Maternal-Fetal Outcomes and Antibody Transfer, Depending on the Trimester of SARS-CoV-2 Infection in Non-Vaccinated Women—A Danish Nationwide Prospective Cohort Study
Abstract
1. Introduction
2. Results
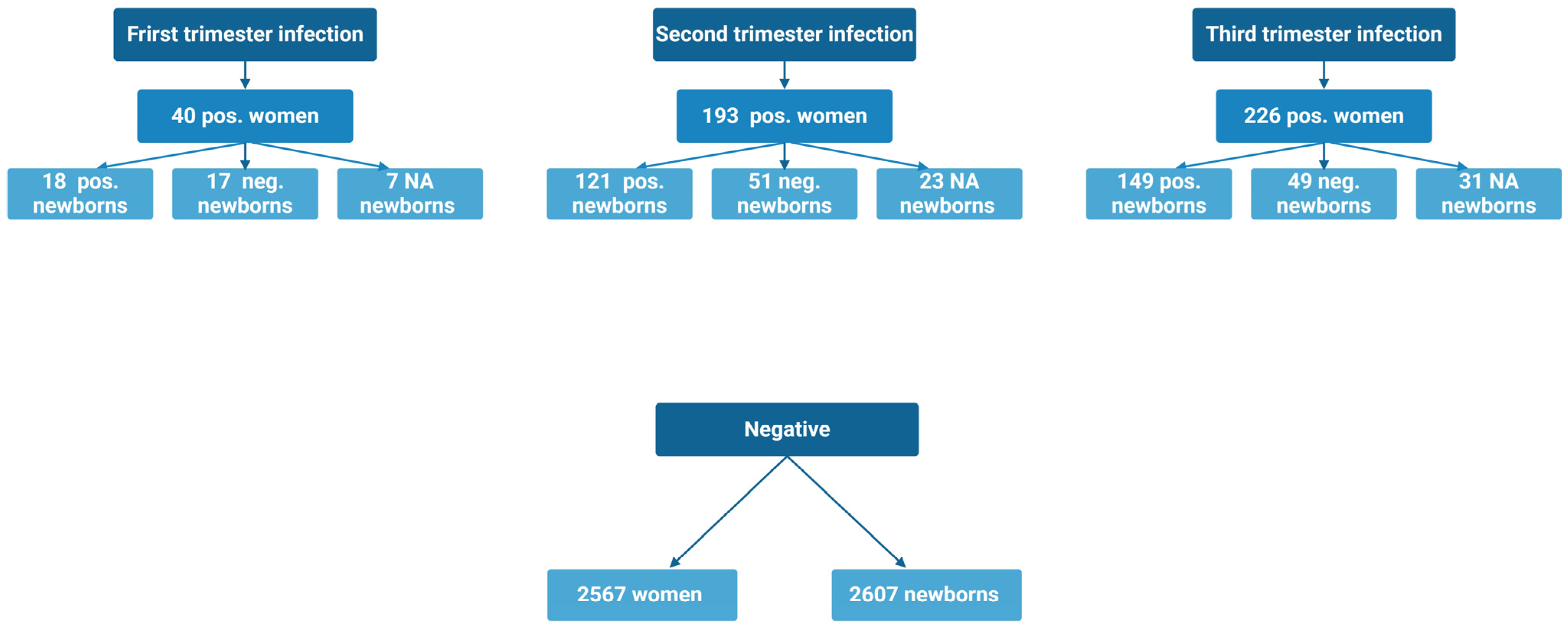
2.1. Characteristics Based on Trimester of Infection and SARS-CoV-2 Positive vs. Negative (Table 1 and Table 2)
| Characteristics | SARS-CoV-2 Pos. in 1st Trimester | SARS-CoV-2 Pos. in 2nd Trimester | SARS-CoV-2 Pos. in 3rd Trimester | p-Value (Bonferroni Corrected) |
|---|---|---|---|---|
| n = 40 | n = 193 | n = 226 | ||
| Age (years) | 30.70 (4.73) | 30.83 (4.58) | 31.28 (4.89) | 1.000 1 |
| Smoking | 2 (5) | 7 (3.6) | 8 (3.5) | |
| Smoking before pregnancy | 2 (5) | 14 (7.3) | 20 (8.8) | 1.000 4 |
| Chronic disease | 12 (30) | 29 (15) | 32 (14.2) | 0.750 3 |
| BMI, preconceptional (kg/m2) | 25.46 (22.20:30.52) | 23.88 (21.22:28.05) | 24.05 (21.82:27.68) | 1.000 2 |
| Characteristics | SARS-CoV-2 Negative | SARS-CoV-2 Positive | p-Value (Bonferroni Corrected) |
|---|---|---|---|
| n = 2567 | n = 459 | ||
| Age (years) | 32.33 (4.59) | 31.04 (4.74) | 0.020 1 |
| Smoking | 91 (3.5) | 17 (3.7) | |
| Smoking before pregnancy | 315 (12.3) | 36 (7.8) | 0.484 3 |
| Chronic disease * | 764 (29.7) | 73 (15.9) | 0.020 3 |
| BMI, preconceptional(kg/m2) | 23.00 (20.90:26.05) | 24.03 (21.55:27.96) | 0.020 2 |
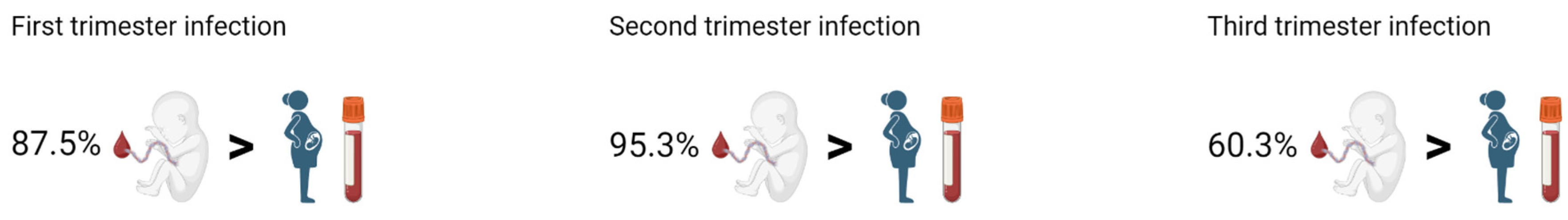
2.2. Vertical Transmission of SARS-CoV-2 Antibodies and Maternal-Fetal Outcomes When Infected in the First Trimester (Table 3 and Table 4)
| Outcomes | SARS-CoV-2 Pos. in 1st Trimester | SARS-CoV-2 Pos. in 2nd Trimester | SARS-CoV-2 Pos. in 3rd Trimester | p-Value (Bonferroni Corrected) |
|---|---|---|---|---|
| n = 40 | n = 193 | n = 226 | ||
| Gestational age at delivery (days) | 276.50 (270.75:284.00) | 279.00 (272.00:286.00) | 281.00 (274.00:287.00) | 0.758 2 |
| GDM | 1 (2.5) | 10 (5.2) | 17 (7.5) | 1.000 4 |
| Gestational hypertension | 0 (0) | 5 (2.6) | 6 (2.7) | 1.000 4 |
| Preeclampsia | 0 (0) | 6 (3.1) | 12 (5.3) | 1.000 4 |
| Preterm PROM | 2 (5) | 1 (0.5) | 1 (0.4) | 0.794 4 |
| Spontaneous labor | 32 (84.2) | 140 (72.5) | 173 (77.6) | |
| Induced labor | 6 (15.8) | 53 (27.5) | 50 (22.4) | 1.000 3 |
| Vaginal delivery | 29 (72.5) | 162 (83.9) | 183 (81) | |
| CS delivery | 11 (27.5) | 31 (16.1) | 43 (19) | 1.000 3 |
| Planned CS | 4 (36.4) | 13 (41.9) | 15 (34.9) | |
| Emergency CS grade 3 | 3 (27.3) | 15 (48.4) | 18 (41.9) | |
| Emergency CS grade 2 | 4 (36.4) | 2 (6.5) | 9 (20.9) | |
| Emergency CS grade 1 | 0 (0) | 1 (3.2) | 1 (2.3) | 1.000 4 |
| Accumulated acute CS | 7 (17.5) | 18 (9.3) | 28 (12.4) | 1.000 4 |
| Vacuum extraction | 3 (7.5) | 9 (4.7) | 17 (7.5) | 1.000 4 |
| Outcome Singleton alive | 38 (95) | 191 (99) | 221 (97.8) | |
| Outcome Singleton stillbirth | 0 (0) | 0 (0) | 2 (0.9) | |
| Outcome Gemelli alive | 2 (5) | 2 (1) | 3 (1.3) | 1.000 4 |
| PPH | 8 (20) | 13 (6.7) | 11 (4.9) | 0.049 3 |
| Any pregnancy complications * | 3 (7.5) | 23 (11.9) | 35 (15.5) | 1.000 3 |
| Liver affected | 0 (0) | 4 (2.1) | 5 (2.2) | 1.000 4 |
| Characteristics/Outcomes | SARS-CoV-2 Pos. in 1st Trimester | SARS-CoV-2 Pos. in 2nd Trimester | SARS-CoV-2 Pos. in 3rd Trimester | p-Value (Bonferroni Corrected) |
|---|---|---|---|---|
| n = 42 | n = 195 | n = 229 | ||
| Male | 25 (59.5) | 102 (52.3) | 121 (52.8) | |
| Female | 17 (40.5) | 93 (47.7) | 108 (47.2) | 1.000 3 |
| Gestational age at delivery (days) | 275.00 (270.00:284.00) | 279.00 (272.00:286.00) | 281.00 (274.00:287.00) | 0.292 2 |
| Preterm birth ¨ | 4 (9.5) | 7 (3.6) | 12 (5.2) | 1.000 4 |
| Neonatal admission | 6 (14.3) | 9 (4.6) | 17 (7.5) | 0.877 4 |
| Any neonatal complications * | 10 (23.8) | 19 (9.7) | 30 (13.2) | 0.524 3 |
| Apgar score < 7 at 5 min | 1 (2.4) | 1 (0.5) | 3 (1.3) | 1.000 4 |
| Apgar score 5 min | 10.00 (10.00:10.00) | 10.00 (10.00:10.00) | 10.00 (10.00:10.00) | 1.000 2 |
| Umbilical artery pH < 7 | 0 (0) | 0 (0) | 1 (0.4) | 1.000 4 |
| Umbilical artery pH | 7.25 (7.20:7.27) | 7.24 (7.19:7.29) | 7.23 (7.18:7.28) | 1.000 2 |
| Malformation | 1 (2.4) | 2 (1) | 5 (2.2) | 1.000 4 |
| Low birth weight < 2500 g | 5 (11.9) | 7 (3.6) | 9 (3.9) | 0.979 4 |
| Birth weight (gram) | 3306.19 (660.80) | 3567.58 (490.37) | 3555.53 (527.66) | 0.133 1 |
2.3. Vertical Transmission of SARS-CoV-2 Antibodies and Maternal-Fetal Outcomes When Infected in the Second Trimester (Table 3 and Table 4)
2.4. Vertical Transmission of SARS-CoV-2 Antibodies and Maternal-Fetal Outcomes When Infected in the Third Trimester (Table 3 and Table 4)
2.5. Vertical Transmission of SARS-CoV-2 Antibodies and Maternal-Fetal Outcomes When Accumulating All SARS-CoV-2 Positive Women (Table 5 and Table 6)
| Outcomes | SARS-CoV-2 Negative | SARS-CoV-2 Positive | p-Value (Bonferroni Corrected) |
|---|---|---|---|
| n = 2567 | n = 459 | ||
| Gestational age at delivery (days) | 281.00 (273.00:287.00) | 280.00 (273.00:286.00) | 1.000 2 |
| GDM | 192 (7.5) | 28 (6.1) | 1.000 3 |
| Gestational hypertension | 91 (3.5) | 11 (2.4) | 1.000 3 |
| Preeclampsia | 107 (4.2) | 18 (3.9) | 1.000 3 |
| Preterm PROM | 68 (2.6) | 4 (0.9) | 0.658 3 |
| Spontaneous labor | 1755 (75.8) | 345 (76) | |
| Induced labor | 559 (24.2) | 109 (24) | 1.000 3 |
| Vaginal delivery | 2037 (79.4) | 374 (81.5) | |
| CS delivery | 530 (20.6) | 85 (18.5) | 1.000 3 |
| Planned CS | 223 (42.2) | 32 (37.6) | |
| Emergency CS grade 3 | 190 (35.9) | 36 (42.4) | |
| Emergency CS grade 2 | 103 (19.5) | 15 (17.6) | |
| Emergency CS grade 1 | 13 (2.5) | 2 (2.4) | 1.000 4 |
| Accumulated acute CS | 306 (11.9) | 53 (11.5) | 1.000 3 |
| Vacuum extraction | 292 (11.4) | 29 (6.3) | 0.032 3 |
| Outcome Singleton alive | 2528 (98.4) | 450 (98) | |
| Outcome Singleton stillbirth | 5 (0.2) | 2 (0.4) | |
| Outcome Gemelli alive | 35 (1.4) | 7 (1.5) | |
| Outcome Gemelli 1 alive 1 stillbirth | 1 (0) | 0 (0) | 1.000 4 |
| PPH | 666 (26) | 32 (7) | 0.020 3 |
| Any pregnancy complications * | 436 (17) | 61 (13.3) | 1.000 3 |
| Liver affected | 32 (1.2) | 9 (2) | 1.000 3 |
| Characteristics/Outcomes | Newborns of SARS-CoV-2 Negative | Newborns of SARS-CoV-2 Positive | p-Value (Bonferroni Corrected) |
|---|---|---|---|
| n = 2607 | n = 466 | ||
| Male | 1326 (50.9) | 248 (53.2) | |
| Female | 1281 (49.1) | 218 (46.8) | 1.000 3 |
| Gestational age at delivery | 280.00 (272.00:287.00) | 280.00 (273.00:286.00) | 1.000 2 |
| Preterm birth ¨ | 141 (5.4) | 23 (4.9) | 1.000 3 |
| Neonatal admission | 310 (11.9) | 32 (6.9) | 0.024 3 |
| Any neonatal complications * | 466 (17.9) | 59 (12.7) | 0.088 3 |
| Apgar score < 7 at 5 min | 24 (0.9) | 5 (1.1) | 1.000 4 |
| Apgar score 5 min | 10.00 (10.00:10.00) | 10.00 (10.00:10.00) | 1.000 2 |
| Umbilical artery pH < 7 | 11 (0.4) | 1 (0.2) | 1.000 4 |
| Umbilical artery pH | 7.24 (7.18:7.29) | 7.24 (7.18:7.28) | 1.000 2 |
| Malformation | 61 (2.3) | 8 (1.7) | 1.000 3 |
| Low birth weight < 2500 g | 122 (4.7) | 21 (4.5) | 1.000 3 |
| Birth weight (gram) | 3478.19 (563.36) | 3538.10 (529.82) | 0.396 1 |
2.6. Women with Multiple Blood Samples During Pregnancy
3. Discussion
4. Materials and Methods
4.1. Positive Cohort
4.2. Negative Cohort
4.3. Analyses
4.4. SARS-CoV-2 Variants
4.5. Baseline Characteristics and Outcomes
4.6. Statistics
5. Conclusions
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 10 March 2023).
- Egerup, P.; Fich, L.; Christiansen, A.M.; Westergaard, D.; Severinsen, E.; Hviid, K.; Kolte, A.; Boje, A.; Bertelsen, M.L.; Prætorius, L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibodies at Delivery in Women, Partners, and Newborns. Obstet. Gynecol. 2020, 137, 49–55. [Google Scholar] [CrossRef] [PubMed]
- la Cour Freiesleben, N.; Egerup, P.; Hviid, K.; Severinsen, E.; Kolte, A.; Westergaard, D.; Fich, L.; Prætorius, L.; Zedeler, A.; Christiansen, A.M.; et al. SARS-CoV-2 in first trimester pregnancy: A cohort study. Hum. Reprod. 2021, 36, 40–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsatsaris, V.; Mariaggi, A.A.; Launay, O.; Couffignal, C.; Rousseau, J.; Ancel, P.; Marcault, E.; Ville, Y.; Cordier, A.G.; Vivanti, A.; et al. SARS-CoV-2 IgG antibody response in pregnant women at delivery. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102041. [Google Scholar] [CrossRef] [PubMed]
- Aabakke, A.; Krebs, L.; Petersen, T.; Kjeldsen, F.; Corn, G.; Wøjdemann, K.; Ibsen, M.; Jonsdottir, F.; Rønneberg, E.; Andersen, C.; et al. SARS-CoV-2 infection in pregnancy in Denmark—Characteristics and outcomes after confirmed infection in pregnancy: A nationwide, prospective, population-based cohort study. Acta Obstet. Gynecol. Scand. 2021, 100, 2097–2119. [Google Scholar] [CrossRef]
- Prabhu, M.; Cagino, K.; Matthews, K.; Friedlander, R.; Glynn, S.; Kubiak, J.; Yang, Y.; Zhao, Z.; Baergen, R.; DiPace, J.; et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1548–1556. [Google Scholar] [CrossRef]
- Overtoom, E.M.; Rosman, A.; Zwart, J.; Vogelvang, T.; Schaap, T.; Akker, T.; Bloemenkamp, K. SARS-CoV-2 infection in pregnancy during the first wave of COVID-19 in the Netherlands: A prospective nationwide population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2020, 129, 91–100. [Google Scholar] [CrossRef]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; Muelen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- Lokken, E.M.; Huebner, E.; Taylor, G.; Hendrickson, S.; Vanderhoeven, J.; Kachikis, A.; Coler, B.; Walker, C.; Sheng, J.; Al-Haddad, B.; et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2021, 225, 77.e1–77.e14. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Silva do Vale, M.; Cardona-Perez, J.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Fallach, N.; Segal, Y.; Agassy, J.; Perez, G.; Peretz, A.; Chodick, G.; Gazit, S.; Patalon, T.; Tov, A.; Goldshtein, I.; et al. Pregnancy outcomes after SARS-CoV-2 infection by trimester: A large, population-based cohort study. PLoS ONE 2022, 17, e0270893. [Google Scholar] [CrossRef]
- Lucot-Royer, L.; Nallet, C.; Vouga, M.; Puyraveau, M.; Mauny, F.; Marty-Quinternet, S.; Bertholdt, C.; Bory, J.P.; Devalland, C.; Canaguier, M.; et al. Analysis of the transplacental transmission of SARS-CoV-2 virus and antibody transfer according to the gestational age at maternal infection. Sci. Rep. 2024, 14, 3458. [Google Scholar] [CrossRef] [PubMed]
- Firan, M.; Bawdon, R.; Radu, C.; Ober, R.; Eaken, D.; Antohe, F.; Ghetie, V.; Ward, E. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of γ-globulin in humans. Int. Immunol. 2001, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J.; Oepkes, D.; Lopriore, E.; Bredius, R. Targeting neonatal Fc receptor: Potential clinical applications in pregnancy. Ultrasound Obstet. Gynecol. 2022, 60, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.; Blumberg, R.; Baker, K.; Agresta, M.; Ponzio, M. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and pre-term newborns. Am. J. Reprod. Immunol. 2019, 80, e12972. [Google Scholar] [CrossRef]
- Albrecht, M.; Pagenkemper, M.; Wiessner, C.; Spohn, M.; Lütgehetmann, M.; Jacobsen, H.; Gabriel, G.; Zazara, D.; Haertel, C.; Hecher, K.; et al. Infant immunity against viral infections is advanced by the placenta-dependent vertical transfer of maternal antibodies. Vaccines 2021, 40, 1563–1571. [Google Scholar] [CrossRef]
- Albrecht, M.; Arck, P. Vertically Transferred Immunity in Neonates: Mothers, Mechanism and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef]
- Capretti, M.G.; Marsico, C.; Gabrielli, L.; Vocale, C.; Arcuri, S.; Simonazzi, G.; Piccinini, A.; Brandolini, C.; Lazzarotto, T.; Corvaglia, L. Infants born following SARS-CoV-2 infection in pregnancy. Pediatrics 2022, 150, e2022056206. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Xu, Y.; Theis, K.; Arenas-Hernandez, M.; Miller, D.; Peyvandipour, A.; Bhatti, G.; Galez, J.; Gershater, M.; et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022, 13, 320. [Google Scholar] [CrossRef]
- Alouini, S.; Guinard, J.; Belin, O.; Mesnard, L.; Werner, E.; Prazuck, T.; Pichon, C. Maternal-Fetal Implications of SARS-CoV-2 Infection during Pregnancy, Viral, Serological Analyses of Placenta and Cord Blood. Int. J. Environ. Res. Public Health 2022, 19, 2105. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Zheng, J.; Liu, P.; Wei, C.; Guo, J.; Zhang, Y.; Zhao, D. Dynamic changes of acquires maternal SARS-CoV-2 IgG in Infants. Sci. Rep. 2021, 11, 8021. [Google Scholar] [CrossRef]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.; Mukhopadhyay, S.; Pfeifer, M.; Woodford, E.; Triebwasser, J.; Gerber, J.; Morris, J.; Weirick, M.; et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021, 175, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.; Sager, R.; Kuhn, P.; Nicolaides, K.; Schneider, H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 1996, 36, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Shook, L.; Brigida, S.; Guzman, R.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.; Sheehan, M.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef]
- Zimmermann, P.; Perrett, K.; Messina, N.; Donath, S.; Ritz, N.; van der Klis, F.; Curtis, N. The effect of maternal immunization during pregnancy on infant vaccine responses. eClinicalMedicine 2018, 13, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Li, J.; Collier, A.R.; Atyeo, C.; James, K.; Boatin, A.; Gray, K.; Bordt, E.; Shook, L.; Yonker, L.; et al. Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers with COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmissionduring pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef]
- Strid, P.; Zapata, L.; Tong, V.; Zambrano, L.; Woodworth Riser, A.; Galang, R.; Gilboa, S.; Ellington, S. Coronavirus Disease 2019 (COVID-19) Severity Among Women of Reproductive Age with Symptomatic Laboratory-Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection by Pregnancy Status-United States, 1 January 2020–25 December 2021. Clin. Infect. Dis. 2022, 75 (Suppl. 2), S317–S325. [Google Scholar] [CrossRef]
- Birol Ilter, P.; Prasad, S.; Mutlu, M.; Tekin, A.; O’Brian, P.; von Dadelszen, P.; Magee, L.; Tekin, S.; Tug, N.; Kalafat, E.; et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during Delta and Omicron waves. Ultrasound Obstet. Gynecol. 2022, 60, 96–102. [Google Scholar] [CrossRef]
- Mupanomunda, M.; Fakih, M.; Miller c Ottenbacher, A.; Winegar, A.; Roberts, P.; Kimathi, M.; Gianopoulos, J.; Cahill, A.; Cacchione, J.; Fogel, R.I. Comparison of Severe Maternal Morbidities Associated with Delivery During Periods of Circulation of Specific SARS-CoV-2 Variants. JAMA Netw. Open 2022, 5, e2226436. [Google Scholar] [CrossRef]
- The Danish Health Authority. Retningslinjer for Håndteringaf COVID-19 I Sundhedsvæsenet [Guidelines for the Management of COVID-19 in the Danish National Health Service]. Available online: https://www.sst.dk/da/udgivelser/2022/retningslinjer-for-haandtering-af-covid-19 (accessed on 10 October 2023).
- eSundhed. Available online: https://www.esundhed.dk/Emner/Graviditet-foedsler-og-boern/Nyfoedte-og-foedsler-1997-#tabpanel56464C3A43AE4B7D871FB8ED0BE17DE3 (accessed on 10 March 2023).
- Harritshøj, L.H.; Gybel-Brask, M.; Afzal, S.; Kamstruo, P.; Jørgensen, C.; Thomsen, M.; Hilsted, L.; Friis-Hansen, L.; Szecsi, P.; Pedersen, L.; et al. Comparison of 16 Serological SARS-CoV-2 Immunoassays in 16 Clinical Laboratories. J. Clin. Microbiol. 2021, 59, e02596-20. [Google Scholar] [CrossRef]
- Statens Serum Institut. Status for Udvikling af SARS-CoV-2 Varianter der Overvåges i Danmark. Available online: https://www.ssi.dk/sygdomme-beredskab-og-forskning/sygdomsovervaagning/c/covid-19-virusvarianter (accessed on 10 October 2023).
- Danish COVID-19 Genome Consortium. Available online: https://covid19genomics.dk/statistics (accessed on 28 February 2025).
- Hvidovre Hospital. Birth Department. Available online: https://www.hvidovrehospital.dk/baby (accessed on 10 October 2023).
- Statistics Denmark. Births. Available online: https://www.dst.dk/en/Statistik/emner/borgere/befolkning/foedsler (accessed on 10 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fich, L.; Christiansen, A.-M.H.; Hviid, K.V.R.; Aabakke, A.J.M.; Hoffmann, E.; Ingham, A.; Ollé-López, J.; Bello-Rodríguez, J.; Juul-Larsen, H.G.; Kelstrup, L.; et al. Maternal-Fetal Outcomes and Antibody Transfer, Depending on the Trimester of SARS-CoV-2 Infection in Non-Vaccinated Women—A Danish Nationwide Prospective Cohort Study. Int. J. Mol. Sci. 2025, 26, 2533. https://doi.org/10.3390/ijms26062533
Fich L, Christiansen A-MH, Hviid KVR, Aabakke AJM, Hoffmann E, Ingham A, Ollé-López J, Bello-Rodríguez J, Juul-Larsen HG, Kelstrup L, et al. Maternal-Fetal Outcomes and Antibody Transfer, Depending on the Trimester of SARS-CoV-2 Infection in Non-Vaccinated Women—A Danish Nationwide Prospective Cohort Study. International Journal of Molecular Sciences. 2025; 26(6):2533. https://doi.org/10.3390/ijms26062533
Chicago/Turabian StyleFich, Line, Ann-Marie Hellerung Christiansen, Kathrine Vauvert R. Hviid, Anna J. M. Aabakke, Eva Hoffmann, Andreas Ingham, Joaquim Ollé-López, Judith Bello-Rodríguez, Helle Gybel Juul-Larsen, Louise Kelstrup, and et al. 2025. "Maternal-Fetal Outcomes and Antibody Transfer, Depending on the Trimester of SARS-CoV-2 Infection in Non-Vaccinated Women—A Danish Nationwide Prospective Cohort Study" International Journal of Molecular Sciences 26, no. 6: 2533. https://doi.org/10.3390/ijms26062533
APA StyleFich, L., Christiansen, A.-M. H., Hviid, K. V. R., Aabakke, A. J. M., Hoffmann, E., Ingham, A., Ollé-López, J., Bello-Rodríguez, J., Juul-Larsen, H. G., Kelstrup, L., Perslev, K., Clausen, T. D., Rode, L., Vinter, C., Hedermann, G., Vestgaard, M. J., Farlie, R., Sørensen, A., Sundtoft, I., ... Nielsen, H. S. (2025). Maternal-Fetal Outcomes and Antibody Transfer, Depending on the Trimester of SARS-CoV-2 Infection in Non-Vaccinated Women—A Danish Nationwide Prospective Cohort Study. International Journal of Molecular Sciences, 26(6), 2533. https://doi.org/10.3390/ijms26062533





