Rich, S.S.; Sale, M.M. Association of the proprotein convertase subtilisin/kexin-type 2 (PCSK2) gene with type 2 diabetes in an African American population. Mol. Genet. Metab. 2007, 92, 145–150. [Google Scholar] [CrossRef][Green Version]
- Winters, A.; Ramos-Molina, B.; Jarvela, T.S.; Yerges-Armstrong, L.; Pollin, T.I.; Lindberg, I. Functional analysis of PCSK2 coding variants: A founder effect in the Old Order Amish population. Diabetes Res. Clin. Pract. 2017, 131, 82–90. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, W.; Zhang, S.; Liu, J.; Li, S.; Li, J.; Yang, P.; He, J.; Su, S.; Li, P. Association of type 2 diabetes susceptibility genes (TCF7L2, SLC30A8, PCSK1 and PCSK2) and proinsulin conversion in a Chinese population. Mol. Biol. Rep. 2012, 39, 17–23. [Google Scholar] [CrossRef]
- Joo, Y.; Kim, H.; Lee, S.; Lee, S. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int. J. Obes. 2019, 43, 1769–1782. [Google Scholar] [CrossRef]
- Ng, M.C.; Tam, C.H.; So, W.Y.; Ho, J.S.; Chan, A.W.; Lee, H.M.; Wang, Y.; Lam, V.K.; Chan, J.C.; Ma, R.C. Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J. Clin. Endocrinol. Metab. 2010, 95, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Renstrom, F.; Payne, F.; Nordstrom, A.; Brito, E.C.; Rolandsson, O.; Hallmans, G.; Barroso, I.; Nordstrom, P.; Franks, P.W.; Consortium, G. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum. Mol. Genet. 2009, 18, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Sandholt, C.H.; Vestmar, M.A.; Bille, D.S.; Borglykke, A.; Almind, K.; Hansen, L.; Sandbaek, A.; Lauritzen, T.; Witte, D.; Jorgensen, T.; et al. Studies of metabolic phenotypic correlates of 15 obesity associated gene variants. PLoS ONE 2011, 6, e23531. [Google Scholar] [CrossRef] [PubMed]
- Schlauch, K.A.; Read, R.W.; Lombardi, V.C.; Elhanan, G.; Metcalf, W.J.; Slonim, A.D.; 23 and Me Research Team; Grzymski, J.J. A Comprehensive Genome-Wide and Phenome-Wide Examination of BMI and Obesity in a Northern Nevadan Cohort. G3 Genes Genomes Genet. 2020, 10, 645–664. [Google Scholar] [CrossRef]
- Sharifi, S.; Daghighi, S.; Motazacker, M.M.; Badlou, B.; Sanjabi, B.; Akbarkhanzadeh, A.; Rowshani, A.T.; Laurent, S.; Peppelenbosch, M.P.; Rezaee, F. Superparamagnetic iron oxide nanoparticles alter expression of obesity and T2D-associated risk genes in human adipocytes. Sci. Rep. 2013, 3, 2173. [Google Scholar] [CrossRef]
- Xi, B.; Takeuchi, F.; Meirhaeghe, A.; Kato, N.; Chambers, J.C.; Morris, A.P.; Cho, Y.S.; Zhang, W.; Mohlke, K.L.; Kooner, J.S.; et al. Associations of genetic variants in/near body mass index-associated genes with type 2 diabetes: A systematic meta-analysis. Clin. Endocrinol. 2014, 81, 702–710. [Google Scholar] [CrossRef]
- Kim, Y.J.; Go, M.J.; Hu, C.; Hong, C.B.; Kim, Y.K.; Lee, J.Y.; Hwang, J.Y.; Oh, J.H.; Kim, D.J.; Kim, N.H.; et al. Large-scale genome-wide association studies in East Asians identify new genetic loci influencing metabolic traits. Nat. Genet. 2011, 43, 990–995. [Google Scholar] [CrossRef]
- Cho, Y.S.; Go, M.J.; Kim, Y.J.; Heo, J.Y.; Oh, J.H.; Ban, H.J.; Yoon, D.; Lee, M.H.; Kim, D.J.; Park, M.; et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 2009, 41, 527–534. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kalbfleisch, J.D.; Tai, B. Statistical analysis of illness-death processes and semicompeting risks data. Biometrics 2010, 66, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Lumley, T. Package ‘survival’. R. Top. Doc. 2015, 128, 28–33. [Google Scholar]
- Schoenfeld, D. Partial Residuals for the Proportional Hazards Regression-Model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Cleophas, T.J.; Zwinderman, A.H.; Cleophas, T.J.; Zwinderman, A.H. Cox Regression With/Without Time Dependent Variables (60 Patients). In SPSS for Starters and 2nd Levelers; Springer: Cham, Switzerland, 2016; pp. 339–346. [Google Scholar]
| Variables | NGT to PD | NGT to T2D | PD to T2D | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Fruit | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 0.27 (0.21–0.34) | <0.001 | 0.68 (0.46–1.02) | 0.063 | 0.92 (0.68–1.24) | 0.570 |
| Tertile 3 | 0.18 (0.14–0.24) | <0.001 | 0.60 (0.39–0.93) | 0.022 | 0.99 (0.72–1.36) | 0.951 |
| Vegetable | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 0.97 (0.85–1.10) | 0.601 | 0.68 (0.45–1.03) | 0.067 | 0.96 (0.69–1.33) | 0.810 |
| Tertile 3 | 1.11 (0.97–1.27) | 0.118 | 0.63 (0.40–1.00) | 0.048 | 1.25 (0.90–1.73) | 0.188 |
| Red meat | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 1.07 (0.83–1.38) | 0.584 | 0.90 (0.59–1.38) | 0.636 | 1.15 (0.83–1.58) | 0.396 |
| Tertile 3 | 1.41 (1.08–1.85) | 0.012 | 0.88 (0.54–1.45) | 0.624 | 1.16 (0.80–1.67) | 0.435 |
| White meat | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 1.05 (0.83–1.34) | 0.673 | 1.04 (0.70–1.55) | 0.828 | 0.95 (0.71–1.29) | 0.757 |
| Tertile 3 | 1.61 (1.26–2.07) | <0.001 | 1.21 (0.77–1.90) | 0.415 | 0.98 (0.71–1.37) | 0.925 |
| Grain | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 0.95 (0.84–1.07) | 0.402 | 0.96 (0.62–1.47) | 0.845 | 0.90 (0.66–1.23) | 0.527 |
| Tertile 3 | 1.00 (0.88–1.13) | 0.971 | 1.32 (0.88–1.96) | 0.177 | 1.00 (0.74–1.35) | 0.992 |
| Fish | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 0.86 (0.68–1.10) | 0.235 | 0.98 (0.63–1.53) | 0.923 | 0.88 (0.63–1.23) | 0.454 |
| Tertile 3 | 1.17 (0.91–1.51) | 0.217 | 1.62 (1.00–2.62) | 0.048 | 1.05 (0.74–1.50) | 0.770 |
| Dairy | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 0.62 (0.49–0.78) | <0.001 | 1.22 (0.82–1.80) | 0.333 | 0.92 (0.68–1.24) | 0.583 |
| Tertile 3 | 1.02 (0.81–1.28) | 0.887 | 1.12 (0.73–1.74) | 0.605 | 0.92 (0.67–1.26) | 0.596 |
| Model | Chr a | Pos b | SNP c | Alleles d | Nearest Gene | HR (95% CI) e | p-Value | q-Value |
|---|---|---|---|---|---|---|---|---|
| NGT f → PD g | 7 | 44235668 | rs4607517 | G/A | GCK | 1.27 (1.17–1.37) | 1.37 × 10−9 | 0.0006 |
| 7 | 44257943 | rs758982 | C/T | CAMK2B | 1.27 (1.18–1.38) | 2.38 × 10−8 | 0.0006 | |
| NGT → T2D h | 15 | 42733571 | rs145386384 | A/G | ZNF106 | 3.77 (2.36–6.00) | 2.41 × 10−8 | 0.0123 |
| 19 | 50360989 | rs59595912 | A/G | PTOV1 | 2.64 (1.85–3.77) | 8.22 × 10−8 | 0.0210 | |
| 2 | 83637190 | rs7575023 | T/C | LOC105374834 | 1.88 (1.49–2.38) | 1.49 × 10−7 | 0.0253 | |
| 13 | 95600085 | rs35566993 | A/G | LINC00557 | 2.76 (1.86–4.09) | 4.74 × 10−7 | 0.0445 | |
| 20 | 17374513 | rs11698919 | A/G | PCSK2 | 2.06 (1.55–2.73) | 6.23 × 10−7 | 0.0445 | |
| 1 | 47931749 | rs59813747 | C/T | FOXD2 | 3.66 (2.20–6.10) | 6.29 × 10−7 | 0.0445 | |
| 16 | 58700803 | rs4784964 | C/T | SLC38A7 | 3.15 (2.00–4.94) | 6.47 × 10−7 | 0.0445 | |
| 1 | 72341074 | rs147467153 | A/G | NEGR1 | 4.31 (2.42–7.67) | 6.97 × 10−7 | 0.0445 |
| Model | Chr a | Pos b | SNP c | Nearest Gene | CADD Score d | DANN Score e |
|---|---|---|---|---|---|---|
| NGT f PD g | 7 | 44235668 | rs4607517 | GCK | 14.470 | 0.877 |
| 7 | 44257943 | rs758982 | CAMK2B | 8.908 | 0.743 | |
| NGT T2D h | 15 | 42733571 | rs145386384 | ZNF106 | 5.721 | 0.715 |
| 19 | 50360989 | rs59595912 | PTOV1 | 1.083 | 0.481 | |
| 2 | 83637190 | rs7575023 | LOC105374834 | 0.025 | 0.573 | |
| 13 | 95600085 | rs35566993 | LINC00557 | 0.695 | 0.391 | |
| 20 | 17374513 | rs11698919 | PCSK2 | 5.039 | 0.318 | |
| 1 | 47931749 | rs59813747 | FOXD2 | 12.890 | 0.994 | |
| 16 | 58700803 | rs4784964 | SLC38A7 | 4.847 | 0.802 | |
| 1 | 72341074 | rs147467153 | NEGR1 | 0.733 | 0.481 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Cha, J.; Choi, S. Identification of Novel Genetic Variants and Food Intake Factors Associated with Type 2 Diabetes in South Korean Adults, Using an Illness–Death Model. Int. J. Mol. Sci. 2025, 26, 2597. https://doi.org/10.3390/ijms26062597
Oh J, Cha J, Choi S. Identification of Novel Genetic Variants and Food Intake Factors Associated with Type 2 Diabetes in South Korean Adults, Using an Illness–Death Model. International Journal of Molecular Sciences. 2025; 26(6):2597. https://doi.org/10.3390/ijms26062597
Chicago/Turabian StyleOh, Jeongmin, Junho Cha, and Sungkyoung Choi. 2025. "Identification of Novel Genetic Variants and Food Intake Factors Associated with Type 2 Diabetes in South Korean Adults, Using an Illness–Death Model" International Journal of Molecular Sciences 26, no. 6: 2597. https://doi.org/10.3390/ijms26062597
APA StyleOh, J., Cha, J., & Choi, S. (2025). Identification of Novel Genetic Variants and Food Intake Factors Associated with Type 2 Diabetes in South Korean Adults, Using an Illness–Death Model. International Journal of Molecular Sciences, 26(6), 2597. https://doi.org/10.3390/ijms26062597






