In Vitro Studies of the Effect of Oil Emulsions from Transgenic Flax Varieties on the Treatment of Wound Healing and Care of Human Skin with the Tendency to Inflammation
Abstract
1. Introduction
2. Results
2.1. Viability Assay
2.2. Proliferation Assay
2.3. Cell Cycle
2.4. Migration Assay
2.5. Apoptotic and Necrotic Cells
2.6. Evaluation of the Intracellular Free Radical Level
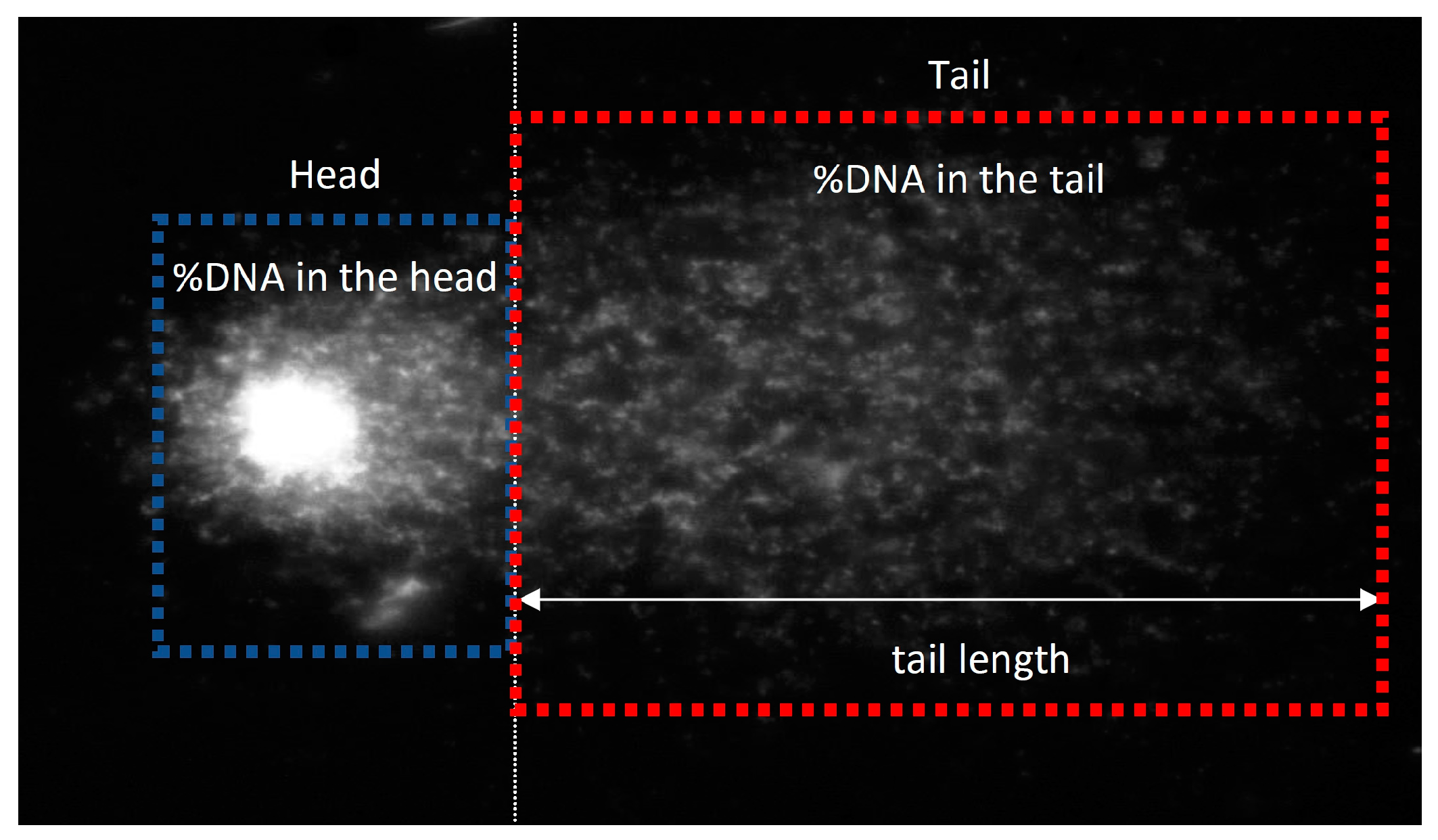
2.7. Genotoxicity Assessment
3. Materials and Methods
3.1. Material
3.2. In Vitro Studies
3.2.1. Cell Line and Conditions
3.2.2. Viability Assay
3.2.3. Proliferation Assay
3.2.4. Cell Cycle
3.2.5. Scratch Assay
3.2.6. Apoptotic and Necrotic Assay
3.2.7. Evaluation of the Intracellular Free Radical Level
3.2.8. Genotoxicity Assessment
3.3. Statistical Analysis
4. Discussion
| Bioactive Compound | Pharmacological Activity | Type of the Study | Study Model | References |
|---|---|---|---|---|
| OA | Wound healing Anti-inflammatory | In vitro | Mouse embryo fibroblasts Balb/c | Cardoso C.R. et al., 2011 [63] |
| In vivo | Balb/c mice | |||
| Wound healing | In vivo | Wistar rats | Pereira L.K.M. et al., 2008 [64] | |
| LA | Wound healing | In vitro | Human Keratinocytes HK, Human Fibroblast HF | Zhao D. et al., 2022 [65] |
| In vivo | Minipigs | |||
| Wound healing | In vivo | Wistar rats | Pereira L.M. et al., 2008 [64] | |
| ALA | Reduce the proliferation and restore the differentiation of psoriatic KCs | In vitro | Psoriatic fibroblasts and KCs from human patients with psoriasis—tissue-engineered reconstructed skin substitutes | Simarad M. et. al. 2021 [73] |
| Anti-inflammatory | In vitro | Psoriatic skin model produced with the addition of activated T cells—biopsis from donors | Morin S. et al., 2022 [74] | |
| Stigmasterol | Antioxidant Anti-inflammatory | In vitro | Human immortalized Keratinocyte cell line HaCaT | Miya G.M. et al., 2023 [81] |
| Anticancer | In vitro | Murine melanoma B16F10, Human melanoma A375 | Han N.R. et al., 2024 [80] | |
| Anti-inflammatory | In vivo | Swiss mice | Milani G.B. et al., 2024 [82] | |
| Chemopreventive skin cancer | In vivo | Swiss albino mice | Ali H. et al., 2015 [79] | |
| β-sitosterol | Antioxidant Anti-inflammatory | In vitro | Human immortal-ized Keratinocyte cell line HaCaT | Miya G.M. et al., 2023 [81] |
| Protective against skin aging | In vitro | Human Dermal Fibroblast HDF, Human immortal-ized Keratinocyte cell line HaCaT | Yo H. et al., 2019 [84] | |
| Anti-inflammatory | In vivo | NC/Nga mice | Han N.R. et al., 2024 [83] | |
| Anti-inflammatory | In vivo | Swiss mice | Milani G.B. et al., 2019 [82] | |
| Induce hair growth in alopecia | In vivo | Wistar rats | Prabahar K. et al., 2023 [85] | |
| Anticancer | In vivo | B16F10 melanoma transplantation to C57BL mice | Iyer D. et al., 2014 [86] | |
| α-Tocopherol | Photoprotection Antioxidant | In vitro | Human immortal-ized Keratinocyte cell line HaCaT | Saleh M.M. et al., 2021 [92] |
| Antioxidant | In vitro | Primary mouse epidermal keratinocytes PMK, Cell line from adult BALB/c mouse skin Normal Mouse Keratinocytes isotated form newborn Balb/c mouse | Maalouf S. et al., 2002 [93] | |
| Antioxidant | In vitro | Human primary fibroblast (HPFs) isolated from perilesional skin of nonmelanoma skin cancer patients_ | Camillo L. et al., 2022 [94] | |
| β-caroten | Antioxidant | In vitro | Normal Human Dermal Fibroblasts FEK4 | Trekli M.C. et al., 2003 [96] |
| Anti-inflammatory | In vivo | Hairless mice HR1 | Kake T. et al., 2019 [97] | |
| Vanillin | Wound healing | In vitro | Human immortal-ized Keratinocyte cell line HaCaT, Primary Skin Fibroblasts | Sinsuebpol C. et al., 2023 [103] |
| Anticancer | In vitro | B16F10 cell line | Pourhadi M. et al., 2022 [105] | |
| In vivo | C57BL6 mice injected murine melanoma B16F10 | |||
| Vanillin acid | Wound healing | In vitro | Human immortal-ized Keratinocyte cell line HaCaT | Zhu X. et al., 2024 [100] |
| Coniferyl aldehyde | Anti-inflammatory | In vitro | Murine macrophages RAW264.7, Human immortal-ized Keratinocyte cell line HaCaT | Akram M. et al 2016 [106] |
| In vivo | Sprague Dawley rats | |||
| p-Coumaric acid | Hypopigmenting | In vitro | Human epidermal melanocytes HEMs | Song K. et al., 2011 [108] |
| Ex vivo | Porcine skin | |||
| Feluric acid | Antioxidant | In vitro | Human immortal-ized Keratinocyte cell line HaCaT | Pluemsamran T. et al., 2012 [110] |
| Antioxidant | In vitro | Normal Human Dermal Fibroblasts NHDF | Hahn H.J. et al 2016 [111] | |
| Antioxidant | In vitro | Human Dermal Fibroblast adult HDFa | Nagarajan R. et al 2014 [112] | |
| Antioxidant | In vitro | Neonatal Normal Human Dermal Fibroblasts HDFn | Calabrese V. et al., 2008 [113] | |
| Wound healing | In vivo | Wistar rats | Ghaisasm M. et al., 2014 [114] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, H.; Wang, M.; Ding, J.; Lin, Y. Hydrogels: A Promising Therapeutic Platform for Inflammatory Skin Diseases Treatment. J. Mater. Chem. B 2024, 12, 8007–8032. [Google Scholar] [CrossRef] [PubMed]
- Nagatake, T.; Kishino, S.; Urano, E.; Murakami, H.; Kitamura, N.; Konishi, K.; Ohno, H.; Tiwari, P.; Morimoto, S.; Node, E.; et al. Intestinal Microbe-Dependent ω3 Lipid Metabolite AKetoA Prevents Inflammatory Diseases in Mice and Cynomolgus Macaques. Mucosal Immunol. 2022, 15, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Skin Diseases: Focus on the Role of Suppressors of Cytokine Signaling (SOCS) Proteins. Cells 2024, 13, 1–20. [Google Scholar] [CrossRef]
- Dissemond, J.; Romanelli, M. Inflammatory Skin Diseases and Wounds. Br. J. Dermatol. 2022, 187, 167–177. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085–2099. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 2018, 9, 1682–1695. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, R.; Tai, Z.; Miao, F.; Shi, X.; Chen, Z.; Zhu, Q. Noval Advance of Histone Modification in Inflammatory Skin Diseases and Related Treatment Methods. Front. Immunol. 2023, 14, 1286776–1286791. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, F.; Meng, X.; Zhou, L. The Associations between Gut Microbiota and Inflammatory Skin Diseases: A Bi-Directional Two-Sample Mendelian Randomization Study. Front. Immunol. 2024, 15, 1297240–1297251. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Rodriguez, E.; Stölzl, D.; Wehkamp, U.; Sun, J.; Gerdes, S.; Sarkar, M.K.; Hübenthal, M.; Zeng, C.; Uppala, R.; et al. Progression of Acute-to-Chronic Atopic Dermatitis Is Associated with Quantitative Rather than Qualitative Changes in Cytokine Responses. J. Allergy Clin. Immunol. 2020, 145, 1406–1415. [Google Scholar] [CrossRef]
- Fox, B.D.; Ashquar, F.; Raviv, Y.; Rozengarten, D.; Straichman, O.; Izhakian, S.; Kramer, M.R. Tacrolimus Levels Are Not Associated with Risk of Malignancy in Lung Transplant Recipients. Ann. Transplant. 2017, 22, 677–681. [Google Scholar] [CrossRef]
- Plötz, S.G.; Wiesender, M.; Todorova, A.; Ring, J. What Is New in Atopic Dermatitis/Eczema? Expert. Opin. Emerg. Drugs 2014, 19, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Yadava, P.; Khan, F.N.; Sharma, S.; Singh, R.; Bana, R.S.; Singh, N.; Kaur, V.; Kalia, S.; Kumar, A. Trends and Advances in Pre- and Post-Harvest Processing of Linseed Oil for Quality Food and Health Products. Crit. Rev. Food Sci. Nutr. 2023, 65, 746–769. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity: Implications for Metabolic Syndrome, Diabetes, Hypertension, Dyslipidemia, Atherosclerosis, and Cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef]
- Naseri, S.; Golpich, M.; Roshancheshm, T.; Joobeni, M.G.; Khodayari, M.; Noori, S.; Zahed, S.A.; Razzaghi, S.; Shirzad, M.; Salavat, F.S.; et al. The Effect of Henna and Linseed Herbal Ointment Blend on Wound Healing in Rats with Second-Degree Burns. Burns 2021, 47, 1442–1450. [Google Scholar] [CrossRef]
- Pointner, T.; Rauh, K.; Auñon-Lopez, A.; Kostadinović Veličkovska, S.; Mitrev, S.; Arsov, E.; Pignitter, M. Comprehensive Analysis of Oxidative Stability and Nutritional Values of Germinated Linseed and Sunflower Seed Oil. Food Chem. 2024, 454, 139790. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Shen, M.; Xie, J.; Yang, J. Evaluation of Trans Fatty Acids, Carbonyl Compounds and Bioactive Minor Components in Commercial Linseed Oils. Food Chem. 2022, 369, 130930–130939. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.M.M.; Abdel-Razek, A.G.; Affifi, S.M.; Qian, Y.; Radziejewska-Kubzdela, E.; Siger, A.; Rudzińska, M.; Abo-Elwafa, G.A.; Grygier, A. Characterization of New Egyptian Linseed Varieties and the Effects of Roasting on Their Pigments, Tocochromanols, Phytosterols, Omega-3 Fatty Acids, and Stability. Molecules 2022, 27, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Influence of Dry Air and Infrared Pre-Treatments on Oxidative Stability, Maillard Reaction Products and Other Chemical Properties of Linseed (Linum usitatissimum L.) Oil. J. Food Sci. Technol. 2022, 59, 366–376. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741–767. [Google Scholar] [CrossRef]
- Saika, A.; Nagatake, T.; Hirata, S.I.; Sawane, K.; Adachi, J.; Abe, Y.; Isoyama, J.; Morimoto, S.; Node, E.; Tiwari, P.; et al. ω3 Fatty Acid Metabolite, 12-Hydroxyeicosapentaenoic Acid, Alleviates Contact Hypersensitivity by Downregulation of CXCL1 and CXCL2 Gene Expression in Keratinocytes via Retinoid X Receptor α. FASEB J. 2021, 35, e21354. [Google Scholar] [CrossRef]
- Horie, Y.; Harauma, A.; Moriguchi, T.; Mitsui, H.; Akase, T. Oral Intake of Linseed Oil Inhibits Skin Barrier Dysfunction in Obese Mice. Cureus 2024, 16, e61392. [Google Scholar] [CrossRef]
- Neukam, K.; De Spirt, S.; Stahl, W.; Bejot, M.; Maurette, J.M.; Tronnier, H.; Heinrich, U. Supplementation of Flaxseed Oil Diminishes Skin Sensitivity and Improves Skin Barrier Function and Condition. Skin. Pharmacol. Physiol. 2011, 24, 67–74. [Google Scholar] [CrossRef]
- Faour, S.; Farahat, M.; Aijaz, A.; Jeschke, M.G. Fibrosis in Burns: An Overview of Mechanisms and Therapies. Am. J. Physiol. Cell Physiol. 2023, 325, C1545–C1557. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wölfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27–48. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Bedelbaeva, K.; Snyder, A.; Gourevitch, D.; Clark, L.; Zhang, X.M.; Leferovich, J.; Cheverud, J.M.; Lieberman, P.; Heber-Katz, E. Lack of P21 Expression Links Cell Cycle Control and Appendage Regeneration in Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 5845–5850. [Google Scholar] [CrossRef]
- Yang, C.; Yin, D.; Zhang, H.; Badea, I.; Yang, S.M.; Zhang, W. Cell Migration Assays and Their Application to Wound Healing Assays—A Critical Review. Micromachines 2024, 15, 720–740. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Mao, J.; Su, Y.; Huang, X.; Xia, W.; Leng, X.; Zan, T. Immunomodulation of Wound Healing Leading to Efferocytosis. Smart Med. 2024, 3, e20230036. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, B.; Qian, S.; Mao, J.; Zhang, L.; Zhang, Y.; Mao, X.; Cui, W.; Sun, X. Biogenerated Oxygen-Related Environmental Stressed Apoptotic Vesicle Targets Endothelial Cells. Adv. Sci. 2024, 11, 2306555. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Recent Advances in Reactive Oxygen Species Scavenging Nanomaterials for Wound Healing. Exploration 2024, 4, 20230066. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.S.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating Skin Regeneration and Wound Healing by Controlled ROS from Photodynamic Treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef]
- Iqbal, U. Infectious Disease Epidemiology: History, Transmission and Prevention. Clin. Case Rep. Trails 2024, 3. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.; Shaposhnikov, M.; Moskalev, A. Genome-Protecting Compounds as Potential Geroprotectors. Int. J. Mol. Sci. 2020, 21, 4484. [Google Scholar] [CrossRef]
- Gębarowski, T.; Wiatrak, B.; Jęśkowiak-Kossakowska, I.; Grajzer, M.; Prescha, A. Oils from Transgenic Flax Lines as Potential Chemopreventive Agents in Colorectal Cancer. Biomedicines 2023, 11, 2592–2615. [Google Scholar] [CrossRef]
- Lorenc-Kukula, K.; Amarowicz, R.; Oszmianski, J.; Doermann, P.; Starzycki, M.; Skala, J.; Zuk, M.; Kulma, A.; Szopa, J. Pleiotropic Effect of Phenolic Compounds Content Increases in Transgenic Flax Plant. J. Agric. Food Chem. 2005, 53, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Skorkowska-Telichowska, K.; Hasiewicz-Derkacz, K.; Gębarowski, T.; Kulma, A.; Moreira, H.; Kostyn, K.; Gębczak, K.; Szyjka, A.; Wojtasik, W.; Gąsiorowski, K. Emulsions Made of Oils from Seeds of GM Flax Protect V79 Cells against Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 7510759–7510772. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Appl. Biochem. Biotechnol. Part B Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The Wound Microbiota: Microbial Mechanisms of Impaired Wound Healing and Infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing Natural Polymers for Skin Wound Healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Rajeshkumar, S. Nanomaterials for Wound Healing: Current Status and Futuristic Frontier. Biomed. Technol. 2024, 6, 26–45. [Google Scholar] [CrossRef]
- Jonidi Shariatzadeh, F.; Currie, S.; Logsetty, S.; Spiwak, R.; Liu, S. Enhancing Wound Healing and Minimizing Scarring: A Comprehensive Review of Nanofiber Technology in Wound Dressings. Prog. Mater. Sci. 2025, 147, 101350. [Google Scholar] [CrossRef]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable Butters and Oils in Skin Wound Healing: Scientific Evidence for New Opportunities in Dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Abioye, R.O.; Okagu, I.U.; Obeme-Nmom, J.I. Bioaccessibility of Bioactive Peptides: Recent Advances and Perspectives. Curr. Opin. Food Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Beroual, K.; Agabou, A.; Bachtarzi, K.; Haouam, S.; Hamdi-Pacha, Y. Safety Assessment of Linum usitatissimum (Linn.) Ingestion in New Zealand Rabbits. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 151–155. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Milajerdi, A.; Reiner, Ž.; Dadgostar, E.; Amirani, E.; Asemi, Z.; Mirsafaei, L.; Mansournia, M.A.; Dana, P.M.; Sadoughi, F.; et al. Effects of Flaxseed Oil Supplementation on Biomarkers of Inflammation and Oxidative Stress in Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2020, 40, 27–33. [Google Scholar] [CrossRef]
- Bardaa, S.; Moalla, D.; Ben Khedir, S.; Rebai, T.; Sahnoun, Z. The Evaluation of the Healing Proprieties of Pumpkin and Linseed Oils on Deep Second-Degree Burns in Rats. Pharm. Biol. 2016, 54, 581–587. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Taghikhani, H.; Habibi, M.; Zandieh, M.A. Wound Healing Activity of Flaxseed Linum usitatissimum L. in Rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2386–2389. [Google Scholar]
- Czemplik, M.; Kulma, A.; Bazela, K.; Szopa, J. The Biomedical Potential of Genetically Modified Flax Seeds Overexpressing the Glucosyltransferase Gene. BMC Complement. Altern. Med. 2012, 12, 251. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Zuk, M.; Kulma, A.; Bugajska-Prusak, A.; Ratajczak, K.; Ga̧siorowski, K.; Kostyn, K.; Szopa, J. New Dressing Materials Derived from Transgenic Flax Products to Treat Long-Standing Venous Ulcers—A Pilot Study. Wound Repair. Regen. 2010, 18, 168–179. [Google Scholar] [CrossRef]
- Gębarowski, T.; Jęśkowiak, I.; Wiatrak, B. Investigation of the Properties of Linen Fibers and Dressings. Int. J. Mol. Sci. 2022, 23, 10480. [Google Scholar] [CrossRef]
- Gębarowski, T.; Wiatrak, B.; Janeczek, M.; Żuk, M.; Pistor, P.; Gąsiorowski, K. Were Our Ancestors Right in Using Flax Dressings? Research on the Properties of Flax Fibre and Its Usefulness in Wound Healing. Oxid. Med. Cell Longev. 2020, 2020, 1682317. [Google Scholar] [CrossRef] [PubMed]
- Skórkowska-Telichowska, K.; Mierziak-Darecka, J.; Wrobel-Kwiatkowska, M.; Gebarowski, T.; Szopa, J.; Zuk, M. Wound Coverage by the Linen Dressing Accelerates Ulcer Healing. Postepy Dermatol. Alergol. 2021, 38, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Sabre, H.M. Review: Medical Benefits of Stearic Acid for Skin and Its Role in Treatment of Some Diseases. Int. Sci. Heal. J. 2024, 2, 82–87. [Google Scholar] [CrossRef]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef]
- Ikeda, K.; Morizane, S.; Akagi, T.; Hiramatsu-Asano, S.; Tachibana, K.; Yahagi, A.; Iseki, M.; Kaneto, H.; Wada, J.; Ishihara, K.; et al. Obesity and Dyslipidemia Synergistically Exacerbate Psoriatic Skin Inflammation. Int. J. Mol. Sci. 2022, 23, 4312–4328. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Song, L. A Review of Fatty Acids Influencing Skin Condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Lovászi, M.; Mattii, M.; Eyerich, K.; Gácsi, A.; Csányi, E.; Kovács, D.; Rühl, R.; Szegedi, A.; Kemény, L.; Ståhle, M.; et al. Sebum Lipids Influence Macrophage Polarization and Activation. Br. J. Dermatol. 2017, 177, 1671–1682. [Google Scholar] [CrossRef]
- Tanojo, H.; Junginger, H.E.; Bodde, H.E. In vivo human skin permeability enhancement by oleic acid: Transepidermal water loss and fourier-transform infrared spectroscopy studies. J. Control. Release 1997, 47, 31–39. [Google Scholar] [CrossRef]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid Functions in Skin: Differential Effects of n-3 Polyunsaturated Fatty Acids on Cutaneous Ceramides, in a Human Skin Organ Culture Model. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1679–1689. [Google Scholar] [CrossRef]
- Giri, V.P.; Pandey, S.; Shukla, P.; Gupta, S.C.; Srivastava, M.; Rao, C.V.; Shukla, S.V.; Dwivedi, A.; Mishra, A. Facile Fabrication of Sandalwood Oil-Based Nanoemulsion to Intensify the Fatty Acid Composition in Burned and Rough Skin. ACS Omega 2024, 9, 6305–6315. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; He, H. The Role of Linoleic Acid in Skin and Hair Health: A Review. Int. J. Mol. Sci. 2025, 26, 246. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Favoreto, S.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic Acid Modulation of the Immune Response in Wound Healing: A New Approach for Skin Repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of Oleic and Linoleic Acids on the Inflammatory Phase of Wound Healing in Rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xiao, J.; Qiang, L.; Deng, X.; An, J.; Zhang, Q.; Zhao, F.; Ma, J.; Fang, C.; Guan, G.; et al. Walnut Ointment Promotes Full-Thickness Burning Wound Healing: Role of Linoleic Acid. Acta Cir. Bras. 2022, 37, e370902–e370914. [Google Scholar] [CrossRef]
- Ruthig, D.J.; Meckling-Gill, K.A. Both (n-3) and (n-6) Fatty Acids Stimulate Wound Healing in the Rat Intestinal Epithelial Cell Line, IEC-6. J. Nutr. 1999, 129, 1791–1798. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, S.; Yang, L.; Jiang, M.; Xin, Y.; Liao, X.; Li, Y.; Lu, J. The Antitumor Effects of α-Linolenic Acid. J. Pers. Med. 2024, 14, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Sun, H.L.; Hsu, Y.H.; Liu, S.H.; Lii, C.K.; Tsai, C.H.; Liu, K.L.; Huang, C.S.; Li, C.C. α-Linolenic Acid Inhibits the Migration of Human Triple-Negative Breast Cancer Cells by Attenuating Twist1 Expression and Suppressing Twist1-Mediated Epithelial-Mesenchymal Transition. Biochem. Pharmacol. 2020, 180, 114152–114165. [Google Scholar] [CrossRef]
- Feng, S.; Xie, X.; Chen, C.; Zuo, S.; Zhao, X.; Li, H. Alpha-Linolenic Acid Inhibits Hepatocellular Carcinoma Cell Growth through Farnesoid X Receptor/β-Catenin Signaling Pathway. Nutr. Metab. 2022, 19, 57. [Google Scholar] [CrossRef]
- González-Fernández, M.J.; Ortea, I.; Guil-Guerrero, J.L. α-Linolenic and γ-Linolenic Acids Exercise Differential Antitumor Effects on HT-29 Human Colorectal Cancer Cells. Toxicol. Res. 2020, 9, 474–483. [Google Scholar] [CrossRef]
- Park, K.S.; Lim, J.W.; Kim, H. Inhibitory Mechanism of Omega-3 Fatty Acids in Pancreatic Inflammation and Apoptosis. Ann. N.Y. Acad. Sci. 2009, 1171, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.J.; Yan, C.; He, C.; Li, P.; Chen, M.; Su, H.; Wan, J.B. Preventive Effect of α-Linolenic Acid-Rich Flaxseed Oil against Ethanol-Induced Liver Injury Is Associated with Ameliorating Gut-Derived Endotoxin-Mediated Inflammation in Mice. J. Funct. Foods 2016, 23, 532–541. [Google Scholar] [CrossRef]
- Simard, M.; Rioux, G.; Morin, S.; Martin, C.; Guérin, S.L.; Flamand, N.; Julien, P.; Fradette, J.; Pouliot, R. Investigation of Omega-3 Polyunsaturated Fatty Acid Biological Activity in a Tissue-Engineered Skin Model Involving Psoriatic Cells. J. Investig. Dermatol. 2021, 141, 2391–2401.e13. [Google Scholar] [CrossRef]
- Morin, S.; Simard, M.; Rioux, G.; Julien, P.; Pouliot, R. Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model. Cells 2022, 11, 1513. [Google Scholar] [CrossRef]
- Ando, H.; Ryu, A.; Akira Hashimoto, ·A.; Oka, M.; Ichihashi, M. Linoleic acid and α-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Sinclaira, A.J.; Attar-Bashia, N.M.; Li, D. What Is the Role of α-Linolenic Acid for Mammals? Lipids 2002, 37, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Takahashi, K.; Tanaka, H.; Ihara, Y.; Ikemoto, A.; Fujii, Y.; Okuyama, H. Dietary, but Not Topical, Alpha-Linolenic Acid Suppresses UVB-Induced Skin Injury in Hairless Mice When Compared with Linoleic Acid. Photochem Photobiol. 2002, 76, 657–663. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Ali, H.; Dixit, S.; Ali, D.; Alqahtani, S.M.; Alkahtani, S.; Alarifi, S. Isolation and Evaluation of Anticancer Efficacy of Stigmasterol in a Mouse Model of DMBA-Induced Skin Carcinoma. Drug Des. Devel Ther. 2015, 9, 2793–2800. [Google Scholar] [CrossRef]
- Han, N.R.; Park, H.J.; Ko, S.G.; Moon, P.D. Stigmasterol Exerts an Anti-Melanoma Property through Down-Regulation of Reactive Oxygen Species and Programmed Cell Death Ligand 1 in Melanoma Cells. Antioxidants 2024, 13, 380. [Google Scholar] [CrossRef]
- Miya, G.M.; Oriola, A.O.; Payne, B.; Cuyler, M.; Lall, N.; Oyedeji, A.O. Steroids and Fatty Acid Esters from Cyperus Sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules 2023, 28, 3434–3448. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.B.; Camponogara, C.; Piana, M.; Silva, C.R.; Oliveira, S.M. Cariniana Domestica Fruit Peels Present Topical Anti-Inflammatory Efficacy in a Mouse Model of Skin Inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 513–528. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, H.M.; Jeong, H.J. The β-Sitosterol Attenuates Atopic Dermatitis-like Skin Lesions through down-Regulation of TSLP. Exp. Biol. Med. 2014, 239, 454–464. [Google Scholar] [CrossRef]
- Yu, H.; Shen, X.; Liu, D.; Hong, M.; Lu, Y. The Protective Effects of β-Sitosterol and Vermicularin from Thamnolia Vermicularis (Sw.) Ach. Against Skin Aging in Vitro. An. Acad. Bras. Cienc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Prabahar, K.; Uthumansha, U.; Elsherbiny, N.; Qushawy, M. Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia. Pharmaceuticals 2023, 16, 563. [Google Scholar] [CrossRef] [PubMed]
- Iyer, D.; Patil, U.K. Efficacy of β-Sitosterol Isolated from Evolvulus Alsinoides L. as Anti-Hyperlipidemic and Anti-Tumor Agent: Evidence from Animal Studies. Chin. J. Integr. Med. 2014, 145–152. [Google Scholar] [CrossRef]
- Akhtar, N.; Menaa, F.; Akhtar, N.; Javed, N.; Sethi, A.; Khan, M.S. Tocopherol Succinate-Loaded Ethosomal Gel Synthesized by Cold Method Technique: Deeper Biophysical Characterizations for Translational Application on Human Skin. J. Cosmet. Dermatol. 2024, 23, 1015–1028. [Google Scholar] [CrossRef]
- Draelos, Z.; Bogdanowicz, P.; Saurat, J.H. Top Weapons in Skin Aging and Actives to Target the Consequences of Skin Cell Senescence. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 15–22. [Google Scholar] [CrossRef]
- Riedl, R.; Wallert, M.; Lorkowski, S.; Wiegand, C. Effects of Histamine and the α-Tocopherol Metabolite α-13′-COOH in an Atopic Dermatitis Full-Thickness Skin Model. Molecules 2023, 28, 440. [Google Scholar] [CrossRef]
- Shakir, M.; Adam, F.; Gaweesh, Y. Clinical Evaluation of adjunctive oral systemic vitamin e therapy in patients with erosive oral lichen planus (a randomized controlled clinicalc trial). Alex. Dent. J. 2024, 1–6. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 10, 3160. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.M.; Lawrence, K.P.; Jones, S.A.; Young, A.R. The Photoprotective Properties of α-Tocopherol Phosphate against Long-Wave UVA1 (385 Nm) Radiation in Keratinocytes in Vitro. Sci. Rep. 2021, 11, 22400–22411. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, S.; El-Sabban, M.; Darwiche, N.; Gali-Muhtasib, H. Protective Effect of Vitamin E on Ultraviolet B Light-Induced Damage in Keratinocytes. Mol. Carcinog. 2002, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Camillo, L.; Grossini, E.; Farruggio, S.; Marotta, P.; Gironil, C.; Zavattaro, E.; Savoia, P. Alpha-Tocophenol Protects Human Dermal Fibroblasts by Modulating Nitric Oxide Release, Mitochondtrial Function, Redox Status, and Inflammation. Skin Pharmacol. Physiol. 2022, 35, 1–12. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Trekli, M.C.; Riss, G.; Goralczyk, R.; Tyrrell, R.M. Beta-Carotene Suppresses UVA-Induced HO-1 Gene Expression in Cultured FEK4. Free Radic. Biol. Med. 2003, 34, 456–464. [Google Scholar] [CrossRef]
- Kake, T.; Imai, M.; Takahashi, N. Effects of β-carotene on oxazolone-induced atopic dermatitis in hairless mice. Exp. Dermatol. 2019, 28, 1044–1050. [Google Scholar] [CrossRef]
- Darban, Z.; Singh, H.; Singh, U.; Bhatia, D.; Gaur, R.; Kuddushi, M.; Dhanka, M.; Shahabuddin, S. β-Carotene Laden Antibacterial and Antioxidant Gelatin/Polyglyceryl Stearate Nano-Hydrogel System for Burn Wound Healing Application. Int. J. Biol. Macromol. 2024, 255, 128019. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865–900. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, J.; Huang, J. Vanillic Acid Promotes Keratinocyte Migration, Proliferation, and Angiogenesis. Ital. J. Food Sci. 2024, 36, 82–91. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.; Joo, M.D.; Sidrat, T.; Mesalam, A.A.; Kong, I.K. Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development. Nutrients 2023, 15, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Paz, I.; Valle-Jiménez, X.; Brunauer, R.; Alavez, S. Vanillic Acid Improves Stress Resistance and Substantially Extends Life Span in Caenorhabditis Elegans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78, 1100–1107. [Google Scholar] [CrossRef]
- Sinsuebpol, C.; Burapapadh, K.; Chowjaroen, V.; Changsan, N. The Radical Scavenging Activity of Vanillin and Its Impact on the Healing Properties of Wounds. J. Adv. Pharm. Technol. Res. 2023, 14, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheng, K.; Mannarino, M.; Jarzem, P.; Cherif, H.; Haglund, L. O-Vanillin Modulates Cell Phenotype and Extracellular Vesicles of Human Mesenchymal Stem Cells and Intervertebral Disc Cells. Cells 2022, 11, 3589–3608. [Google Scholar] [CrossRef]
- Pourhadi, M.; Ghasemi, A.; Abediny, R.; Javanmard, S.H.; Vaseghi, G. The Inhibitory Effects of Vanillin on the Growth of Melanoma by Reducing Nuclear Factor-?B Activation. Adv. Biomed. Res. 2022, 11, 68. [Google Scholar] [CrossRef]
- Akram, M.; Kim, K.A.; Kim, E.S.; Shin, Y.J.; Noh, D.; Kim, E.; Kim, J.H.; Majid, A.; Chang, S.Y.; Kim, J.K.; et al. Selective Inhibition of JAK2/STAT1 Signaling and INOS Expression Mediates the Anti-Inflammatory Effects of Coniferyl Aldehyde. Chem. Biol. Interact. 2016, 256, 102–110. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Song, K.; An, S.M.; Kim, M.; Koh, J.S.; Boo, Y.C. Comparison of the Antimelanogenic Effects of P-Coumaric Acid and Its Methyl Ester and Their Skin Permeabilities. J. Dermatol. Sci. 2011, 63, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic Acid and Ferulic Acid Inhibit UVA-Induced Matrix Metalloproteinase-1 through Regulation of Antioxidant Defense System in Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of Ferulic Acid Protects Human Dermal Fibroblasts against Ultraviolet a Irradiation. Ann. Dermatol. 2016, 28, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Ambothi, K. Ferulic Acid Prevents Ultraviolet-B Radiation Induced Oxidative DNA Damage in Human Dermal Fibroblasts. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 203. [Google Scholar] [CrossRef]
- Calabrese, V.; Calafato, S.; Puleo, E.; Cornelius, C.; Sapienza, M.; Morganti, P.; Mancuso, C. Redox Regulation of Cellular Stress Response by Ferulic Acid Ethyl Ester in Human Dermal Fibroblasts: Role of Vitagenes. Clin. Dermatol. 2008, 26, 358–363. [Google Scholar] [CrossRef]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of Wound Healing Activity of Ferulic Acid in Diabetic Rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef]
- Maia, M.B.D.S.; Franco, E.D.S.; De Aquino, C.M.F.; De Medeiros, P.L.; Evêncio, L.B.; Góes, A.J.D.S. Effect of a Semisolid Formulation of Linum usitatissimum L. (Linseed) Oil on the Repair of Skin Wounds. Evid. Based Complement. Altern. Med. 2012, 2012, 270752. [Google Scholar] [CrossRef]
- Tou, K.A.S.; Rehman, K.; Ishak, W.M.W.; Zulfakar, M.H. Influence of Omega Fatty Acids on Skin Permeation of a Coenzyme Q10 Nanoemulsion Cream Formulation: Characterization, in Silico and Ex Vivo Determination. Drug Dev. Ind. Pharm. 2019, 45, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Murashova, N.M. Lecithin Microemulsions as Drug Carriers. Colloid J. 2023, 85, 746–756. [Google Scholar] [CrossRef]
- Savić, V.; Ilić, T.; Nikolić, I.; Marković, B.; Čalija, B.; Cekić, N.; Savić, S. Tacrolimus-Loaded Lecithin-Based Nanostructured Lipid Carrier and Nanoemulsion with Propylene Glycol Monocaprylate as a Liquid Lipid: Formulation Characterization and Assessment of Dermal Delivery Compared to Referent Ointment. Int. J. Pharm. 2019, 569, 118624. [Google Scholar] [CrossRef]
- Breternitz, M.; Kowatzki, D.; Langenauer, M.; Elsner, P.; Fluhr, J.W. Fax +41 61 306 12 34 E-Mail Karger@karger.Ch Placebo-Controlled, Double-Blind, Randomized, Prospective Study of a Glycerol-Based Emollient on Eczematous Skin in Atopic Dermatitis: Biophysical and Clinical Evaluation. Skin. Pharmacol. Physiol. 2008, 21, 39–45. [Google Scholar] [CrossRef]
- Youssef, R.; Hafez, V.; Elkholy, Y.; Mourad, A. Glycerol 85% Efficacy on Atopic Skin and Its Microbiome: A Randomized Controlled Trial with Clinical and Bacteriological Evaluation. J. Dermatol. Treat. 2021, 32, 730–736. [Google Scholar] [CrossRef]
- Gebarowski, T.; Gebczak, K.; Wiatrak, B.; Kulma, A.; Pelc, K.; Czuj, T.; Szopa, J.; Gasiorowski, K. Flax Oil From Transgenic Linum usitatissimum Selectively In Vitro Proliferation of Human Cancer Cell Lines. Acta Pol. Pharm. Drug Res. 2017, 74, 653–659. [Google Scholar]
- Riwaldt, S.; Corydon, T.J.; Pantalone, D.; Sahana, J.; Wise, P.; Wehland, M.; Krüger, M.; Melnik, D.; Kopp, S.; Infanger, M.; et al. Role of Apoptosis in Wound Healing and Apoptosis Alterations in Microgravity. Front. Bioeng. Biotechnol. 2021, 9, 679650. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Francis, M.; DiPietro, L.A. Differential Apoptosis in Mucosal and Dermal Wound Healing. Adv. Wound Care (New Rochelle) 2014, 3, 751–761. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Food Processing Antioxidants. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 81, pp. 31–64. [Google Scholar]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Liu, J.; Pan, X.; Zhao, Q. Stigmasterol Exerts Neuro-Protective Effect against Ischemic/Reperfusion Injury through Reduction of Oxidative Stress and Inactivation of Autophagy. Neuropsychiatr. Dis. Treat. 2019, 15, 2991–3001. [Google Scholar] [CrossRef]
- Tabassum, A.; Jameela, M.; Jat, R.K. Chemopreventive Effect of Acetohexamide and Stigmasterol Against Uvb-Induced Skin Cancer. Front. J. Pharm. Sci. Res. 2019, 2, 47–51. [Google Scholar]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on Its Anti-Tumor Effect and Mechanism of Action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Kulma, A.; Gębarowski, T.; Wojtasik, W.; Kostyn, K.; Moreira, H.; Szyjka, A.; Boba, A.; Preisner, M.; Mierziak, J.; et al. V79 Fibroblasts Are Protected Against Reactive Oxygen Species by Flax Fabric. Appl. Biochem. Biotechnol. 2018, 184, 366–385. [Google Scholar] [CrossRef] [PubMed]
- Matar, D.Y.; Ng, B.; Darwish, O.; Wu, M.; Orgill, D.P.; Panayi, A.C. Skin Inflammation with a Focus on Wound Healing. Adv. Wound Care 2023, 12, 269–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Li, P.; Liu, W.; Zhang, Y.; Dong, A. Dual-Light-Triggered In Situ Structure and Function Regulation of Injectable Hydrogels for High-Efficient Anti-Infective Wound Therapy. Adv. Healthc. Mater. 2022, 11, 2101722. [Google Scholar] [CrossRef]
- Marvella, J.I.; Kim, H.K. Synthesis of New Multifunctional Linolenic Acid Vanillyl Ester and Investigation of Antioxidant and Antibacterial Activities. Appl. Biochem. Biotechnol. 2024, 196, 7877–7895. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Cao, W.; Gao, J.; Wang, D.; Lin, M.; Liang, C.; Li, N.; Xu, R. Dual-Functional Nanofibrous Patches for Accelerating Wound Healing. Int. J. Mol. Sci. 2022, 23, 10983. [Google Scholar] [CrossRef] [PubMed]
- Alawlaqi, M.M.; Al-Rajhi, A.M.H.; Abdelghany, T.M.; Ganash, M.; Moawad, H. Evaluation of Biomedical Applications for Linseed Extract: Antimicrobial, Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities In Vitro. J. Funct. Biomater. 2023, 14, 300. [Google Scholar] [CrossRef]
- Han, B.; Yu, B.; Liu, L.; Xiu, Y.; Wang, H. Experimental Investigation of the Strong Stability, Antibacterial and Anti-Inflammatory Effect and High Bioabsorbability of a Perilla Oil or Linseed Oil Nanoemulsion System. RSC Adv. 2019, 9, 25739–25749. [Google Scholar] [CrossRef]
- Hady, A.A.; Darweesh, M.F.; Motar, A.A. The Antibacterial of Essential Fatty Acid Semicarbazide Extracted from Flaxseed Oil Against Some Nosocomial Infection Bacteria in Iraq. Int. J. Curr. Pharm. Rev. Res. 2017, 8, 31–39. [Google Scholar] [CrossRef][Green Version]
- Kaithwas, G.; Mukerjee, A.; Kumar, P.; Majumdar, D.K. Linum usitatissimum (Linseed/Flaxseed) Fixed Oil: Antimicrobial Activity and Efficacy in Bovine Mastitis. Inflammopharmacology 2011, 19, 45–52. [Google Scholar] [CrossRef]
- Joshi, Y.; Garg, R.; Juyal, D. Evaluation of Synergistic Antimicrobial Activity of Gemifloxacin with Linum usitatissimum Seed Oil. J. Phytopharm. 2014, 3, 384–388. [Google Scholar] [CrossRef]
- Padmanabha, V.A.; Sankar, J.; Malakar, J. Investigation of Wound Healing Activity of Linseed Oil (Linum usitatissimum) in Antiseptic Cream. World J. Pharm. Res. 2017, 6, 106–113. [Google Scholar] [CrossRef]
- De Spirt, S.; Stahl, W.; Tronnier, H.; Sies, H.; Bejot, M.; Maurette, J.M.; Heinrich, U. Intervention with Flaxseed and Borage Oil Supplements Modulates Skin Condition in Women. Br. J. Nutr. 2009, 101, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Rouphael, Y.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial Properties, Cytotoxic Effects, and Fatty Acids Composition of Vegetable Oils from Purslane, Linseed, Luffa, and Pumpkin Seeds. Appl. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Pavan, M.; Novello, G.; Massa, N.; Rocchetti, A.; Berta, G.; Gamalero, E. Sensitivity of Candida Albicans to Essential Oils: Are They an Alternative to Antifungal Agents? J. Appl. Microbiol. 2016, 121, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Luo, C.H.; Thio, C.L.P.; Chang, Y.J. Immunomodulatory Role of Staphylococcus Aureus in Atopic Dermatitis. Pathogens 2022, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Schlievert, P.M.; Yoshida, T.; Lussier, S.; Boguniewicz, M.; Hata, T.; Fuxench, Z.; De Benedetto, A.; Ong, P.Y.; Ko, J.; et al. Rapid Reduction in Staphylococcus Aureus in Atopic Dermatitis Subjects Following Dupilumab Treatment. J. Allergy Clin. Immunol. 2023, 152, 1179–1195. [Google Scholar] [CrossRef]
- Klisic, A.; Bakic, M.; Karanikolic, V. Comparative Analysis of Redox Homeostasis Biomarkers in Patients with Psoriasis and Atopic Dermatitis. Antioxidants 2023, 12, 1875. [Google Scholar] [CrossRef] [PubMed]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Galiniak, S.; Mołoń, M.; Biesiadecki, M.; Bożek, A.; Rachel, M. The Role of Oxidative Stress in Atopic Dermatitis and Chronic Urticaria. Antioxidants 2022, 11, 1590. [Google Scholar] [CrossRef]
- Stankiewicz, D. GMO—Korzyści i Zagrożenia; Biuro Analiz Sejmowych, Infos: Warszawa, Poland, 2007; ISSN 1896-6659. [Google Scholar]
- Serafin-Andrzejewska, M.; Jama-Rodzeńska, A.; Helios, W.; Kozak, M.; Lewandowska, S.; Zalewski, D.; Kotecki, A. Influence of Nitrogen Fertilization, Seed Inoculation and the Synergistic Effect of These Treatments on Soybean Yields under Conditions in South-Western Poland. Sci. Rep. 2024, 14, 6672. [Google Scholar] [CrossRef]
- Kowalski, A. Legal Responses to Emerging Biotechnologies: Regulating Genetic Data and Biotechnology in the Digital Age. Leg. Stud. Digit. Age 2024, 4, 1–8. [Google Scholar]
- Planz, V.; Franzen, L.; Windbergs, M. Novel in Vitro Approaches for the Simulation and Analysis of Human Skin Wounds. Skin. Pharmacol. Physiol. 2015, 28, 91–96. [Google Scholar] [CrossRef]
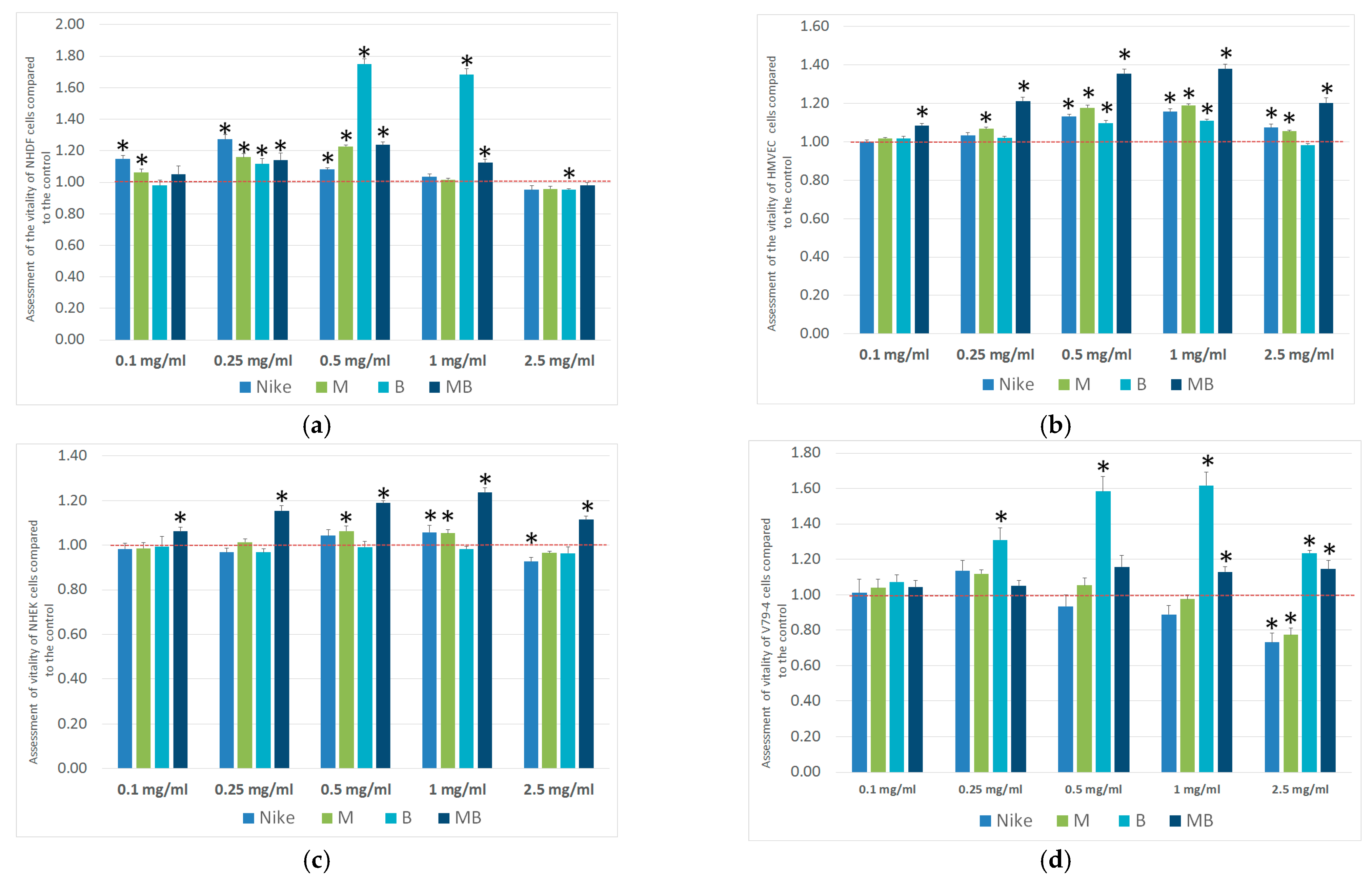
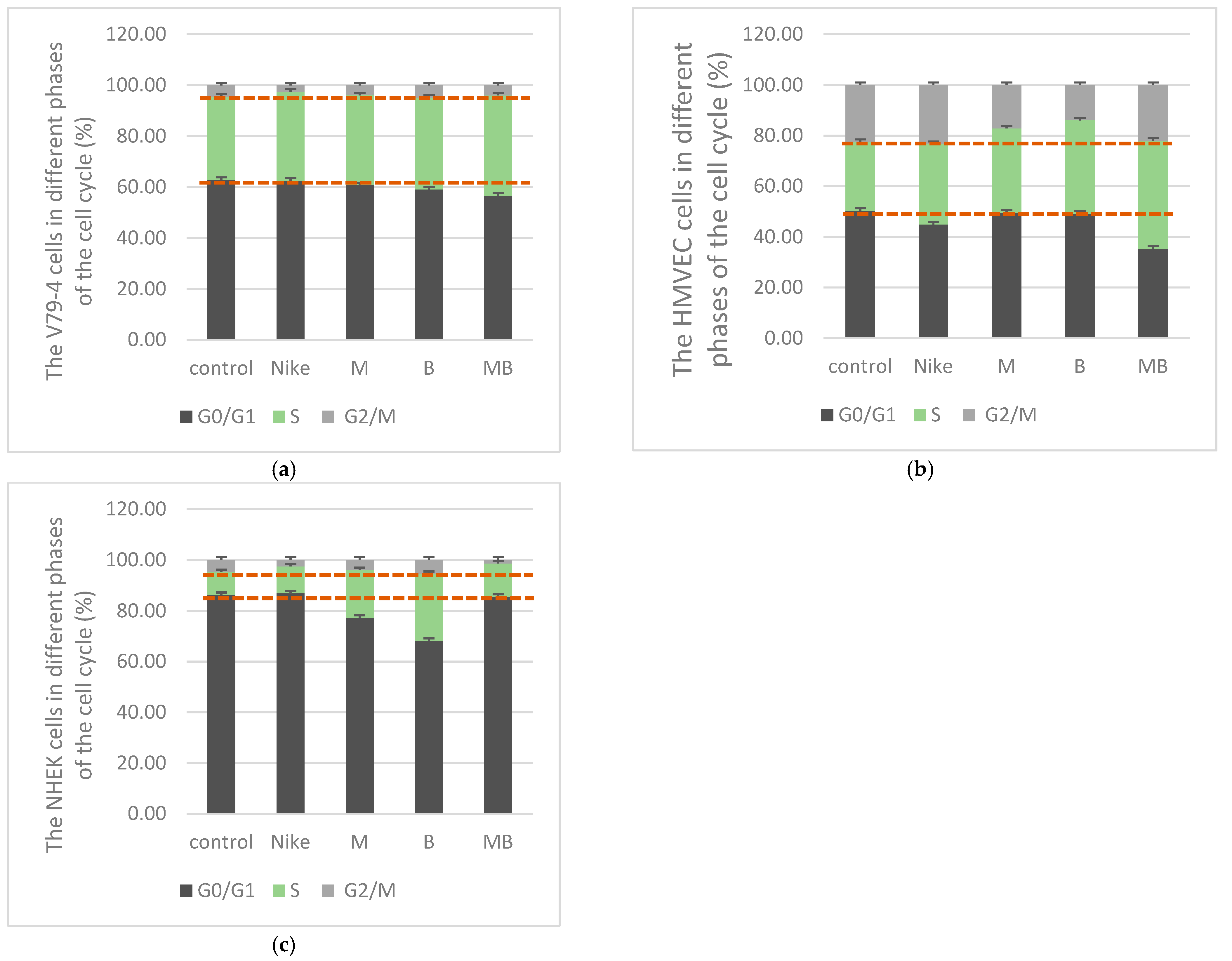
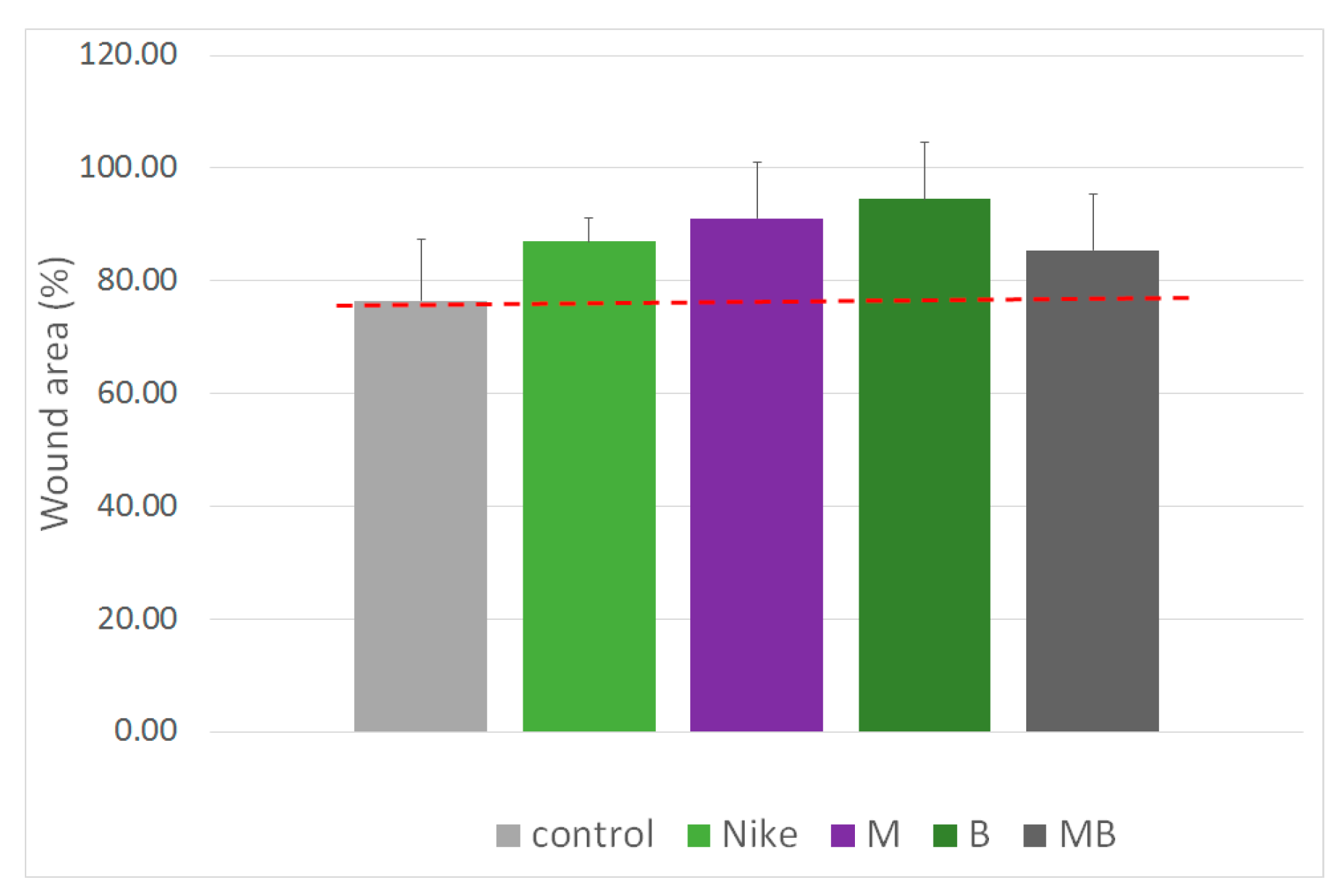
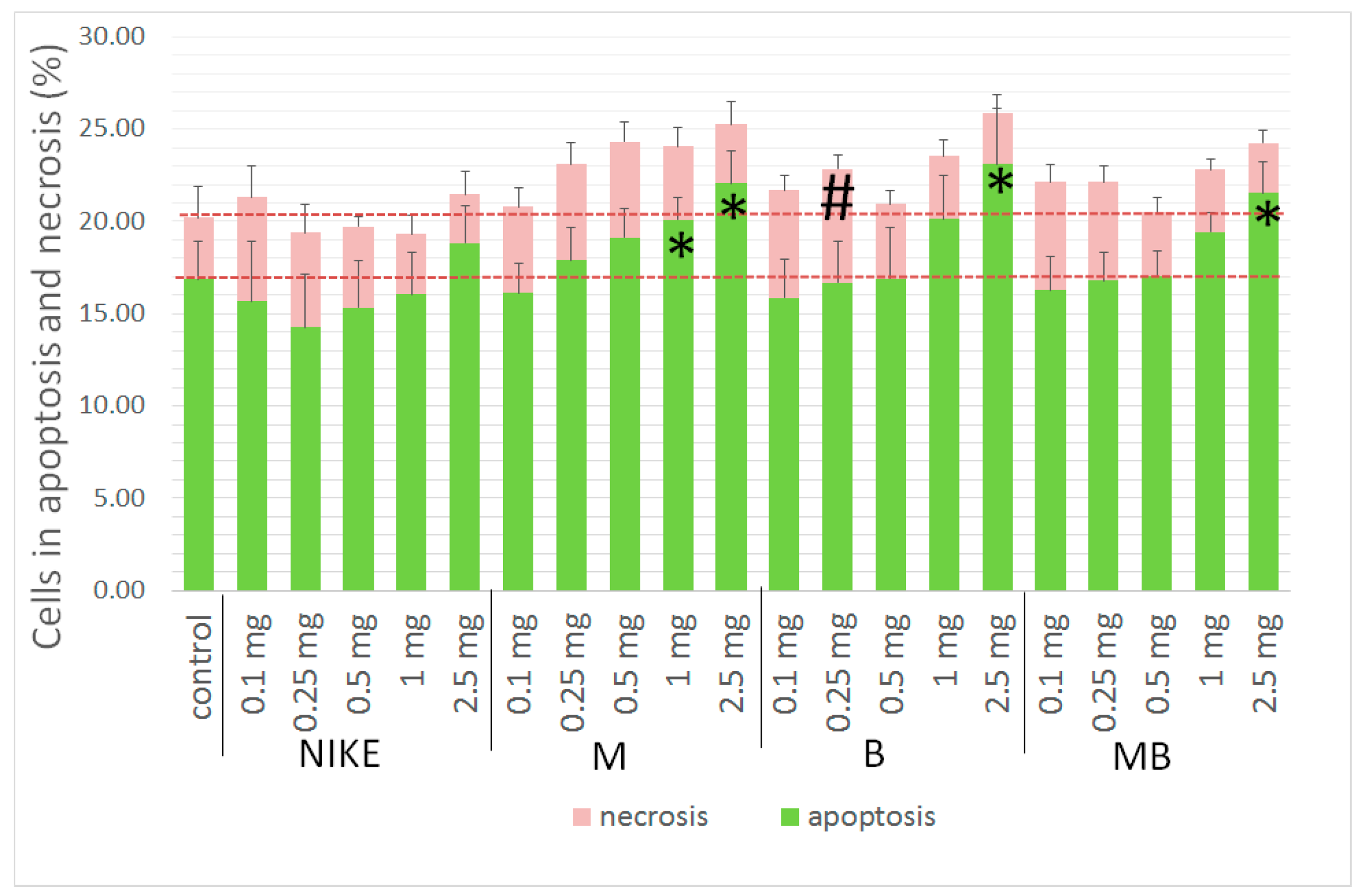
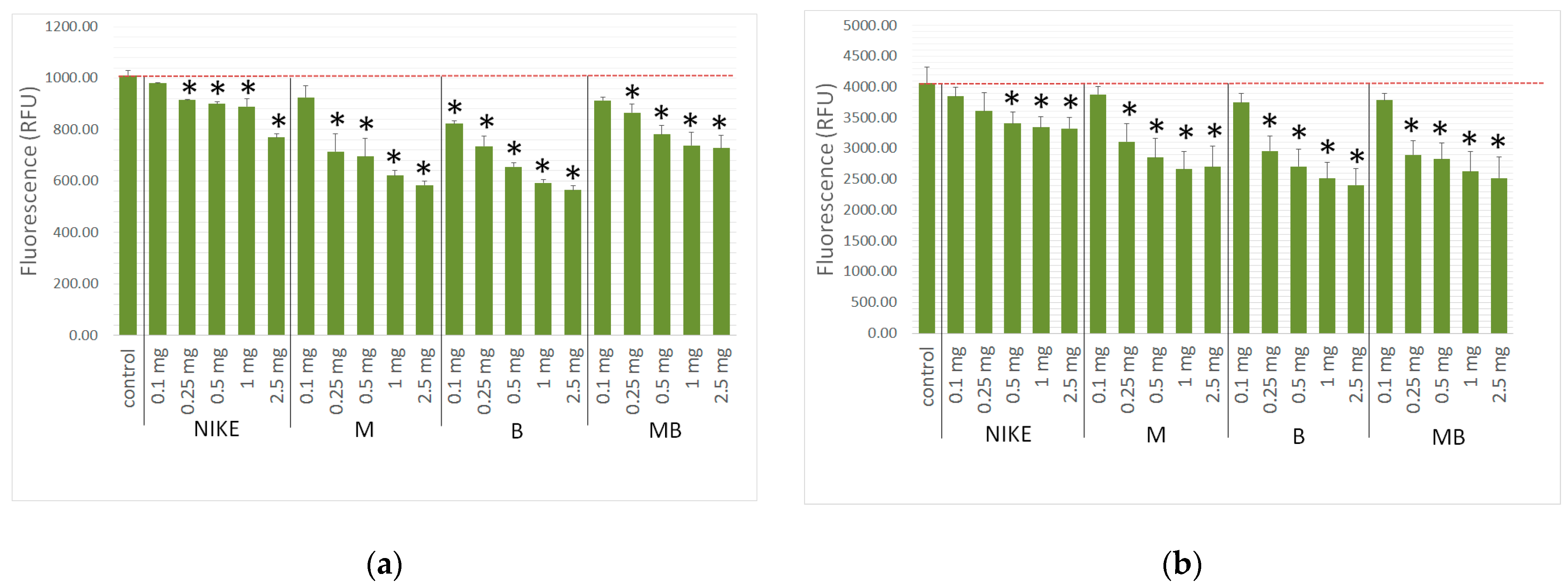
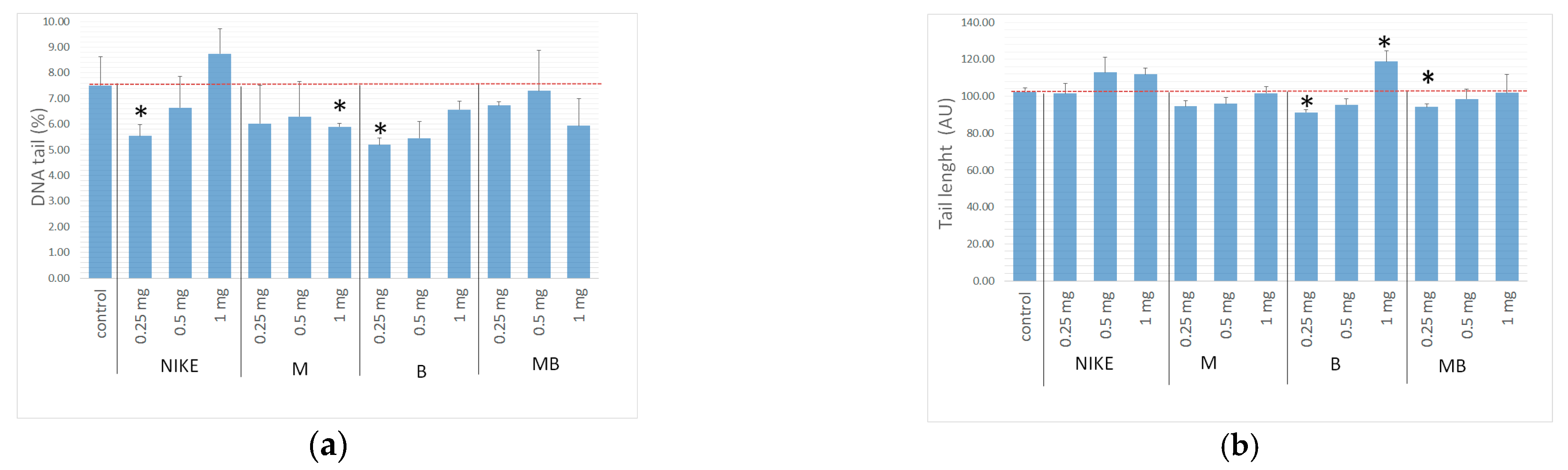
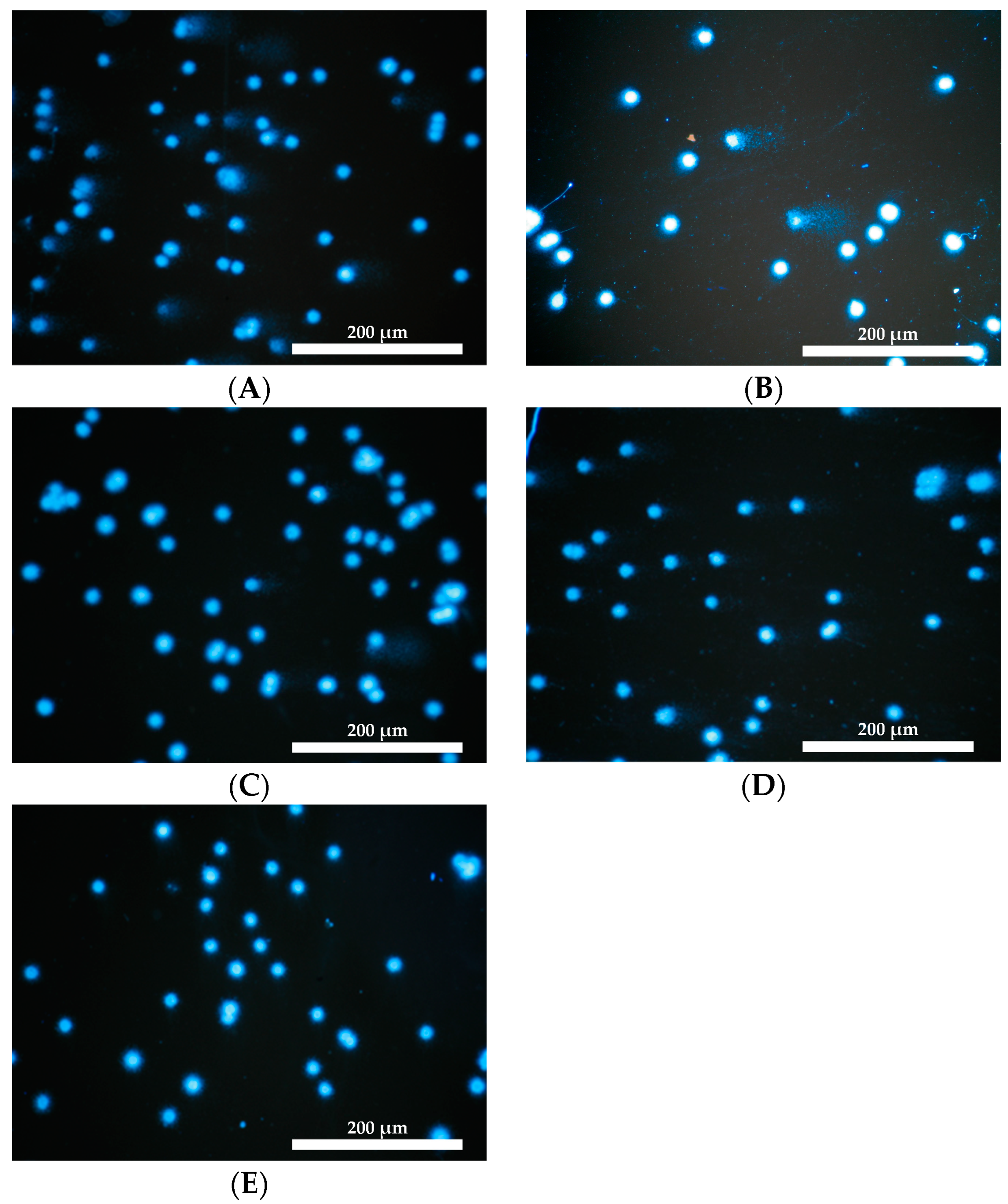
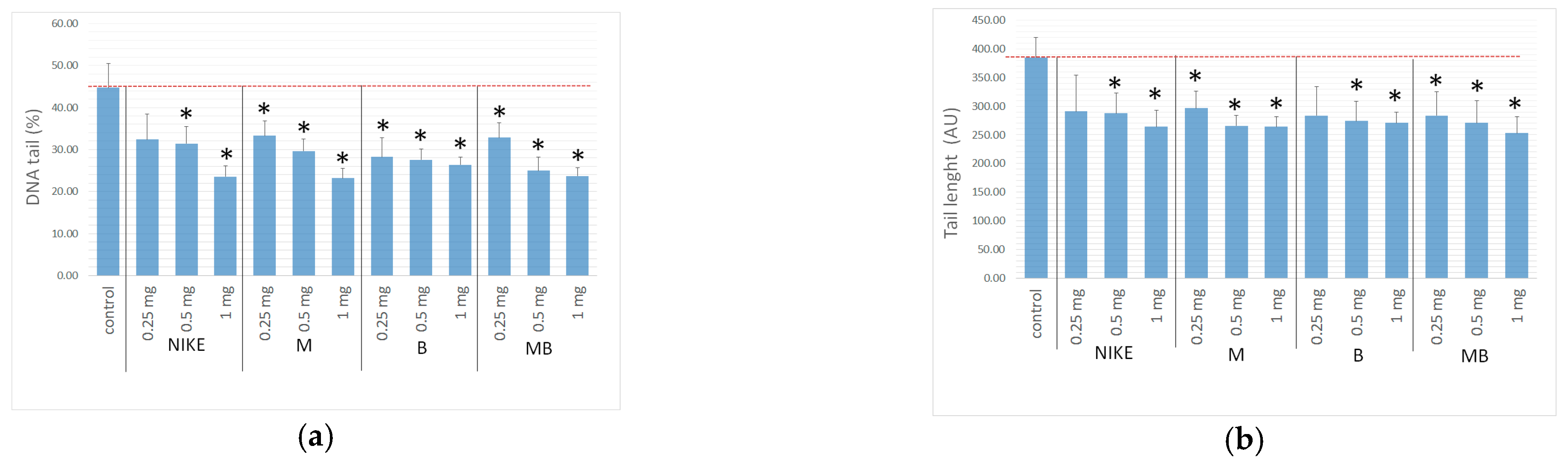

| Cell Line | IC50 [mg/mL] | |||
|---|---|---|---|---|
| Linseed Oil Emulsions | ||||
| NIKE | M | B | MB | |
| NHDF | NA | NA | NA | NA |
| HMVEC | NA | NA | NA | NA |
| NHEK | NA | NA | NA | NA |
| V79-4 | 4.79 ± 0.33 | 5.48 ± 0.28 | NA | NA |
| THP1 | 2.82 ± 0.50 | 2.25 ± 0.52 | NA | NA |
| A431 | 3.21 ± 0.25 | 2.39 ± 0.77 | 1.90 ± 0.26 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jęśkowiak-Kossakowska, I.; Gębarowski, T.; Skórkowska-Telichowska, K.; Wiatrak, B. In Vitro Studies of the Effect of Oil Emulsions from Transgenic Flax Varieties on the Treatment of Wound Healing and Care of Human Skin with the Tendency to Inflammation. Int. J. Mol. Sci. 2025, 26, 2544. https://doi.org/10.3390/ijms26062544
Jęśkowiak-Kossakowska I, Gębarowski T, Skórkowska-Telichowska K, Wiatrak B. In Vitro Studies of the Effect of Oil Emulsions from Transgenic Flax Varieties on the Treatment of Wound Healing and Care of Human Skin with the Tendency to Inflammation. International Journal of Molecular Sciences. 2025; 26(6):2544. https://doi.org/10.3390/ijms26062544
Chicago/Turabian StyleJęśkowiak-Kossakowska, Izabela, Tomasz Gębarowski, Katarzyna Skórkowska-Telichowska, and Benita Wiatrak. 2025. "In Vitro Studies of the Effect of Oil Emulsions from Transgenic Flax Varieties on the Treatment of Wound Healing and Care of Human Skin with the Tendency to Inflammation" International Journal of Molecular Sciences 26, no. 6: 2544. https://doi.org/10.3390/ijms26062544
APA StyleJęśkowiak-Kossakowska, I., Gębarowski, T., Skórkowska-Telichowska, K., & Wiatrak, B. (2025). In Vitro Studies of the Effect of Oil Emulsions from Transgenic Flax Varieties on the Treatment of Wound Healing and Care of Human Skin with the Tendency to Inflammation. International Journal of Molecular Sciences, 26(6), 2544. https://doi.org/10.3390/ijms26062544









