The Role of Calcified Nodules in Acute Coronary Syndrome: Diagnosis and Management
Abstract
1. Introduction
2. Prevalence and Risk Factors
3. Pathophysiologic Mechanisms and Plaque Characteristics
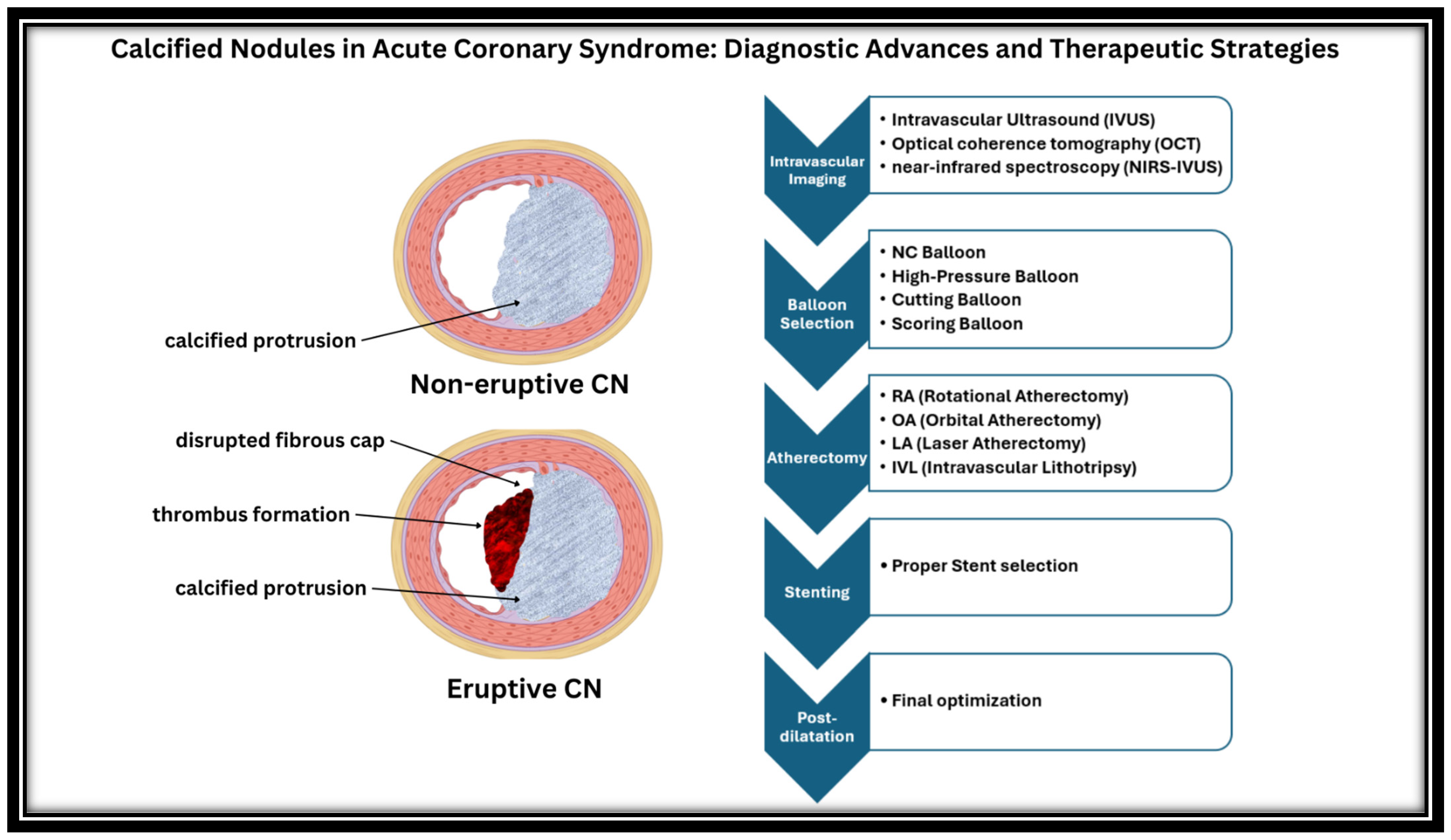
4. Intravascular Imaging in CNs
5. Revascularization Difficulties
6. Intra-Procedural Modification of Calcified Nodules
6.1. Non-Compliant Balloons (NC)
6.2. High-Pressure Balloons
6.3. Cutting Balloons
6.4. Scoring Balloons
6.5. Rotational Atherectomy (RA)
6.6. Orbital Atherectomy
6.7. Intravascular Lithotripsy (IVL)
6.8. Laser Atherectomy
7. Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward from Mechanisms to Precision Treatment. Circulation 2017, 136, 1155. [Google Scholar] [CrossRef]
- Sato, Y.; Kawakami, R.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawai, K.; Ghosh, S.; Romero, M.E.; Kolodgie, F.D.; Finn, A.V.; et al. Sex Differences in Coronary Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Davis, H.R.; Arbustini, E.; Virmani, R. Sex Differences in Coronary Artery Disease: Pathological Observations. Atherosclerosis 2015, 239, 260–267. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Chasikidis, C.; Tsioufis, K.; Tousoulis, D. Pathophysiology of Acute Coronary Syndromes—Diagnostic and Treatment Considerations. Life 2023, 13, 1543. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takano, M.; Tsurumi, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Okazaki, H.; Shirakabe, A.; Seino, Y.; Hata, N.; et al. Features and Outcomes of Patients with Calcified Nodules at Culprit Lesions of Acute Coronary Syndrome: An Optical Coherence Tomography Study. Cardiology 2018, 139, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients with Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Torii, S.; Sato, Y.; Otsuka, F.; Kolodgie, F.D.; Jinnouchi, H.; Sakamoto, A.; Park, J.; Yahagi, K.; Sakakura, K.; Cornelissen, A.; et al. Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death. J. Am. Coll Cardiol. 2021, 77, 1599–1611. [Google Scholar] [CrossRef]
- Sugane, H.; Kataoka, Y.; Otsuka, F.; Nakaoku, Y.; Nishimura, K.; Nakano, H.; Murai, K.; Honda, S.; Hosoda, H.; Matama, H.; et al. Cardiac Outcomes in Patients with Acute Coronary Syndrome Attributable to Calcified Nodule. Atherosclerosis 2021, 318, 70–75. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Tanaka, A.; Taruya, A.; Hikimoto, S.; Morimoto, J.; Mori, K.; Asae, Y.; Higashioka, D.; Tamaki, T.; Aoki, H.; et al. Abstract 13826: Patient Characteristics and Prognosis of OCT Verified Calcified Nodules in Acute Coronary Syndrome. Circulation 2015, 132. [Google Scholar] [CrossRef]
- Lee, T.; Mintz, G.S.; Matsumura, M.; Zhang, W.; Cao, Y.; Usui, E.; Kanaji, Y.; Murai, T.; Yonetsu, T.; Kakuta, T.; et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc. Imaging 2017, 10, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Gatto, L.; Fabbiocchi, F.; Vergallo, R.; Paoletti, G.; Ruscica, G.; Marco, V.; Romagnoli, E.; Boi, A.; Fineschi, M.; et al. Clinical Outcomes of Calcified Nodules Detected by Optical Coherence Tomography: A Sub-Analysis of the CLIMA Study. EuroIntervention 2020, 16, 380–386. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons From Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Puentes, J.; Garreau, M.; Lebreton, H.; Roux, C. Understanding Coronary Artery Movement: A Knowledge-Based Approach. Artif. Intell. Med. 1998, 13, 207–237. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and Its Progression: What Does It Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has Our Understanding of Calcification in Human Coronary Atherosclerosis Progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef]
- New, S.E.P.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-Derived Matrix Vesicles: An Alternative Novel Mechanism for Microcalcification in Atherosclerotic Plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Hayashi, H.; Izumi, C.; Kondo, H.; Tamura, T.; Enomoto, S.; Amano, M.; Nishimura, S.; Tanaka, Y.; Isshiki, T.; et al. Four-Dimensional Computed Tomography-Based Finite Element Modeling of the Behavior of the Right Coronary Artery. Circ. J. 2017, 81, 1059–1061. [Google Scholar] [CrossRef]
- Chinikar, M.; Sadeghipour, P. Coronary Stent Fracture: A Recently Appreciated Phenomenon with Clinical Relevance. Curr. Cardiol. Rev. 2014, 10, 349–354. [Google Scholar] [CrossRef]
- Nakazawa, G.; Yazdani, S.K.; Finn, A.V.; Vorpahl, M.; Kolodgie, F.D.; Virmani, R. Pathological Findings at Bifurcation Lesions: The Impact of Flow Distribution on Atherosclerosis and Arterial Healing After Stent Implantation. J. Am. Coll Cardiol. 2010, 55, 1679–1687. [Google Scholar] [CrossRef]
- Nakazawa, G.; Finn, A.V.; Vorpahl, M.; Ladich, E.; Kutys, R.; Balazs, I.; Kolodgie, F.D.; Virmani, R. Incidence and Predictors of Drug-Eluting Stent Fracture in Human Coronary Artery: A Pathologic Analysis. J. Am. Coll Cardiol. 2009, 54, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-Eluting Coronary Stents: Insights from Preclinical and Pathology Studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef]
- Moses, J.W.; Usui, E.; Maehara, A. Recognition of Recurrent Stent Failure Due to Calcified Nodule: Between a Rock and a Hard Place. JACC Case Rep. 2020, 2, 1879–1881. [Google Scholar] [CrossRef]
- Pengchata, P.; Pongakasira, R.; Wongsawangkit, N.; Phichaphop, A.; Wongpraparut, N. Characteristics and Pattern of Calcified Nodule and/or Nodular Calcification Detected by Intravascular Ultrasound on the Device-Oriented Composite Endpoint (DoCE) in Patients with Heavily Calcified Lesions Who Underwent Rotational Atherectomy-Assisted Percutaneous Coronary Intervention. J. Interv. Cardiol. 2023, 2023, 6456695. [Google Scholar] [CrossRef]
- Johnson, T.W.; Räber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.; et al. Clinical Use of Intracoronary Imaging. Part 2: Acute Coronary Syndromes, Ambiguous Coronary Angiography Findings, and Guiding Interventional Decision-Making: An Expert Consensus Document of the European Association of Percutaneous Cardiovascular Interventions: Endorsed by the Chinese Society of Cardiology, the Hong Kong Society of Transcatheter Endocardiovascular Therapeutics (HKSTENT) and the Cardiac Society of Australia and New Zealand. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Guagliumi, G.; Pellegrini, D.; Maehara, A.; Mintz, G.S. All Calcified Nodules Are Made Equal and Require the Same Approach: Pros and Cons. EuroIntervention 2023, 19, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mintz, G.S.; Tam, A.; Mcpherson, J.A.; Iñiguez, A.; Fajadet, J.; Fahy, M.; Weisz, G.; De Bruyne, B.; Serruys, P.W.; et al. Prevalence, Distribution, Predictors, and Outcomes of Patients with Calcified Nodules in Native Coronary Arteries: A 3-Vessel Intravascular Ultrasound Analysis from Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT). Circulation 2012, 126, 537–545. [Google Scholar] [CrossRef]
- Lee, J.B.; Mintz, G.S.; Lisauskas, J.B.; Biro, S.G.; Pu, J.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Goldstein, J.; Stone, G.W.; et al. Histopathologic Validation of the Intravascular Ultrasound Diagnosis of Calcified Coronary Artery Nodules. Am. J. Cardiol. 2011, 108, 1547–1551. [Google Scholar] [CrossRef]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients with ST-Segment Elevation Myocardial Infarction Incidence, Morphologic Characteristics, and Outcomes after Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar] [CrossRef]
- Wang, L.; Parodi, G.; Maehara, A.; Valenti, R.; Migliorini, A.; Vergara, R.; Carrabba, N.; Mintz, G.S.; Antoniucci, D. Variable Underlying Morphology of Culprit Plaques Associated with ST-Elevation Myocardial Infarction: An Optical Coherence Tomography Analysis from the SMART Trial. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1381–1389. [Google Scholar] [CrossRef]
- Kajander, O.A.; Pinilla-Echeverri, N.; Jolly, S.S.; Bhindi, R.; Huhtala, H.; Niemelä, K.; Fung, A.; Vijayaraghavan, R.; Alexopoulos, D.; Sheth, T. Culprit Plaque Morphology in STEMI - an Optical Coherence Tomography Study: Insights from the TOTAL-OCT Substudy. EuroIntervention 2016, 12, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.I.; Didier, R.; Ota, H.; Magalhaes, M.A.; Popma, C.J.; Kollmer, M.R.; Spad, M.A.; Torguson, R.; Suddath, W.; Satler, L.F.; et al. Role of Near-Infrared Spectroscopy in Intravascular Coronary Imaging. Cardiovasc. Revasc. Med. 2015, 16, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Madder, R.D.; Goldstein, J.A.; Madden, S.P.; Puri, R.; Wolski, K.; Hendricks, M.; Sum, S.T.; Kini, A.; Sharma, S.; Rizik, D.; et al. Detection by Near-Infrared Spectroscopy of Large Lipid Core Plaques at Culprit Sites in Patients with Acute St-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2013, 6, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Ten Cate, T.; Powers, E.; Kim, C.; et al. Identification of Patients and Plaques Vulnerable to Future Coronary Events with Near-Infrared Spectroscopy Intravascular Ultrasound Imaging: A Prospective, Cohort Study. Lancet 2019, 394, 1629–1637. [Google Scholar] [CrossRef]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. Cardiovasc. Imaging 2021, 14, 1440–1450. [Google Scholar] [CrossRef]
- Demuyakor, A.; Hu, S.; Koniaeva, E.; Liu, M.; Weng, Z.; Zhao, C.; Feng, X.; He, L.; Xu, Y.; Zeng, M.; et al. Impact of Nodular Calcification in Patients with Acute Coronary Syndrome (ACS) Treated with Primary Percutaneous Coronary Intervention (PCI). BMC Cardiovasc. Disord. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Morofuji, T.; Kuramitsu, S.; Shinozaki, T.; Jinnouchi, H.; Sonoda, S.; Domei, T.; Hyodo, M.; Shirai, S.; Ando, K. Clinical Impact of Calcified Nodule in Patients with Heavily Calcified Lesions Requiring Rotational Atherectomy. Catheter. Cardiovasc. Interv. 2021, 97, 10–19. [Google Scholar] [CrossRef]
- Hemetsberger, R.; Abdelghani, M.; Toelg, R.; Mankerious, N.; Allali, A.; Garcia-Garcia, H.M.; Windecker, S.; Lefèvre, T.; Saito, S.; Kandzari, D.; et al. Impact of Coronary Calcification on Clinical Outcomes after Implantation of Newer-generation Drug-eluting Stents. J. Am. Heart Assoc. 2021, 10, 19815. [Google Scholar] [CrossRef]
- Watanabe, M.; Iwai, S.; Okamura, A.; Kyodo, A.; Nogi, K.; Kamon, D.; Hashimoto, Y.; Ueda, T.; Soeda, T.; Okura, H.; et al. Prognostic Impact of Calcified Plaque Morphology After Drug Eluting Stent Implantation―An Optical Coherence Tomography Study―An Optical Coherence Tomography Study. Circ. J. 2021, 85, 2019–2028. [Google Scholar] [CrossRef]
- Nozoe, M.; Nishioka, S.; Oi, K.; Reports, N.S.-C. Effects of Patient Background and Treatment Strategy on Clinical Outcomes after Coronary Intervention for Calcified Nodule Lesions. Circ. Rep. 2021, 3, 699–706. [Google Scholar] [CrossRef]
- Mori, H.; Finn, A.V.; Atkinson, J.B.; Lutter, C.; Narula, J.; Virmani, R. Calcified Nodule: An Early and Late Cause of In-Stent Failure. JACC Cardiovasc. Interv. 2016, 9, e125–e126. [Google Scholar] [CrossRef]
- Sagris, M.; Apostolos, A.; Theofilis, P.; Ktenopoulos, N.; Katsaros, O.; Tsalamandris, S.; Tsioufis, K.; Toutouzas, K.; Tousoulis, D. Myocardial Ischemia–Reperfusion Injury: Unraveling Pathophysiology, Clinical Manifestations, and Emerging Prevention Strategies. Biomedicines 2024, 12, 802. [Google Scholar] [CrossRef]
- Sato, T.; Matsumura, M.; Yamamoto, K.; Shlofmitz, E.; Moses, J.W.; Khalique, O.K.; Thomas, S.V.; Tsoulios, A.; Cohen, D.J.; Mintz, G.S.; et al. Impact of Eruptive vs Noneruptive Calcified Nodule Morphology on Acute and Long-Term Outcomes After Stenting. JACC Cardiovasc. Interv. 2023, 16, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Yin, Y.; Liu, X.; Fang, C.; Jiang, S.; Xu, X.; Sun, S.; Pei, X.; Jia, R.; Tang, C.; et al. Clinical Outcomes of Different Calcified Culprit Plaques in Patients with Acute Coronary Syndrome. J. Clin. Med. 2022, 11, 4018. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.; Huang, H.; Wang, L.; Liu, Z.; Cai, J.; Huang, H. Calcified Nodules in Non-Culprit Lesions with Acute Coronary Syndrome Patients. Rev. Cardiovasc. Med. 2024, 25, 136. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, K.; Yoshida, K.; Yoshidomi, Y.; Koga, Y.; Amari, K.; Tokunou, T.; Sadamatsu, K.; Yoshida, K.; Yoshidomi, Y.; Koga, Y.; et al. Comparison of Pre-Dilation with a Non-Compliant Balloon versus a Dual Wire Scoring Balloon for Coronary Stenting. World J. Cardiovasc. Dis. 2013, 3, 395–400. [Google Scholar] [CrossRef]
- Hoffmann, R.; Mintz, G.S.; Popma, J.J.; Satler, L.F.; Kent, K.M.; Pichard, A.D.; Leon, M.B. Treatment of Calcified Coronary Lesions with Palmaz-Schatz Stents. An Intravascular Ultrasound Study. Eur. Heart J. 1998, 19, 1224–1231. [Google Scholar] [CrossRef]
- Felekos, I.; Karamasis, G.V.; Pavlidis, A.N. When Everything Else Fails: High-Pressure Balloon for Undilatable Lesions. Cardiovasc. Revasc. Med. 2018, 19, 306–313. [Google Scholar] [CrossRef]
- Raja, Y.; Routledge, H.C.; Doshi, S.N. A Noncompliant, High Pressure Balloon to Manage Undilatable Coronary Lesions. Catheter. Cardiovasc. Interv. 2010, 75, 1067–1073. [Google Scholar] [CrossRef]
- Diaz, J.F.; Gómez-Menchero, A.; Cardenal, R.; Sánchez-González, C.; Sanghvi, A. Extremely High-Pressure Dilation with a New Noncompliant Balloon. Tex. Heart Inst. J. 2012, 39, 635. [Google Scholar]
- Secco, G.G.; Buettner, A.; Parisi, R.; Pistis, G.; Vercellino, M.; Audo, A.; Kambis, M.; Garbo, R.; Porto, I.; Tarantini, G.; et al. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc. Revasc. Med. 2019, 20, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’Ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very High-Pressure Dilatation for Undilatable Coronary Lesions: Indications and Results with a New Dedicated Balloon. EuroIntervention 2016, 12, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Caiazzo, G.; Kilic, I.D.; Serdoz, R.; Secco, G.G.; Sinagra, G.; Lee, R.; Foin, N.; Di Mario, C. Is High Pressure Postdilation Safe in Bioresorbable Vascular Scaffolds? Optical Coherence Tomography Observations after Noncompliant Balloons Inflated at More than 24 Atmospheres. Catheter. Cardiovasc. Interv. 2016, 87, 839–846. [Google Scholar] [CrossRef]
- Okura, H.; Hayase, M.; Shimodozono, S.; Kobayashi, T.; Sano, K.; Matsushita, T.; Kondo, T.; Kijima, M.; Nishikawa, H.; Kurogane, H.; et al. Mechanisms of Acute Lumen Gain Following Cutting Balloon Angioplasty in Calcified and Noncalcified Lesions: An Intravascular Ultrasound Study. Catheter. Cardiovasc. Interv. 2002, 57, 429–436. [Google Scholar] [CrossRef]
- Albiero, R.; Silber, S.; Di Mario, C.; Cernigliaro, C.; Battaglia, S.; Reimers, B.; Frasheri, A.; Klauss, V.; Auge, J.M.; Rubartelli, P.; et al. Cutting Balloon versus Conventional Balloon Angioplasty for the Treatment of In-Stent Restenosis: Results of the Restenosis Cutting Balloon Evaluation Trial (RESCUT). J. Am. Coll Cardiol. 2004, 43, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Yamaguchi, T.; Suzuki, T.; Nakamura, M.; Kitayama, M.; Nishikawa, H.; Inoue, T.; Kara, K.; Usuba, F.; Sakurada, M.; et al. Impact of Cutting Balloon Angioplasty (CBA) Prior to Bare Metal Stenting on Restenosis. Circ. J. 2007, 71, 1–8. [Google Scholar] [CrossRef]
- Karvouni, E.; Stankovic, G.; Albiero, R.; Takagi, T.; Corvaja, N.; Vaghetti, M.; Di Mario, C.; Colombo, A. Cutting Balloon Angioplasty for Treatment of Calcified Coronary Lesions. Catheter. Cardiovasc. Interv. 2001, 54, 473–481. [Google Scholar] [CrossRef]
- Tang, Z.; Bai, J.; Su, S.P.; Wang, Y.; Liu, M.H.; Bai, Q.C.; Tian, J.W.; Xue, Q.; Gao, L.; An, C.X.; et al. Cutting-Balloon Angioplasty before Drug-Eluting Stent Implantation for the Treatment of Severely Calcified Coronary Lesions. J. Geriatr. Cardiol. 2014, 11, 44. [Google Scholar]
- Mauri, L.; Bonan, R.; Weiner, B.H.; Legrand, V.; Bassand, J.P.; Popma, J.J.; Niemyski, P.; Prpic, R.; Ho, K.K.L.; Chauhan, M.S.; et al. Cutting Balloon Angioplasty for the Prevention of Restenosis: Results of the Cutting Balloon Global Randomized Trial. Am. J. Cardiol. 2002, 90, 1079–1083. [Google Scholar] [CrossRef]
- Ishihara, T.; Iida, O.; Takahara, M.; Tsujimura, T.; Okuno, S.; Kurata, N.; Asai, M.; Okamoto, S.; Nanto, K.; Mano, T. Improved Crossability with Novel Cutting Balloon versus Scoring Balloon in the Treatment of Calcified Lesion. Cardiovasc. Interv. Ther. 2021, 36, 198–207. [Google Scholar] [CrossRef]
- Takano, M.; Yamamoto, M.; Murakami, D.; Takano, H.; Asai, K.; Yasutake, M.; Seino, Y.; Mizuno, K. Optical Coherence Tomography after New Scoring Balloon Angioplasty for In-Stent Restenosis and de Novo Coronary Lesions. Int. J. Cardiol. 2010, 141, e51-3. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; R, C., Jr.; Abizaid, A.; Feres, F.; Abizaid, A.S.; Costa, R.; Staico, R.; Mattos, L.A.; Sousa, A.G.; Grube, E.; et al. Intravascular Ultrasound Assessment of the Novel AngioSculpt Scoring Balloon Catheter for the Treatment of Complex Coronary Lesions. J. Invasive Cardiol. 2008, 20, 21–27. [Google Scholar] [PubMed]
- Kawase, Y.; Saito, N.; Watanabe, S.; Bao, B.; Yamamoto, E.; Watanabe, H.; Higami, H.; Matsuo, H.; Ueno, K.; Kimura, T. Utility of a Scoring Balloon for a Severely Calcified Lesion: Bench Test and Finite Element Analysis. Cardiovasc. Interv. Ther. 2014, 29, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Koyama, T.; Imoto, Y.; Katsuki, Y.; Kawahara, M.; Nakamura, K.; Kodama, S.; Noguchi, H.; Iwasaki, K. Prolonged Inflation Technique Using a Scoring Balloon for Severe Calcified Lesion. Int. Heart J. 2017, 58, 982–987. [Google Scholar] [CrossRef]
- Jujo, K.; Saito, K.; Ishida, I.; Kim, A.; Suzuki, Y.; Furuki, Y.; Ouchi, T.; Ishii, Y.; Sekiguchi, H.; Yamaguchi, J.; et al. Intimal Disruption Affects Drug-Eluting Cobalt-Chromium Stent Expansion: A Randomized Trial Comparing Scoring and Conventional Balloon Predilation. Int. J. Cardiol. 2016, 221, 23–31. [Google Scholar] [CrossRef]
- Ashida, K.; Hayase, T.; Shinmura, T. Efficacy of Lacrosse NSE Using the “Leopard-Crawl” Technique on Severely Calcified Lesions. J. Invasive Cardiol. 2013, 25, 555–564. [Google Scholar]
- Sugawara, Y.; Ueda, T.; Soeda, T.; Watanabe, M.; Okura, H.; Saito, Y. Plaque Modification of Severely Calcified Coronary Lesions by Scoring Balloon Angioplasty Using Lacrosse Non-Slip Element: Insights from an Optical Coherence Tomography Evaluation. Cardiovasc. Interv. Ther. 2019, 34, 242–248. [Google Scholar] [CrossRef]
- Farb, A.; Roberts, D.K.; Pichard, A.D.; Kent, K.M.; Virmani, R. Coronary Artery Morphologic Features after Coronary Rotational Atherectomy: Insights into Mechanisms of Lumen Enlargement and Embolization. Am. Heart J. 1995, 129, 1058–1067. [Google Scholar] [CrossRef]
- Writing Committee Members; Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 197–215. [Google Scholar] [CrossRef]
- Reifart, N.; Vandormael, M.; Krajcar, M.; Göhring, S.; Preusler, W.; Schwarz, F.; Störger, H.; Hofmann, M.; Klöpper, J.; Müller, S.; et al. Randomized Comparison of Angioplasty of Complex Coronary Lesions at a Single Center. Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) Study. Circulation 1997, 96, 91–98. [Google Scholar] [CrossRef]
- Dill, T.; Dietz, U.; Hamm, C.W.; Küchler, R.; Rupprecht, H.J.; Haude, M.; Cyran, J.; Özbek, C.; Kuck, K.H.; Berger, J.; et al. A Randomized Comparison of Balloon Angioplasty versus Rotational Atherectomy in Complex Coronary Lesions (COBRA Study). Eur. Heart J. 2000, 21, 1759–1766. [Google Scholar] [CrossRef]
- Mauri, L.; Reisman, M.; Buchbinder, M.; Popma, J.J.; Sharma, S.K.; Cutlip, D.E.; Ho, K.K.L.; Prpic, R.; Zimetbaum, P.J.; Kuntz, R.E. Comparison of Rotational Atherectomy with Conventional Balloon Angioplasty in the Prevention of Restenosis of Small Coronary Arteries: Results of the Dilatation vs Ablation Revascularization Trial Targeting Restenosis (DART). Am. Heart J. 2003, 145, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, P.L.; Bass, T.A.; Kipperman, R.M.; Sharaf, B.L.; Ho, K.K.L.; Cutlip, D.E.; Zhang, Y.; Kuntz, R.E.; Williams, D.O.; Lasorda, D.M.; et al. Results of the Study to Determine Rotablator and Transluminal Angioplasty Strategy (STRATAS). Am. J. Cardiol. 2001, 87, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D.; Feldman, T.; Muller, D.W.M.; Mason, D.; Schreiber, T.; Haik, B.; Mooney, M.; O’Neill, W.W. Coronary Angioplasty and Rotablator Atherectomy Trial (CARAT): Immediate and Late Results of a Prospective Multicenter Randomized Trial. Catheter. Cardiovasc. Interv. 2001, 53, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kini, A.; Mehran, R.; Lansky, A.; Kobayashi, Y.; Marmur, J.D. Randomized Trial of Rotational Atherectomy Versus Balloon Angioplasty for Diffuse In-Stent Restenosis (ROSTER). Am. Heart J. 2004, 147, 16–22. [Google Scholar] [CrossRef]
- Vom Dahl, J.; Dietz, U.; Haager, P.K.; Silber, S.; Niccoli, L.; Buettner, H.J.; Schiele, F.; Thomas, M.; Commeau, P.; Ramsdale, D.R.; et al. Rotational Atherectomy Does Not Reduce Recurrent In-Stent Restenosis: Results of the Angioplasty versus Rotational Atherectomy for Treatment of Diffuse in-Stent Restenosis Trial (ARTIST). Circulation 2002, 105, 583–588. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Seguchi, M.; Tsukui, T.; Jinnouchi, H.; Wada, H.; Momomura, S.; Fujita, H. Comparison of Clinical Outcomes of Intravascular Ultrasound-Calcified Nodule between Percutaneous Coronary Intervention with versus without Rotational Atherectomy in a Propensity-Score Matched Analysis. PLoS ONE 2020, 15, e0241836. [Google Scholar] [CrossRef]
- Cockburn, J.; Hildick-Smith, D.; Cotton, J.; Doshi, S.; Hanratty, C.; Ludman, P.; Robinson, D.; Redwood, S.; De Belder, M.; De Belder, A. Contemporary Clinical Outcomes of Patients Treated with or without Rotational Coronary Atherectomy--an Analysis of the UK Central Cardiac Audit Database. Int. J. Cardiol. 2014, 170, 381–387. [Google Scholar] [CrossRef]
- Tomey, M.I.; Kini, A.S.; Sharma, S.K. Current Status of Rotational Atherectomy. JACC Cardiovasc. Interv. 2014, 7, 345–353. [Google Scholar] [CrossRef]
- Barbato, E.; Carrié, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European Expert Consensus on Rotational Atherectomy. EuroIntervention 2015, 11, 30–36. [Google Scholar] [CrossRef]
- Isogai, T.; Yasunaga, H.; Matsui, H.; Tanaka, H.; Fushimi, K. Relationship between Hospital Volume and Major Cardiac Complications of Rotational Atherectomy: A Nationwide Retrospective Cohort Study in Japan. J. Cardiol. 2016, 67, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Sulimov, D.S.; Abdel-Wahab, M.; Toelg, R.; Kassner, G.; Geist, V.; Richardt, G. Stuck Rotablator: The Nightmare of Rotational Atherectomy. EuroIntervention 2013, 9, 251–258. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Richardt, G.; Joachim Büttner, H.; Toelg, R.; Geist, V.; Meinertz, T.; Schofer, J.; King, L.; Neumann, F.J.; Khattab, A.A. High-Speed Rotational Atherectomy before Paclitaxel-Eluting Stent Implantation in Complex Calcified Coronary Lesions: The Randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) Trial. JACC Cardiovasc. Interv. 2013, 6, 10–19. [Google Scholar] [CrossRef]
- Lee, M.S.; Gordin, J.S.; Stone, G.W.; Sharma, S.K.; Saito, S.; Mahmud, E.; Chambers, J.; Généreux, P.; Shlofmitz, R. Orbital and Rotational Atherectomy during Percutaneous Coronary Intervention for Coronary Artery Calcification. Catheter. Cardiovasc. Interv. 2018, 92, 61–67. [Google Scholar] [CrossRef]
- Yamamoto, M.H.; Maehara, A.; Karimi Galougahi, K.; Mintz, G.S.; Parviz, Y.; Kim, S.S.; Koyama, K.; Amemiya, K.; Kim, S.Y.; Ishida, M.; et al. Mechanisms of Orbital Versus Rotational Atherectomy Plaque Modification in Severely Calcified Lesions Assessed by Optical Coherence Tomography. JACC Cardiovasc. Interv. 2017, 10, 2584–2586. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Toelg, R.; Byrne, R.A.; Geist, V.; El-Mawardy, M.; Allali, A.; Rheude, T.; Robinson, D.R.; Abdelghani, M.; Sulimov, D.S.; et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2018, 11, e007415. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Mahmoudi, M.; Lhermusier, T.; Kiramijyan, S.; Ota, H.; Chen, F.; Torguson, R.; Suddath, W.O.; Satler, L.F.; Pichard, A.D.; et al. Comparison of Rotational Atherectomy, Plain Old Balloon Angioplasty, and Cutting-Balloon Angioplasty Prior to Drug-Eluting Stent Implantation for the Treatment of Heavily Calcified Coronary Lesions. J. Invasive Cardiol. 2015, 27, 387–391. [Google Scholar]
- Beohar, N.; Kaltenbach, L.A.; Wojdyla, D.; Pineda, A.M.; Rao, S.V.; Stone, G.W.; Leon, M.B.; Sanghvi, K.A.; Moses, J.W.; Kirtane, A.J. Trends in Usage and Clinical Outcomes of Coronary Atherectomy: A Report from the National Cardiovascular Data Registry CathPCI Registry. Circ. Cardiovasc. Interv. 2020, 13, E008239. [Google Scholar] [CrossRef]
- Généreux, P.; Lee, A.C.; Kim, C.Y.; Lee, M.; Shlofmitz, R.; Moses, J.W.; Stone, G.W.; Chambers, J.W. Orbital Atherectomy for Treating De Novo Severely Calcified Coronary Narrowing (1-Year Results from the Pivotal ORBIT II Trial). Am. J. Cardiol. 2015, 115, 1685–1690. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Shlofmitz, R.; Lee, M.S. Orbital Atherectomy: A Comprehensive Review. Interv. Cardiol. Clin. 2019, 8, 161–171. [Google Scholar] [CrossRef]
- Meraj, P.M.; Shlofmitz, E.; Kaplan, B.; Jauhar, R.; Doshi, R. Clinical Outcomes of Atherectomy Prior to Percutaneous Coronary Intervention: A Comparison of Outcomes Following Rotational versus Orbital Atherectomy (COAP-PCI Study). J. Interv. Cardiol. 2018, 31, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Kirtane, A.J.; Kandzari, D.E.; Armstrong, E.J.; Krucoff, M.W.; Redfors, B.; Ben-Yehuda, O.; Lerew, D.R.; Ali, Z.A.; Maehara, A.; et al. Randomized Evaluation of Vessel Preparation with Orbital Atherectomy Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Artery Lesions: Design and Rationale of the ECLIPSE Trial. Am. Heart J. 2022, 249, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Chandra, P.; Choksi, N.; Khanna, P.; Chambers, J. Safety and Feasibility of Orbital Atherectomy for the Treatment of Calcified Coronary Lesions: The ORBIT I Trial. Catheter. Cardiovasc. Interv. 2013, 81, 1134–1139. [Google Scholar] [CrossRef]
- Lee, M.; Généreux, P.; Shlofmitz, R.; Phillipson, D.; Anose, B.M.; Martinsen, B.J.; Himmelstein, S.I.; Chambers, J.W. Orbital Atherectomy for Treating de Novo, Severely Calcified Coronary Lesions: 3-Year Results of the Pivotal ORBIT II Trial. Cardiovasc. Revasc. Med. 2017, 18, 261–264. [Google Scholar] [CrossRef]
- Bhatt, P.; Parikh, P.; Patel, A.; Chag, M.; Chandarana, A.; Parikh, R.; Parikh, K. Long-Term Safety and Performance of the Orbital Atherectomy System for Treating Calcified Coronary Artery Lesions: 5-Year Follow-up in the ORBIT I Trial. Cardiovasc. Revasc. Med. 2015, 16, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Feldman, R.L.; Himmelstein, S.I.; Bhatheja, R.; Villa, A.E.; Strickman, N.E.; Shlofmitz, R.A.; Dulas, D.D.; Arab, D.; Khanna, P.K.; et al. Pivotal Trial to Evaluate the Safety and Efficacy of the Orbital Atherectomy System in Treating de Novo, Severely Calcified Coronary Lesions (ORBIT II). JACC Cardiovasc. Interv. 2014, 7, 510–518. [Google Scholar] [CrossRef]
- Kini, A.S.; Vengrenyuk, Y.; Pena, J.; Motoyama, S.; Feig, J.E.; Meelu, O.A.; Rajamanickam, A.; Bhat, A.M.; Panwar, S.; Baber, U.; et al. Optical Coherence Tomography Assessment of the Mechanistic Effects of Rotational and Orbital Atherectomy in Severely Calcified Coronary Lesions. Catheter. Cardiovasc. Interv. 2015, 86, 1024–1032. [Google Scholar] [CrossRef]
- Karimi Galougahi, K.; Patel, S.; Shlofmitz, R.A.; Maehara, A.; Kereiakes, D.J.; Hill, J.M.; Stone, G.W.; Ali, Z.A. Calcific Plaque Modification by Acoustic Shock Waves: Intravascular Lithotripsy in Coronary Interventions. Circ. Cardiovasc. Interv. 2021, 14, E009354. [Google Scholar] [CrossRef]
- Ali, Z.A.; Brinton, T.J.; Hill, J.M.; Maehara, A.; Matsumura, M.; Karimi Galougahi, K.; Illindala, U.; Götberg, M.; Whitbourn, R.; Van Mieghem, N.; et al. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions: First Description. JACC Cardiovasc. Imaging 2017, 10, 897–906. [Google Scholar] [CrossRef]
- Brinton, T.J.; Ali, Z.A.; Hill, J.M.; Meredith, I.T.; Maehara, A.; Illindala, U.; Lansky, A.; Götberg, M.; van Mieghem, N.M.; Whitbourn, R.; et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation 2019, 139, 834–836. [Google Scholar] [CrossRef]
- Ali, Z.A.; Nef, H.; Escaned, J.; Werner, N.; Banning, A.P.; Hill, J.M.; De Bruyne, B.; Montorfano, M.; Lefevre, T.; Stone, G.W.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: The Disrupt CAD II Study. Circ. Cardiovasc. Interv. 2019, 12, e008434. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef]
- Saito, S.; Yamazaki, S.; Takahashi, A.; Namiki, A.; Kawasaki, T.; Otsuji, S.; Nakamura, S.; Shibata, Y. Intravascular Lithotripsy for Vessel Preparation in Severely Calcified Coronary Arteries Prior to Stent Placement—Primary Outcomes From the Japanese Disrupt CAD IV Study. Circ. J. 2021, 85, 826–833. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Di Mario, C.; Riley, R.F.; Fajadet, J.; Shlofmitz, R.A.; Saito, S.; Ali, Z.A.; Klein, A.J.; Price, M.J.; Hill, J.M.; et al. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc. Interv. 2021, 14, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Kereiakes, D.J.; Hill, J.M.; Saito, S.; Di Mario, C.; Honton, B.; Gonzalo, N.; Riley, R.F.; Maehara, A.; Matsumura, M.; et al. Impact of Calcium Eccentricity on the Safety and Effectiveness of Coronary Intravascular Lithotripsy: Pooled Analysis From the Disrupt CAD Studies. Circ. Cardiovasc. Interv. 2023, 16, E012898. [Google Scholar] [CrossRef]
- Huang, W.C.; Kuramitsu, S.; Kanno, D.; Kashima, Y.; Lu, T.M.; Fujita, T. Can Intravascular Lithotripsy Compress Noneruptive Calcified Nodules? Cardiovasc. Interv. 2024, 17, 2307–2308. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Escaned, J.; Tirado, G.; Gonzalo, N. Intravascular Lithotripsy for Recurrent Restenosis Caused by Severe Calcific Neoatherosclerosis. EuroIntervention 2020, 16, E351–E352. [Google Scholar] [CrossRef]
- Ali, Z.A.; McEntegart, M.; Hill, J.M.; Spratt, J.C. Intravascular Lithotripsy for Treatment of Stent Underexpansion Secondary to Severe Coronary Calcification. Eur. Heart J. 2020, 41, 485–486. [Google Scholar] [CrossRef]
- Ali, Z.A.; Kereiakes, D.; Hill, J.; Saito, S.; Di Mario, C.; Honton, B.; Gonzalo, N.; Riley, R.; Maehara, A.; Matsumura, M.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Calcified Nodules. JACC Cardiovasc. Interv. 2023, 16, 1122–1124. [Google Scholar] [CrossRef]
- Blachutzik, F.; Honton, B.; Escaned, J.; Hill, J.M.; Werner, N.; Banning, A.P.; Lansky, A.J.; Schlattner, S.; De Bruyne, B.; Di Mario, C.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy in Eccentric Calcified Coronary Lesions: A Patient-Level Pooled Analysis from the Disrupt CAD I and CAD II Studies. Clin. Res. Cardiol. 2021, 110, 228–236. [Google Scholar] [CrossRef]
- Shlofmitz, R.A.; Saito, S.; Honton, B.; Riley, R.F.; Hill, J.; Ali, Z.A.; Maehara, A.; Stone, G.W.; Kereiakes, D.J. CRT-100.36 Impact of Calcified Nodules on 2-Year Clinical Outcomes After IVL-Assisted Coronary Stenting: Pooled Analysis From the DISRUPT CAD OCT Sub-Studies. Cardiovasc. Interv. 2023, 16, S1. [Google Scholar] [CrossRef]
- Köster, R.; Kähler, J.; Brockhoff, C.; Münzel, T.; Meinertz, T. Laser Coronary Angioplasty: History, Present and Future. Am. J. Cardiovasc. Drugs 2002, 2, 197–207. [Google Scholar] [CrossRef]
- Mintz, G.S.; Kovach, J.A.; Javier, S.P.; Pichard, A.D.; Kent, K.M.; Popma, J.J.; Salter, L.F.; Leon, M.B. Mechanisms of Lumen Enlargement after Excimer Laser Coronary Angioplasty. An Intravascular Ultrasound Study. Circulation 1995, 92, 3408–3414. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.P.; Hobson, A.R.; McKenzie, D.; Shah, N.; Sinha, M.K.; Wells, T.A.; Levy, T.M.; Swallow, R.A.; Talwar, S.; O’Kane, P.D. Beyond the Balloon: Excimer Coronary Laser Atherectomy Used Alone or in Combination with Rotational Atherectomy in the Treatment of Chronic Total Occlusions, Non-Crossable and Non-Expansible Coronary Lesions. EuroIntervention 2013, 9, 243–250. [Google Scholar] [CrossRef]
- Mangieri, A.; Jabbour, R.J.; Tanaka, A.; Aurelio, A.; Colombo, A.; Latib, A. Excimer Laser Facilitated Coronary Angioplasty of a Heavy Calcified Lesion Treated with Bioresorbable Scaffolds. J. Cardiovasc. Med. 2016, 17 (Suppl. S2), e149–e150. [Google Scholar] [CrossRef]
- Azzalini, L.; Ly, H.Q. Laser Atherectomy for Balloon Failure in Chronic Total Occlusion. When the Going Gets Tough. Int. Heart J. 2014, 55, 546–549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niccoli, G.; Di Vito, L.; Montone, R.A.; Porto, I.; Crea, F. Excimer Laser for a Highly Stenotic Saphenous Vein Graft: Evidence of Debulking by Optical Coherence Tomography. EuroIntervention 2014, 9, 1484. [Google Scholar] [CrossRef]
- Mohandes, M.; Rojas, S.; Torres, M.; Moreno, C.; Fernández, F.; Guarinos, J.; Bardají, A. Percutaneous Coronary Intervention of Chronically Occluded Saphenous Vein Grafts Using Excimer Laser Atherectomy as an Adjuvant Therapy. Cardiovasc. Revasc. Med. 2017, 18, 2–6. [Google Scholar] [CrossRef]
- Mohandes, M.; Rojas, S.; Moreno, C.; Fernández, F.; Fuertes, M.; Guarinos, J. Excimer Laser in Percutaneous Coronary Intervention of Device Uncrossable Chronic Total and Functional Occlusions. Cardiovasc. Revasc. Med. 2020, 21, 657–660. [Google Scholar] [CrossRef]
- Lee, T.; Shlofmitz, R.A.; Song, L.; Tsiamtsiouris, T.; Pappas, T.W.; Madrid, A.; Jeremias, A.; Haag, E.S.; Ali, Z.A.; Moses, J.W.; et al. The Effectiveness of Excimer Laser Angioplasty to Treat Coronary In-Stent Restenosis with Peri-Stent Calcium as Assessed by Optical Coherence Tomography. EuroIntervention 2019, 15, E279–E288. [Google Scholar] [CrossRef]
- Latib, A.; Takagi, K.; Chizzola, G.; Tobis, J.; Ambrosini, V.; Niccoli, G.; Sardella, G.; DiSalvo, M.E.; Armigliato, P.; Valgimigli, M.; et al. Excimer Laser LEsion Modification to Expand Non-Dilatable Stents: The ELLEMENT Registry. Cardiovasc. Revasc. Med. 2014, 15, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, J.; Armstrong, E.; Truong, H.T.; Parachini, J.M.; Alame, A.; Danek, B.; Karatasakis, A.; Nguyen-Trong, P.-K.; Iwnetu, R.; Resendes, E.; et al. Contemporary Use of Laser During Percutaneous Coronary Intervention: Results from the Laser Veterans Affairs (Lava) Multicenter Registry. J. Am. Coll Cardiol. 2017, 69, 1115. [Google Scholar] [CrossRef]
| Technique | Mechanism of Action | Key Evidence | Advantages | Limitations/Complications | Clinical Use |
|---|---|---|---|---|---|
| Non-Compliant Balloons | High-pressure inflation reshapes calcified plaques. | Effective for mild/moderate calcifications but limited for severe calcifications. | Safe for mild calcifications; low cost. | Dissection and perforation risks at high pressure. | Predilation before stenting in mildly calcified lesions. |
| High-Pressure Balloons | Twin-layer technology withstands pressures up to 35 atm. | A 90% success rate in non-dilatable lesions; rare coronary rupture risk. | Effective where NC balloons fail. | Limited data; potential coronary rupture. | Treating resistant calcified lesions and optimizing stent expansion. |
| Cutting Balloons | Blades incise calcified plaque to aid dilation. | Larger lumen gain; 0.8% risk of perforation. | Precise, focused luminal gain. | Increased risk of perforation and device entrapment. | Focal calcifications, ostial lesions, and in-stent restenosis (ISR). |
| Scoring Balloons | Scoring elements concentrate force to fracture calcifications. | Safer alternative to cutting balloons; proven efficacy in ISR. | Lower dissection risk than cutting balloons. | Not effective for dense calcifications. | Moderate calcifications or ISR; safer luminal gain in eccentric nodules. |
| Intravascular Lithotripsy (IVL) | Acoustic waves fracture calcium without damaging soft tissue. | A 92.4% procedural success rate with minimal complications. | Uniform energy delivery; minimal embolization risk. | Limited deliverability in tortuous vessels. | Severe or eccentric calcifications; adjunct to atherectomy for resistant CNs. |
| Rotational Atherectomy | Diamond-tipped burr ablates calcifications, reducing rigidity. | Gold standard for severe calcifications; PREPARE-CALC trial confirms procedural success. | Effective for deep, dense calcifications. | Risk of slow/no-reflow events; operator dependent. | Severe calcifications resistant to balloon angioplasty; may facilitate device delivery in CNs. |
| Orbital Atherectomy | Elliptical crown ablates calcifications while sparing pliable tissue. | Comparable safety and efficacy to RA; no large RCTs yet. | Lower thermal injury risk than RA. | Requires further evidence; risk of distal embolization. | Treating deep, eccentric, or superficial calcium; better modification of CNs. |
| Laser Atherectomy | UV laser photoablates plaque by vaporizing water and breaking carbon bonds. | LAVA registry shows 90% technical success in undilatable ISR. | Effective for ISR and chronic occlusions. | Niche application; requires specialized equipment. | Balloon-uncrossable or undilatable lesions and ISR. |
| Category | Key Features | Clinical Implications | Diagnostic Approaches | Therapeutic Strategies |
|---|---|---|---|---|
| Pathophysiology |
|
|
|
|
| Isolated Calcified Nodules |
|
|
|
|
| Calcified Nodules with Plaque Rupture or Erosion |
|
|
|
|
| Therapeutic Challenges |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsaros, O.; Sagris, M.; Karakasis, P.; Ktenopoulos, N.; Soulaidopoulos, S.; Theofilis, P.; Apostolos, A.; Tzoumas, A.; Patsourakos, N.; Toutouzas, K.; et al. The Role of Calcified Nodules in Acute Coronary Syndrome: Diagnosis and Management. Int. J. Mol. Sci. 2025, 26, 2581. https://doi.org/10.3390/ijms26062581
Katsaros O, Sagris M, Karakasis P, Ktenopoulos N, Soulaidopoulos S, Theofilis P, Apostolos A, Tzoumas A, Patsourakos N, Toutouzas K, et al. The Role of Calcified Nodules in Acute Coronary Syndrome: Diagnosis and Management. International Journal of Molecular Sciences. 2025; 26(6):2581. https://doi.org/10.3390/ijms26062581
Chicago/Turabian StyleKatsaros, Odysseas, Marios Sagris, Paschalis Karakasis, Nikolaos Ktenopoulos, Stergios Soulaidopoulos, Panagiotis Theofilis, Anastasios Apostolos, Andreas Tzoumas, Nikolaos Patsourakos, Konstantinos Toutouzas, and et al. 2025. "The Role of Calcified Nodules in Acute Coronary Syndrome: Diagnosis and Management" International Journal of Molecular Sciences 26, no. 6: 2581. https://doi.org/10.3390/ijms26062581
APA StyleKatsaros, O., Sagris, M., Karakasis, P., Ktenopoulos, N., Soulaidopoulos, S., Theofilis, P., Apostolos, A., Tzoumas, A., Patsourakos, N., Toutouzas, K., Tsioufis, K., & Tousoulis, D. (2025). The Role of Calcified Nodules in Acute Coronary Syndrome: Diagnosis and Management. International Journal of Molecular Sciences, 26(6), 2581. https://doi.org/10.3390/ijms26062581












