Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury
Abstract
1. Introduction
2. Results
2.1. RCI Resulted in Higher Mortality and Lower Body Weight than RI and Slowed Down Skin Wound Healing
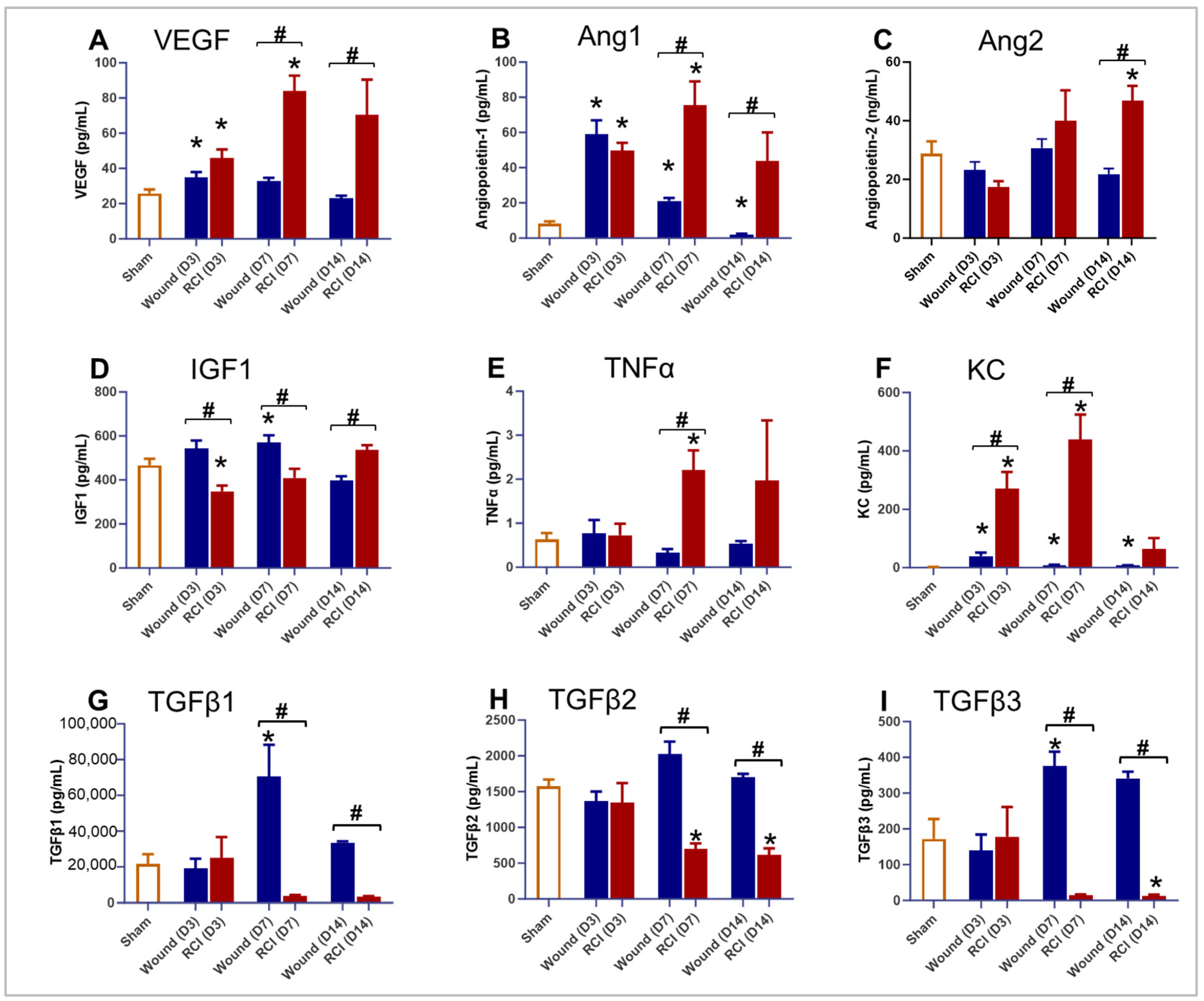
2.2. The Proinflammatory Factors and Endothelial Damage Markers Were Significantly Increased and Persistent in RCI Mice Compared to Wound-Alone Mice
2.3. RCI Hindered Granulation Tissue Formation Compared to Wounds Alone
2.4. RCI Did Not Affect the Infiltration of Neutrophils and Macrophages to the Skin Wounds Compared to Wounds Alone
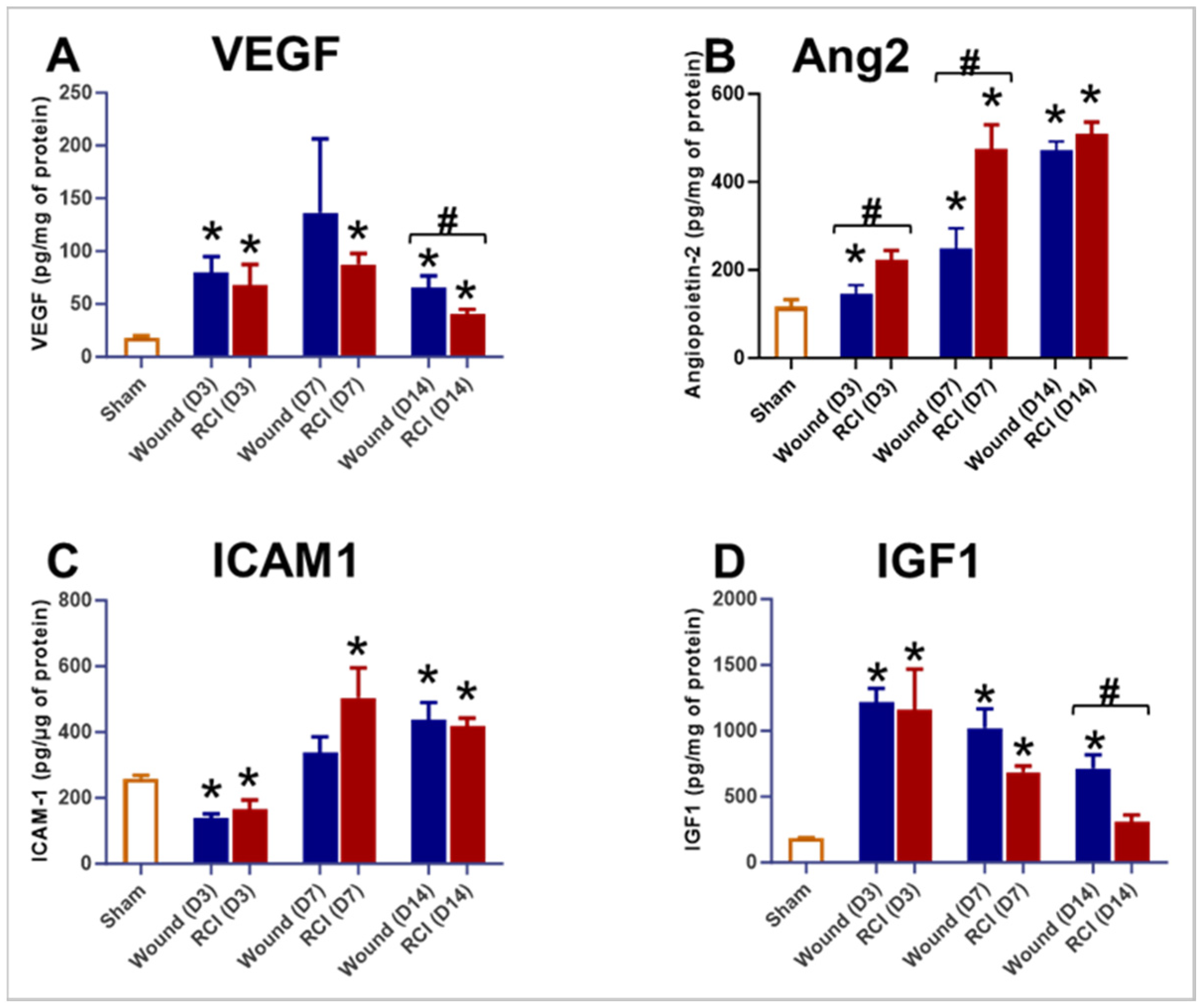
2.5. RCI Induced an Insufficient Endothelial Response in Skin Wounds Compared to Wounds Alone
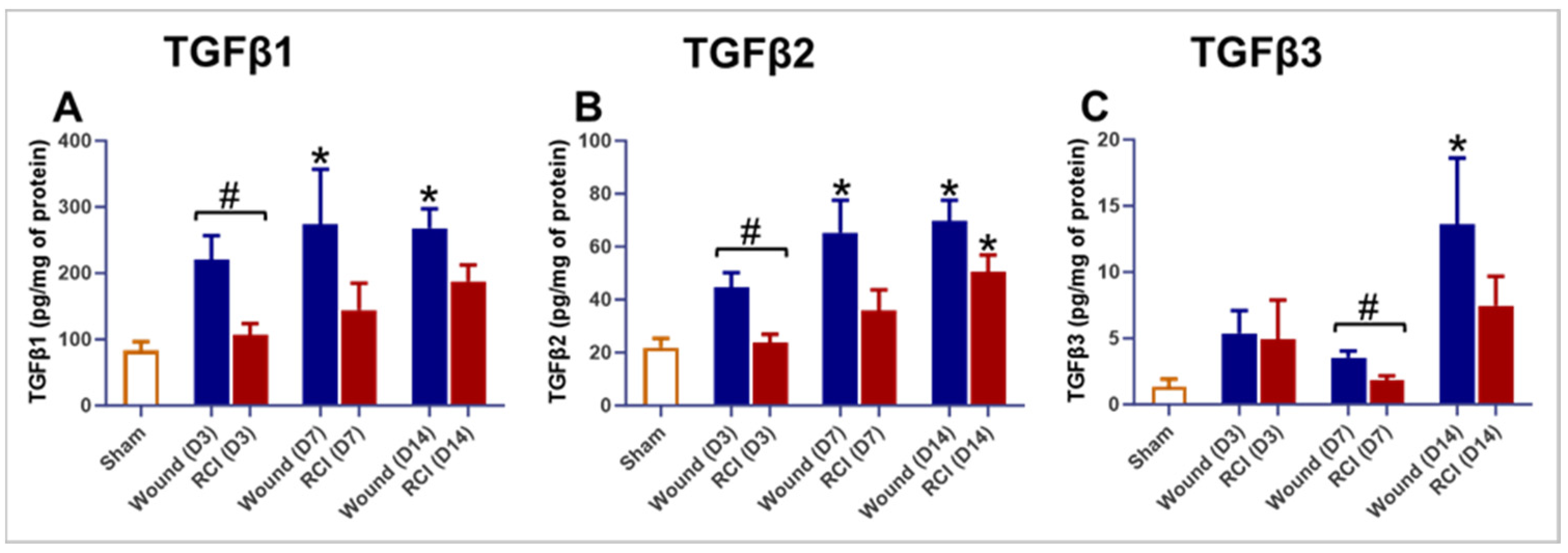
2.6. Altered TGFβ Signaling in the Skin of RCI Mice Compared to the Wound-Alone Group
2.7. RCI Reduced CTGF but Not Fibronectin Levels in Skin Wounds Compared to Wounds Alone
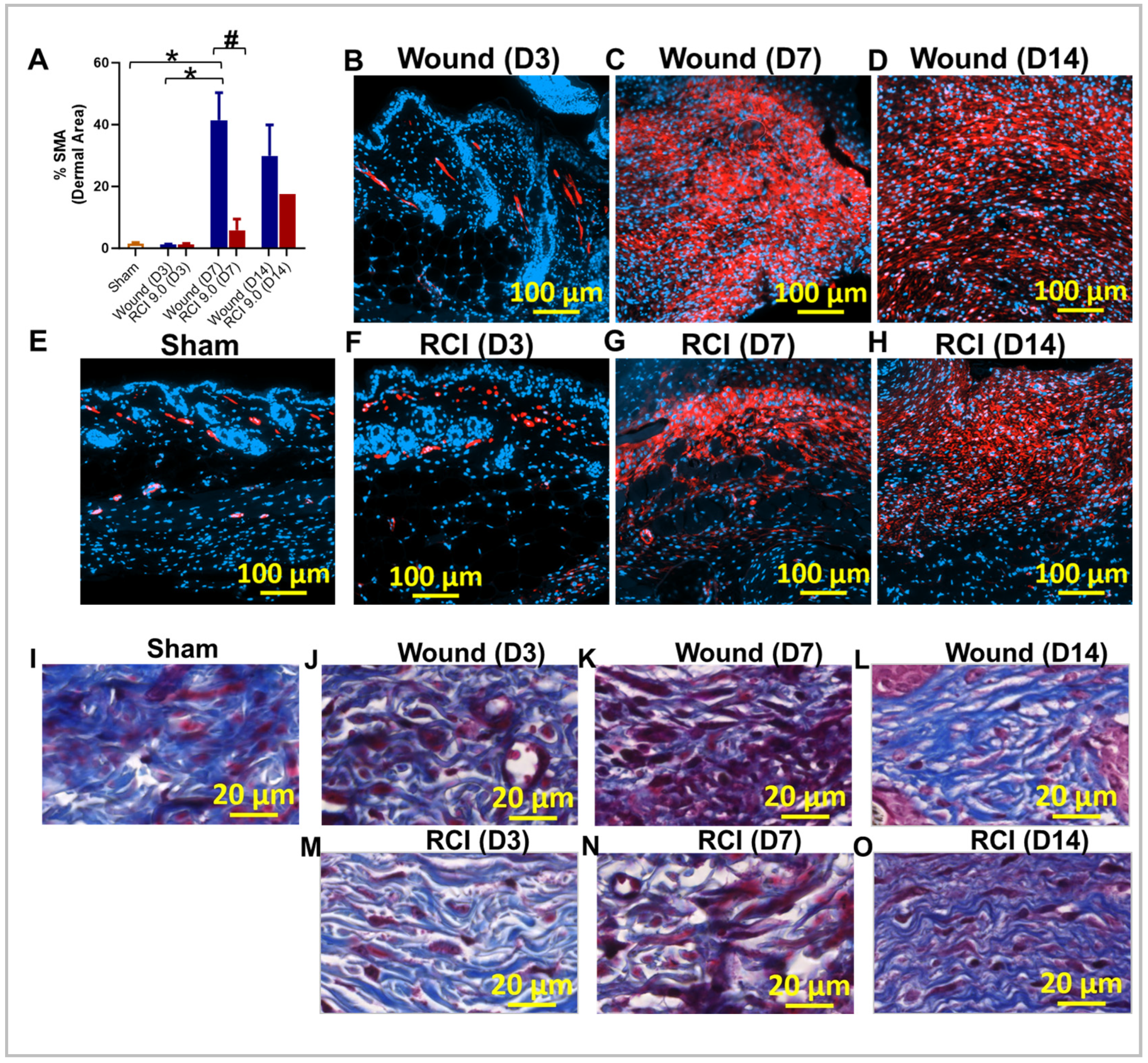
2.8. RCI Reduced Myofibroblast Presence and Delays Collagen Deposition in Skin Wounds Compared to Wounds Alone
3. Discussion
4. Materials and Methods
4.1. Mice and Ethics Statement
4.2. Experimental Design
4.3. Total-Body Irradiation
4.4. Skin Wounding
4.5. Thirty-Day Survival
4.6. Body Weight and Wound Size Measurement
4.7. Blood and Tissue Collection, Peripheral Blood Cell Count, and Serum Preparation
4.8. Skin Tissue Lysate Preparation
4.9. N-Acetylglucosaminidase Activity and Myeloperoxidase Activity Assays
4.10. Enzyme-Linked Immunosorbent Assay
4.11. Histological Examination of Skin
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Lin, B.; Zhai, M.; Cui, W.; Hull, L.; Zizzo, A.; Li, X.; Kiang, J.G.; Xiao, M. Deteriorative Effects of Radiation Injury Combined with Skin Wounding in a Mouse Model. Toxics 2022, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhai, M.; Lin, B.; Cui, W.; Hull, L.; Li, X.; Anderson, M.N.; Smith, J.T.; Umali, M.V.; Jiang, S.; et al. PEG-G-CSF and L-Citrulline Combination Therapy for Mitigating Skin Wound Combined Radiation Injury in a Mouse Model. Radiat. Res. 2021, 196, 113–127. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, A.L.; Ramakrishnan, N.; Hatchett, R.J. Radiation combined injury: Overview of NIAID research. Health Phys. 2010, 98, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Blakely, W.F. Combined radiation injury and its impacts on radiation countermeasures and biodosimetry. Int. J. Radiat. Biol. 2023, 99, 1055–1065. [Google Scholar] [CrossRef]
- Wang, J.; Boerma, M.; Fu, Q.; Hauer-Jensen, M. Radiation responses in skin and connective tissues: Effect on wound healing and surgical outcome. Hernia 2006, 10, 502–506. [Google Scholar] [CrossRef]
- Ledney, G.D.; Elliott, T.B. Combined injury: Factors with potential to impact radiation dose assessments. Health Phys. 2010, 98, 145–152. [Google Scholar] [CrossRef]
- Kiang, J.G.; Jiao, W.; Cary, L.H.; Mog, S.R.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat. Res. 2010, 173, 319–332. [Google Scholar] [CrossRef]
- Xiao, M.; Li, X.; Wang, L.; Lin, B.; Zhai, M.; Hull, L.; Zizzo, A.; Cui, W.; Kiang, J.G. Skin Wound following Irradiation Aggravates Radiation-Induced Brain Injury in a Mouse Model. Int. J. Mol. Sci. 2023, 24, 10701. [Google Scholar] [CrossRef]
- Kiang, J.G.; Fukumoto, R. Ciprofloxacin increases survival after ionizing irradiation combined injury: Gamma-H2AX formation, cytokine/chemokine, and red blood cells. Health Phys. 2014, 106, 720–726. [Google Scholar] [CrossRef]
- Kiang, J.G.; Zhai, M.; Bolduc, D.L.; Smith, J.T.; Anderson, M.N.; Ho, C.; Lin, B.; Jiang, S. Combined Therapy of Pegylated G-CSF and Alxn4100TPO Improves Survival and Mitigates Acute Radiation Syndrome after Whole-Body Ionizing Irradiation Alone and Followed by Wound Trauma. Radiat. Res. 2017, 188, 476–490. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Cannon, G.; Anderson, M.N.; Ho, C.; Zhai, M.; Cui, W.; Xiao, M. Ghrelin, a novel therapy, corrects cytokine and NF-kappaB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Swindell, W.R. Putting a strain on diversity. EMBO J. 2018, 37, e100862. [Google Scholar] [CrossRef] [PubMed]
- Sloin, H.E.; Bikovski, L.; Levi, A.; Amber-Vitos, O.; Katz, T.; Spivak, L.; Someck, S.; Gattegno, R.; Sivroni, S.; Sjulson, L.; et al. Hybrid Offspring of C57BL/6J Mice Exhibit Improved Properties for Neurobehavioral Research. eNeuro 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Alhajj, M.; Goyal, A. Physiology, Granulation Tissue. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2024. [Google Scholar] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Douglass, M.S.; Kaplowitz, M.R.; Zhang, Y.; Fike, C.D. Impact of l-citrulline on nitric oxide signaling and arginase activity in hypoxic human pulmonary artery endothelial cells. Pulm. Circ. 2023, 13, e12221. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bouten, R.M.; Young, E.F.; Selwyn, R.; Iacono, D.; Rittase, W.B.; Day, R.M. Chapter Two—Effects of radiation on endothelial barrier and vascular integrity. In Tissue Barriers in Disease, Injury and Regeneration; Gorbunov, N.V., Ed.; Elsevier BV: Cambridge, MA, USA, 2021; pp. 43–94. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Korpela, E.; Liu, S.K. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat. Oncol. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.P.; Himburg, H.A. Organ-Specific Endothelial Dysfunction Following Total Body Irradiation Exposure. Toxics 2022, 10, 747. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.I.; Krishnan, S. Radiation-Induced Endothelial Vascular Injury: A Review of Possible Mechanisms. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef]
- Wang, J.; Boerma, M.; Fu, Q.; Hauer-Jensen, M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 2007, 13, 3047–3055. [Google Scholar] [CrossRef]
- Ogawa, R.; Akaishi, S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis—Keloids and hypertrophic scars may be vascular disorders. Med. Hypotheses 2016, 96, 51–60. [Google Scholar] [CrossRef]
- Blum, A.; Zarqh, O.; Peleg, A.; Sirchan, R.; Blum, N.; Salameh, Y.; Ganaem, M. Vascular inflammation and endothelial dysfunction in fracture healing. Am. J. Orthop. 2012, 41, 87–91. [Google Scholar]
- Amon, M.; Laschke, M.W.; Harder, Y.; Vollmar, B.; Menger, M.D. Impact of severity of local soft-tissue trauma on long-term manifestation of microcirculatory and microlymphatic dysfunctions. J. Trauma 2006, 61, 924–932. [Google Scholar] [CrossRef]
- Yokoi, H.; Mukoyama, M.; Sugawara, A.; Mori, K.; Nagae, T.; Makino, H.; Suganami, T.; Yahata, K.; Fujinaga, Y.; Tanaka, I.; et al. Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2002, 282, F933–F942. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; McCoy, M.; Murtazina, R.; Podrez, E.A.; Byzova, T.V. Timely Wound Healing Is Dependent on Endothelial but Not on Hair Follicle Stem Cell Toll-Like Receptor 2 Signaling. J. Investig. Dermatol. 2022, 142, 3082–3092.e1. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Lingen, M.W. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch. Pathol. Lab. Med. 2001, 125, 67–71. [Google Scholar] [CrossRef]
- Flanagan, M. The physiology of wound healing. J. Wound Care 2000, 9, 299–300. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care (New Rochelle) 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Fu, M.; Peng, D.; Lan, T.; Wei, Y.; Wei, X. Multifunctional regulatory protein connective tissue growth factor (CTGF): A potential therapeutic target for diverse diseases. Acta Pharm. Sin. B 2022, 12, 1740–1760. [Google Scholar] [CrossRef]
- Rebolledo, D.L.; Lipson, K.E.; Brandan, E. Driving fibrosis in neuromuscular diseases: Role and regulation of Connective tissue growth factor (CCN2/CTGF). Matrix Biol. Plus 2021, 11, 100059. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Kau, H.C.; Wei, Y.H. Essential role of connective tissue growth factor (CTGF) in transforming growth factor-beta1 (TGF-beta1)-induced myofibroblast transdifferentiation from Graves’ orbital fibroblasts. Sci. Rep. 2018, 8, 7276. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral Health 2015, 7, 75–80. [Google Scholar]
- Goumans, M.J.; Liu, Z.; ten Dijke, P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009, 19, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabe-Garcia, A.; Nicolas, F.J. Role of TGF-beta in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv. Wound Care (New Rochelle) 2013, 2, 195–214. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Sim, S.L.; Kumari, S.; Kaur, S.; Khosrotehrani, K. Macrophages in Skin Wounds: Functions and Therapeutic Potential. Biomolecules 2022, 12, 1659. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Pathak, R.; Sridharan, V.; Jones, T.; Mao, X.W.; Nelson, G.; Boerma, M.; Hauer-Jensen, M.; Zhou, D.; et al. Whole body proton irradiation causes acute damage to bone marrow hematopoietic progenitor and stem cells in mice. Int. J. Radiat. Biol. 2017, 93, 1312–1320. [Google Scholar] [CrossRef]
- Vegesna, V.; Withers, H.R.; Holly, F.E.; McBride, W.H. The effect of local and systemic irradiation on impairment of wound healing in mice. Radiat. Res. 1993, 135, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Nilsson, J.; Sturesson, C. Non-invasive imaging of microcirculation: A technology review. Med. Devices 2014, 7, 445–452. [Google Scholar] [CrossRef]
- Kumar, V.P.; Wuddie, K.; Tsioplaya, A.; Weaver, A.; Holmes-Hampton, G.P.; Ghosh, S.P. Development of a Multi-Organ Radiation Injury Model with Precise Dosimetry with Focus on GI-ARS. Radiat. Res. 2024, 201, 19–34. [Google Scholar] [CrossRef] [PubMed]




| 8.5 Gy (#) | 8.85 Gy (#) | 9.25 Gy (#) | 9.5 Gy (#) | Total (#) | |
|---|---|---|---|---|---|
| RI | 20 | 20 | 20 | 20 | 80 |
| RCI | 20 | 20 | 20 | 20 | 80 |
| Total (#) | 40 | 40 | 40 | 40 | 160 |
| Sham (#) | Wound (#) | 9.0 Gy RCI (#) | Total (#) | |
|---|---|---|---|---|
| Day 3 | 5 | 5 | 10 | |
| Day 7 | 5 | 5 | 10 | |
| Day 14 | 5 * | 5 | 10 ^ | 20 |
| Total (#) | 5 | 15 | 20 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lin, B.; Zhai, M.; Hull, L.; Cui, W.; Xiao, M. Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury. Int. J. Mol. Sci. 2024, 25, 12498. https://doi.org/10.3390/ijms252312498
Wang L, Lin B, Zhai M, Hull L, Cui W, Xiao M. Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury. International Journal of Molecular Sciences. 2024; 25(23):12498. https://doi.org/10.3390/ijms252312498
Chicago/Turabian StyleWang, Li, Bin Lin, Min Zhai, Lisa Hull, Wanchang Cui, and Mang Xiao. 2024. "Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury" International Journal of Molecular Sciences 25, no. 23: 12498. https://doi.org/10.3390/ijms252312498
APA StyleWang, L., Lin, B., Zhai, M., Hull, L., Cui, W., & Xiao, M. (2024). Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury. International Journal of Molecular Sciences, 25(23), 12498. https://doi.org/10.3390/ijms252312498






