Metabolomic Characterization and Bioinformatic Studies of Bioactive Compounds in Two Varieties of Psidium guajava L. Leaf by GC–MS Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening in Guava Leaves by GC–MS Analysis
2.2. Statistical Multivariate Analysis
2.3. Network Pharmacology and Target Protein Prediction
2.4. Molecular Docking Analysis
2.5. Interaction of Bioactive Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Methanol Extracts
3.3. Analysis and Compound Identification by GC–MS Chromatography
3.4. GC–MS Statistical Multivariate Analysis
3.5. Molecular Docking Analysis
3.6. Interaction of Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| GC–MS | Gas Chromatography coupled with Mass Spectrometry |
| PCA | Principal Component Analysis |
| BC | β-caryophyllene |
| CB2 | Cannabinoid Receptor 2 |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| BAX | Bcl2-Associated Protein X |
| BCL2 | B-cell Lymphoma 2 |
| AKT1 | Protein Kinase B |
References
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics Analysis and Biological Investigation of Three Malvaceae Plants. Phytochem. Anal. 2020, 31, 204–214. [Google Scholar] [CrossRef]
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Torres-León, C.; Ramírez-Guzmán, K.N.; Martínez, G.A.; Aguilar, C.N. Guava (Psidium Guajava L.) Fruit and Valorization of Industrialization By-Products. Processes 2021, 9, 1075. [Google Scholar] [CrossRef]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The Phytochemistry and Medicinal Value of Psidium Guajava (Guava). Clin. Phytoscience 2018, 4, 32. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M.S.; Maheshwari, C.; Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; et al. Guava (Psidium Guajava L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Foods 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Toledo, J.R.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Aceves-Hernandez, F.J. Caracterización de La Guayaba Cultivada En México. Rev. Mex. Cienc. Agric. 2022, 13, 1233–1245. [Google Scholar] [CrossRef]
- Padilla-Ramírez, J.S. Caracterización Morfológica de Fruto de la Colección Ex Situ de Psidium Guajava L. Agro Product. 2016, 9, 4. [Google Scholar]
- Pérez-Barraza, M.H.; Osuna-García, J.A.; Padilla-Ramírez, J.S.; Sánchez-Lucio, R.; Nolasco-González, Y.; González-Gaona, E. Fenología, productividad y calidad de fruto de guayaba pulpa crema y rosa en clima tropical en México. Interciencia 2015, 40, 198–203. [Google Scholar]
- Padilla Ramírez, J.S.; González Gaona, E.; Perales de la Cruz, M.Á. Nuevas variedades de guayaba (Psidium guajava L.). In Folleto Técnico 42; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Coyoacán, Mexican, 2010; ISBN 978-607-37-0634-6. [Google Scholar]
- Padilla-Ramírez, J.S.; González-Gaona, E.; Esquivel-Villagrana, F.; Mercado-Silva, E.; Hernández-Delgado, S.; Mayek-Pérez, N. Caracterización de Germoplasma Sobresaliente de Guayabo de la Región Calvillo-Cañones, México. Rev. Fitotec. Mex. 2002, 25, 393–399. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Xie, C.; Zhou, Y.; Yang, R.; Wu, J.; Xu, J.; Tu, K. Metabolomics Analysis of Different Quinoa Cultivars Based on UPLC-ZenoTOF-MS/MS and Investigation into Their Antioxidant Characteristics. Plants 2024, 13, 240. [Google Scholar] [CrossRef]
- Hill, C.B.; Roessner, U. Metabolic Profiling of Plants by GC–MS. In The Handbook of Plant Metabolomics; Wiley Online Books: Wiley, NJ, USA, 2013; pp. 1–23. [Google Scholar]
- Abadie, C.; Lalande, J.; Tcherkez, G. Exact Mass GC-MS Analysis: Protocol, Database, Advantages, and Application to Plant Metabolic Profiling. Plant Cell Environ. 2022, 45, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaye, A.F.; Hameed, I.H.; Kadhim, M.J. A Review: Uses of Gas Chromatography-Mass Spectrometry (GC-MS) Technique for Analysis of Bioactive Natural Compounds of Some Plants. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 81–85. [Google Scholar] [CrossRef]
- Sobrinho, A.C.G.; Corpes, R.S.; dos Santos, K.I.P.; Barra, I.M.M.; Miyagawa, H.K.; Santos, A.S.S. Untargeted GC-MS Metabolomics Applied to Wild Leaves and Callus Produced by Plant Tissue Culture of Hibiscus Sabdariffa L. Arab. J. Chem. 2022, 15, 104103. [Google Scholar] [CrossRef]
- Afzal, M.; Iqbal, R.; Mahmood, Z.; Zeshan, B.; Wattoo, J.I. Study of GC-MS and HPLC Characterized Metabolic Compounds In Guava (Psidium guajava L.) Leaves. Pak. J. Agric. Sci. 2019, 56, 3. [Google Scholar]
- Satyal, P.; Paudel, P.; Lamichhane, B.; Setzer, W.N. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Am. J. Essent. Oils Nat. Prod. 2015, 3, 11–14. [Google Scholar]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2021, 35, 1393–1397. [Google Scholar] [CrossRef]
- Xu, C.; Liang, Z.; Tang, D.; Xiao, T.; Tsunoda, M.; Zhang, Y.; Zhao, L.; Deng, S.; Song, Y. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Volatile Components from Guava Leaves. J. Essent. Oil Bear. Plants 2017, 20, 1536–1546. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-Mediated Plant–Plant Interactions: Volatile Organic Compounds as Modulators of Receiver Plant Defence, Growth, and Reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Dunkić, V.; Nazlić, M.; Ruščić, M.; Vuko, E.; Akrap, K.; Topić, S.; Milović, M.; Vuletić, N.; Puizina, J.; Grubešić, R.J.; et al. Hydrodistillation and Microwave Extraction of Volatile Compounds: Comparing Data for Twenty-One Veronica Species from Different Habitats. Plants 2022, 11, 902. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; Ávila-Oviedo, J.L.; Campos-Mendoza, F.J.; Valencia-Cantero, E. Microbial Volatile Organic Compounds: Insights into Plant Defense. Plants 2024, 13, 2013. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile Terpenes–Mediators of Plant-to-plant Communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Padovan, A.; Keszei, A.; Külheim, C.; Foley, W.J. The Evolution of Foliar Terpene Diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Wallis, I.R.; Foley, W.J. Mosaic Eucalypt Trees Suggest Genetic Control at a Point That Influences Several Metabolic Pathways. J. Chem. Ecol. 2012, 38, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Estrada, M. Limitations of Plant Stress Tolerance upon Heat and CO2 Exposure in Black Poplar: Assessment of Photosynthetic Traits and Stress Volatile Emissions. Plants 2024, 13, 1165. [Google Scholar] [CrossRef]
- Reddy, S.G.; Dolma, S.K.; Koundal, R.; Singh, B. Chemical Composition and Insecticidal Activities of Essential Oils against Diamondback Moth, Plutella Xylostella (L.) (Lepidoptera: Yponomeutidae). Nat. Prod. Res. 2016, 30, 1834–1838. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High Toxicity of Camphene and γ-Elemene from Wedelia Prostrata Essential Oil against Larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2018, 25, 10383–10391. [Google Scholar] [CrossRef]
- Tanabe, K.; Hayashi, C.; Katahira, T.; Sasaki, K.; Igami, K. Multiblock Metabolomics: An Approach to Elucidate Whole-Body Metabolism with Multiblock Principal Component Analysis. Comput. Struct. Biotechnol. J. 2021, 19, 1956–1965. [Google Scholar] [CrossRef]
- Li, D.S.; Shi, L.L.; Guo, K.; Luo, S.H.; Liu, Y.C.; Chen, Y.G.; Liu, Y.; Li, S.H. A New Sesquiterpene Synthase Catalyzing the Formation of (R)-β-Bisabolene from Medicinal Plant Colquhounia Coccinea Var. Mollis and Its Anti-Adipogenic and Antibacterial Activities. Phytochemistry 2023, 211, 113681. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a Sesquiterpene from the Essential Oil Extract of Opoponax (Commiphora Guidottii), Exhibits Cytotoxicity in Breast Cancer Cell Lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic Properties of the Terpenoids Aromadendrene and 1,8-Cineole from the Essential Oil of Eucalyptus Globulus against Antibiotic-Susceptible and Antibiotic-Resistant Pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Aromadendrene Oxide 2, Induces Apoptosis in Skin Epidermoid Cancer Cells through ROS Mediated Mitochondrial Pathway. Life Sci. 2018, 197, 19–29. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant Activity of Limonene on Normal Murine Lymphocytes: Relation to H2O2 Modulation and Cell Proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Singh, P.; Molla, M.; Yimer, Y.; Ewunetie, A.; Tadesse, T.Y.; Ayele, T.M.; Kefale, B. Nutraceuticals: A Source of Benefaction for Neuropathic Pain and Fibromyalgia. J. Funct. Foods 2022, 97, 105260. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Paula-Freire, L.I.G.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L.A. The Oral Administration of Trans-Caryophyllene Attenuates Acute and Chronic Pain in Mice. Phytomedicine 2014, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ettitaou, A.; Kabdy, H.; Oubella, K.; Raoui, K.; Oubahmane, M.; Aboufatima, R.; Elyazouli, L.; Garzoli, S.; Chait, A. Molecular docking of quercetin: A promising approach for the development of new anti-inflammatory and analgesic drugs. Nat. Prod. Res. 2024, 38, 1–10. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Thomas, A. Therapeutic applications for agents that act at CB1 and CB2 receptors. In The Cannabinoid Receptors; Reggio, P.H., Ed.; The Receptors; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Rigano, D.; Sirignano, C.; Taglialatela-Scafati, O. The potential of natural products for targeting PPARα. Acta Pharm. Sin. B 2017, 7, 427–438. [Google Scholar] [CrossRef]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Honda, A.; Kamata, S.; Akahane, M.; Machida, Y.; Uchii, K.; Shiiyama, Y.; Habu, Y.; Miyawaki, S.; Kaneko, C.; Oyama, T.; et al. Functional and Structural Insights into Human PPARα/δ/γ Subtype Selectivity of Bezafibrate, Fenofibric Acid, and Pemafibrate. Int. J. Mol. Sci. 2022, 23, 4726. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Mohammad, T.; Padder, R.A.; Hassan, M.I.; Husain, M. Thymoquinone and quercetin induce enhanced apoptosis in non-small cell lung cancer in combination through the Bax/Bcl2 cascade. J. Cell. Biochem. 2022, 123, 259–274. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, L.; Gao, M.; Wang, S.; Meng, L.; Guo, L. Molecular docking and in vitro experiments verified that kaempferol induced apoptosis and inhibited human HepG2 cell proliferation by targeting BAX, CDK1, and JUN. Mol. Cell. Biochem. 2023, 478, 767–780. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, X.; Li, Y.; Wang, R. Analysis of the Binding Sites on BAX and the Mechanism of BAX Activators through Extensive Molecular Dynamics Simulations. J. Chem. Inf. Model. 2022, 62, 5208–5222. [Google Scholar] [CrossRef] [PubMed]
- Rosdi, M.N.M.; Arif, S.M.; Abu Bakar, M.H.; Razali, S.A.; Zulkifli, R.M.; Ya’akob, H. Molecular docking studies of bioactive compounds from Annona muricata Linn as potential inhibitors for Bcl-2, Bcl-w and Mcl-1 antiapoptotic proteins. Apoptosis 2018, 23, 27–40. [Google Scholar] [CrossRef]
- Sathishkumar, N.; Sathiyamoorthy, S.; Ramya, M.; Yang, D.U.; Lee, H.N.; Yang, D.C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzym. Inhib. Med. Chem. 2012, 27, 685–692. [Google Scholar] [CrossRef]
- Liu, W.; Bulgaru, A.; Haigentz, M.; Stein, C.A.; Perez-Soler, R.; Mani, S. The BCL2-family of protein ligands as cancer drugs: The next generation of therapeutics. Curr. Med. Chem.-Anti-Cancer Agents 2003, 3, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Karim, S. Structure-Based Profiling of Potential Phytomolecules with AKT1 a Key Cancer Drug Target. Molecules 2023, 28, 2597. [Google Scholar] [CrossRef]
- Mahajan, P.; Wadhwa, B.; Barik, M.R.; Malik, F.; Nargotra, A. Combining ligand- and structure-based in silico methods for the identification of natural product-based inhibitors of Akt1. Mol. Divers. 2020, 24, 45–60. [Google Scholar] [CrossRef]
- Whiting, Z.M.; Yin, J.; de la Harpe, S.M.; Vernall, A.J.; Grimsey, N.L. Developing the Cannabinoid Receptor 2 (CB2) Pharmacopoeia: Past, Present, and Future. Trends Pharmacol. Sci. 2022, 43, 754–771. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Porto, D.S.; Port, B.D.C.B.; Conte, J.; Argenta, D.F.; Balleste, M.P.; Micke, G.A.; Campos, Â.M.M.; Caumo, K.S.; Caon, T. Development of Ophthalmic Nanoemulsions of β-Caryophyllene for the Treatment of Acanthamoeba Keratitis. Int. J. Pharm. 2024, 659, 124252. [Google Scholar] [CrossRef] [PubMed]
- Ricardi, C.; Barachini, S.; Consoli, G.; Marazziti, D.; Polini, B.; Chiellini, G. Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders. Int. J. Mol. Sci. 2024, 25, 3203. [Google Scholar] [CrossRef] [PubMed]
- Kamata, S.; Oyama, T.; Saito, K.; Honda, A.; Yamamoto, Y.; Suda, K.; Ishikawa, R.; Itoh, T.; Watanabe, Y.; Shibata, T.; et al. PPARα Ligand-Binding Domain Structures with Endogenous Fatty Acids and Fibrates. iScience 2020, 23, 101727. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef]
- Xu, J.; Chavis, J.A.; Racke, M.K.; Drew, P.D. Peroxisome Proliferator-Activated Receptor-α and Retinoid X Receptor Agonists Inhibit Inflammatory Responses of Astrocytes. J. Neuroimmunol. 2006, 176, 95–105. [Google Scholar] [CrossRef]
- Straus, D.S.; Glass, C.K. Anti-Inflammatory Actions of PPAR Ligands: New Insights on Cellular and Molecular Mechanisms. Trends Immunol. 2007, 28, 551–558. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.-J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of Metabolism and As Therapeutic Targets in Cardiovascular Disease. Part I: PPAR-α. Futur. Cardiol. 2017, 13, 259–278. [Google Scholar] [CrossRef]
- Cheng, A.Y.Y.; Leiter, L.A. PPAR-alpha: Therapeutic Role in Diabetes-related Cardiovascular Disease. Diabetes Obes. Metab. 2008, 10, 691–698. [Google Scholar] [CrossRef]
- Al-Harbi, L.N. Morin Prevents Non-Alcoholic Hepatic Steatosis in Obese Rats by Targeting the Peroxisome Proliferator-Activated Receptor Alpha (PPARα). Life 2024, 14, 945. [Google Scholar] [CrossRef]
- Spaner, D.E.; Lee, E.; Shi, Y.; Wen, F.; Li, Y.; Tung, S.; McCaw, L.; Wong, K.; Gary-Gouy, H.; Dalloul, A.; et al. PPAR-Alpha Is a Therapeutic Target for Chronic Lymphocytic Leukemia. Leukemia 2013, 27, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Spitz, A.Z.; Gavathiotis, E. Physiological and Pharmacological Modulation of BAX. Trends Pharmacol. Sci. 2022, 43, 206–220. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Ye, N.; Wild, C.; Chen, H.; Zhou, J. Direct Activation of Bax Protein for Cancer Therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, L.; Liu, D.; Luan, S.; Huang, M.; Zhao, L. Directly Targeting BAX for Drug Discovery: Therapeutic Opportunities and Challenges. Acta Pharm. Sin. B 2024, 14, 2378–2401. [Google Scholar] [CrossRef]
- Reed, J.C. Proapoptotic Multidomain Bcl-2/Bax-Family Proteins: Mechanisms, Physiological Roles, and Therapeutic Opportunities. Cell Death Differ. 2006, 13, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Dinsdale, D.; Dyer, M.J.S.; Cohen, G.M. Bcl-2 Inhibitors: Small Molecules with a Big Impact on Cancer Therapy. Cell Death Differ. 2009, 16, 360–367. [Google Scholar] [CrossRef]
- Campàs, C.; Cosialls, A.M.; Barragán, M.; Iglesias-Serret, D.; Santidrián, A.F.; Coll-Mulet, L.; de Frias, M.; Domingo, A.; Pons, G.; Gil, J. Bcl-2 Inhibitors Induce Apoptosis in Chronic Lymphocytic Leukemia Cells. Exp. Hematol. 2006, 34, 1663–1669. [Google Scholar] [CrossRef]
- Tahir, S.K.; Yang, X.; Anderson, M.G.; Morgan-Lappe, S.E.; Sarthy, A.V.; Chen, J.; Warner, R.B.; Ng, S.-C.; Fesik, S.W.; Elmore, S.W.; et al. Influence of Bcl-2 Family Members on the Cellular Response of Small-Cell Lung Cancer Cell Lines to ABT-737. Cancer Res. 2007, 67, 1176–1183. [Google Scholar] [CrossRef]
- D’Aguanno, S.; Del Bufalo, D. Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer. Cells 2020, 9, 1287. [Google Scholar] [CrossRef]
- Hill, M.M.; Hemmings, B.A. Inhibition of Protein Kinase B/Akt. Implications for cancer therapy. Pharmacol. Ther. 2002, 93, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.F.; Bilodeau, M.T.; Lindsley, C.W. The Akt/PKB Family of Protein Kinases: A Review of Small Molecule Inhibitors and Progress Towards Target Validation. Curr. Top. Med. Chem. 2010, 10, 458–477. [Google Scholar] [CrossRef]
- Hinz, N.; Jücker, M. Distinct Functions of AKT Isoforms in Breast Cancer: A Comprehensive Review. Cell Commun. Signal. 2019, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Kaur, P.; Shukla, S.; Gupta, S. Plant Flavonoid Apigenin Inactivates Akt to Trigger Apoptosis in Human Prostate Cancer: An in Vitro and in Vivo Study. Carcinogenesis 2008, 29, 2210–2217. [Google Scholar] [CrossRef]
- Lakshmi, T.P.; Kumar, A.; Vijaykumar, V.; Natarajan, S.; Krishna, R. Identification of Natural Allosteric Inhibitor for Akt1 Protein through Computational Approaches and in Vitro Evaluation. Int. J. Biol. Macromol. 2017, 96, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Alwhaibi, A.; Verma, A.; Adil, M.S.; Somanath, P.R. The Unconventional Role of Akt1 in the Advanced Cancers and in Diabetes-Promoted Carcinogenesis. Pharmacol. Res. 2019, 145, 104270. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeong, J.H.; Seo, J.W.; Shin, C.G.; Kim, Y.S.; In, J.G.; Yang, D.C.; Yi, J.S.; Choi, Y.E. Enhanced Triterpene and Phytosterol Biosynthesis in Panax Ginseng Overexpressing Squalene Synthase Gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef]
- Aminfar, Z.; Tohidfar, M. In Silico Analysis of Squalene Synthase in Fabaceae Family Using Bioinformatics Tools. J. Genet. Eng. Biotechnol. 2018, 16, 739–747. [Google Scholar] [CrossRef]
- Hazra, A.; Dutta, M.; Dutta, R.; Bhattacharya, E.; Bose, R.; Biswas, S.M. Squalene Synthase in Plants–Functional Intricacy and Evolutionary Divergence While Retaining a Core Catalytic Structure. Plant Gene 2023, 33, 100403. [Google Scholar] [CrossRef]
- Patel, N.; Patel, P.; Kendurkar, S.V.; Thulasiram, H.V.; Khan, B.M. Overexpression of Squalene Synthase in Withania somnifera Leads to Enhanced Withanolide Biosynthesis. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 409–420. [Google Scholar] [CrossRef]
- Seo, J.W.; Jeong, J.H.; Shin, C.G.; Lo, S.C.; Han, S.S.; Yu, K.W.; Harada, E.; Han, J.Y.; Choi, Y.E. Overexpression of Squalene Synthase in Eleutherococcus senticosus Increases Phytosterol and Triterpene Accumulation. Phytochemistry 2005, 66, 869–877. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Palazon, J.; Bonfill, M.; Hidalgo-Martinez, D. Enhancing Centelloside Production in Centella asiatica Hairy Root Lines through Metabolic Engineering of Triterpene Biosynthetic Pathway Early Genes. Plants 2023, 12, 3363. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A.; Gupta, P. Advances in Biosynthesis, Regulation, and Metabolic Engineering of Plant Specialized Terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Zhang, A.; Xiong, Y.; Fang, J.; Jiang, X.; Wang, T.; Liu, K.; Peng, H.; Zhang, X. Diversity and Functional Evolution of Terpene Synthases in Rosaceae. Plants 2022, 11, 736. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 258–303. [Google Scholar]
- Canal, D.; Escudero, F.L.G.; Mendes, L.A.; da Silva Ferreira, M.F.; Turchetto-Zolet, A.C. Genome-Wide Identification, Expression Profile and Evolutionary Relationships of TPS Genes in the Neotropical Fruit Tree Species Psidium cattleyanum. Sci. Rep. 2023, 13, 3930. [Google Scholar] [CrossRef] [PubMed]
- Canal, D.; dos Santos, P.H.D.; de Avelar Carpinetti, P.; Silva, M.A.; Fernandes, M.; Brustolini, O.J.B.; Ferreira, A.; da Silva Ferreira, M.F. Exploring the Versatility of Sesquiterpene Biosynthesis in Guava Plants: A Comparative Genome-Wide Analysis of Two Cultivars. Sci. Rep. 2024, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Afendi, F.M.; Altaf-UI-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of medicinal plants: The importance of multivariate analysis of analytical chemistry data. Curr. Comput.-Aided Drug Des. 2010, 6, 179–196. [Google Scholar] [CrossRef]
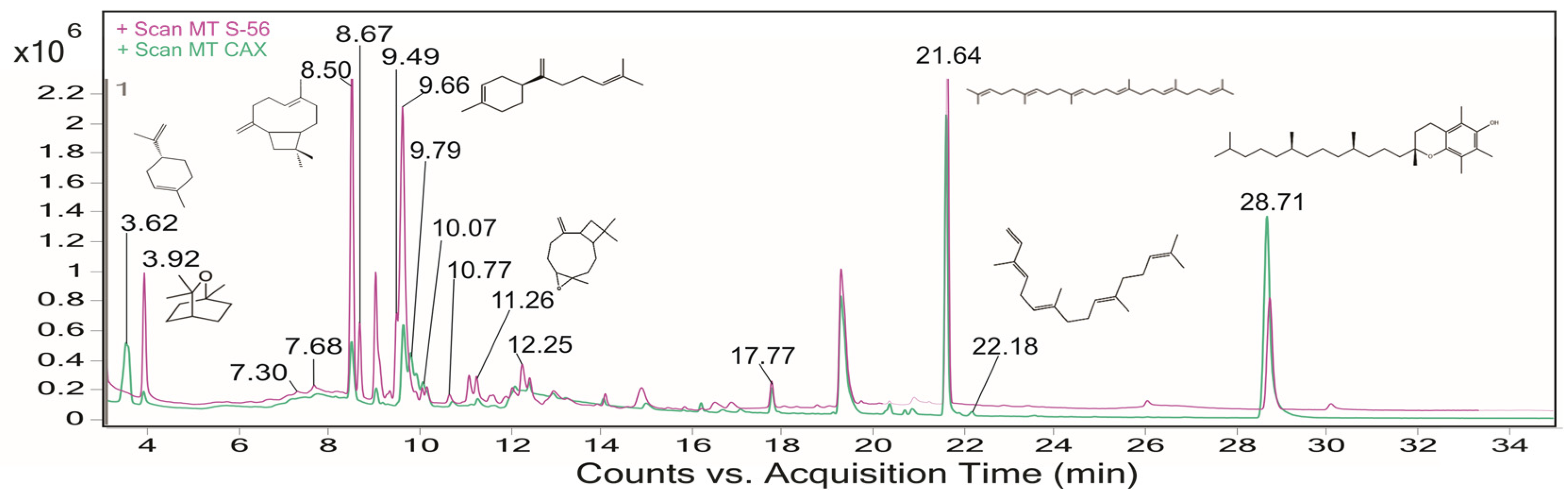
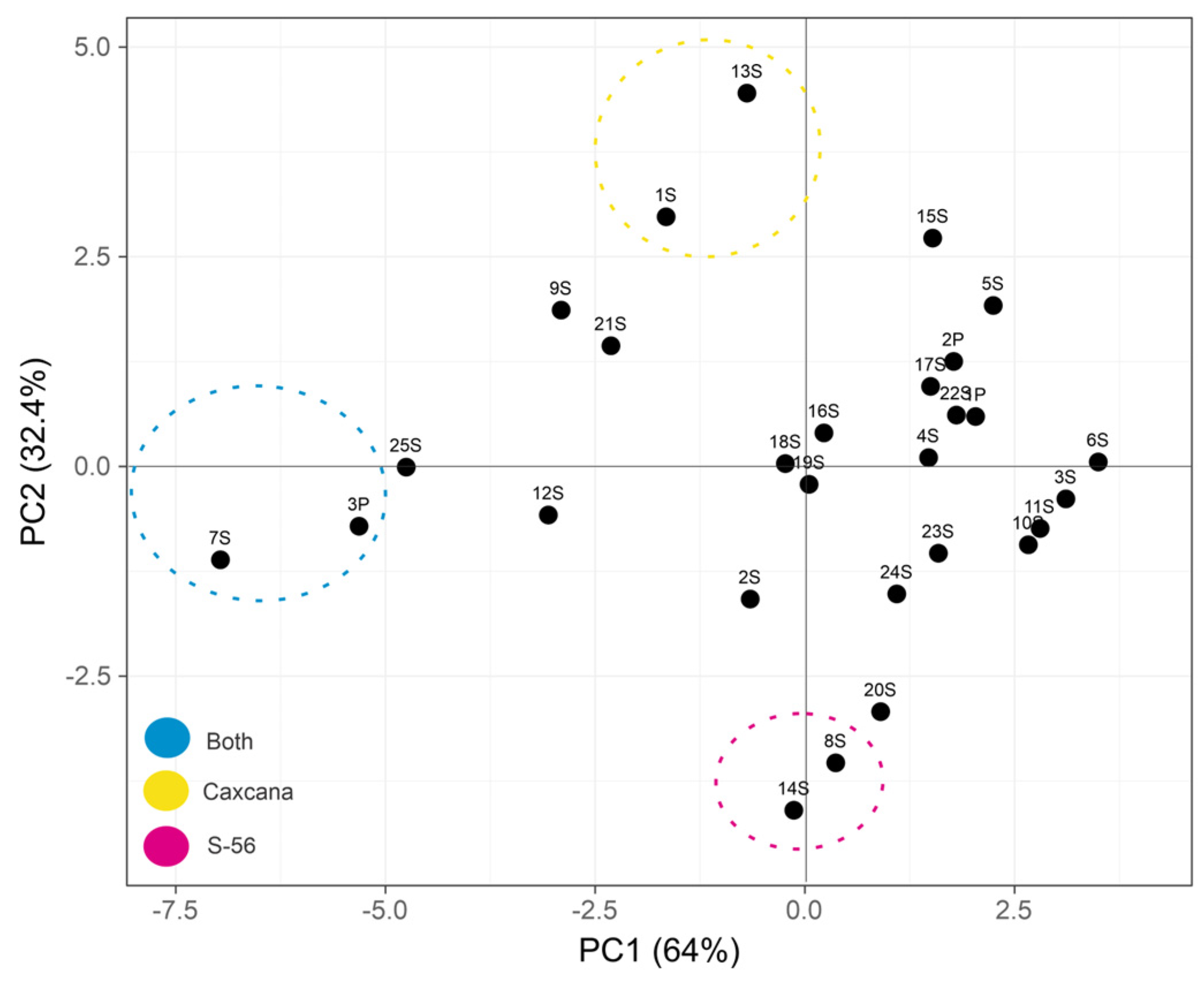
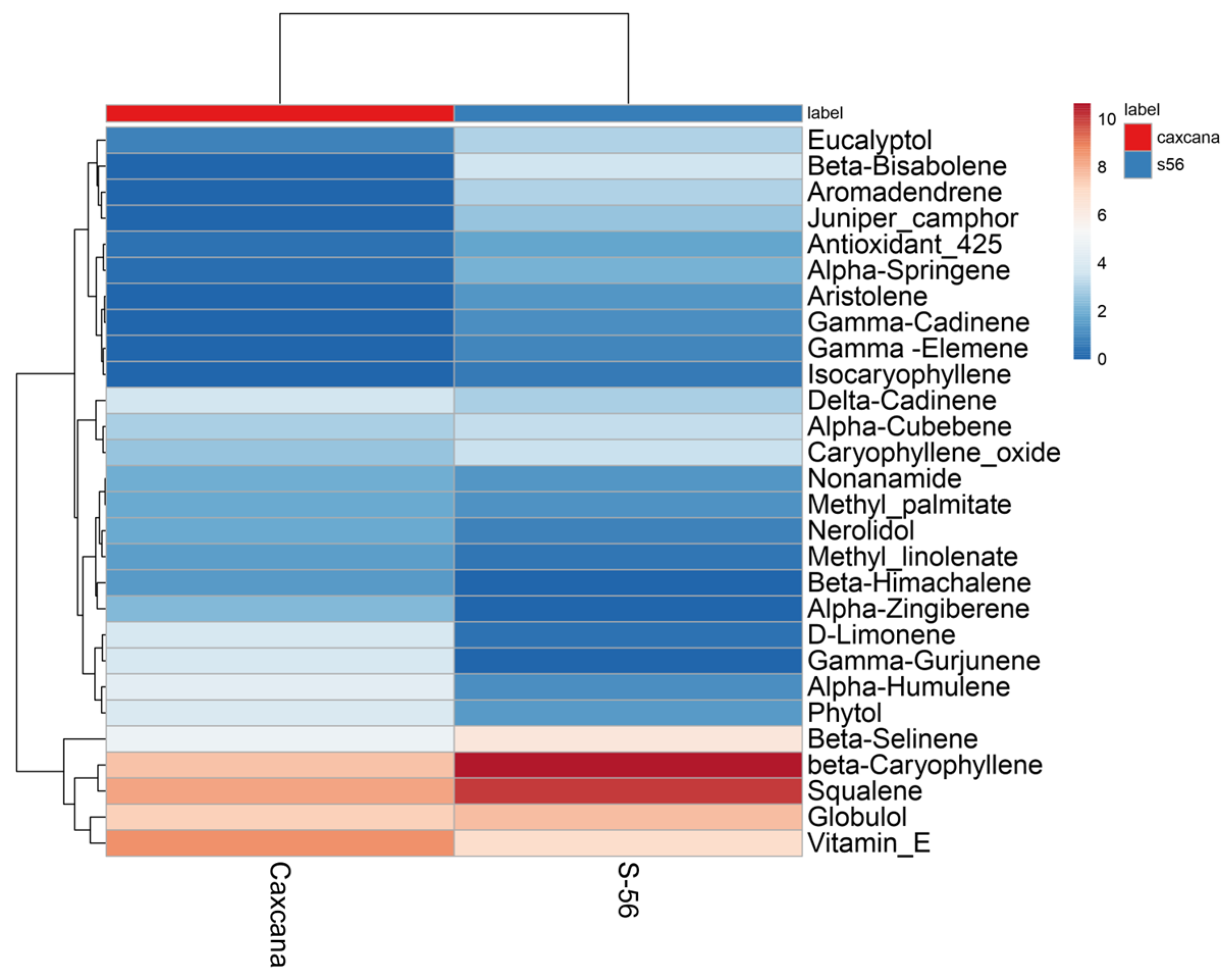

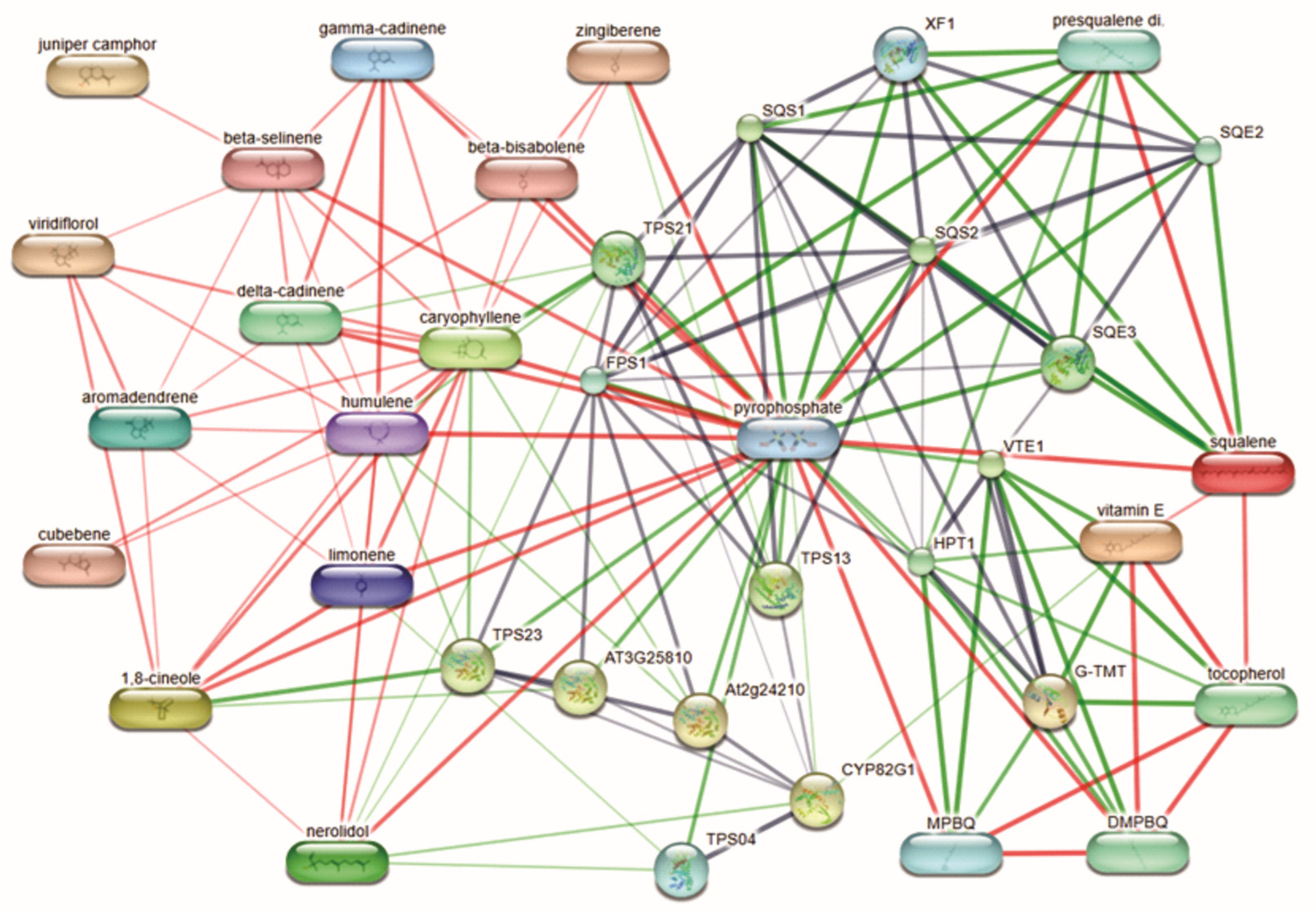

| Code | RT | CAS | Compounds | % Area Caxcana | % Area S-56 | M+ | m/z (Abundance) |
|---|---|---|---|---|---|---|---|
| Primary Metabolites | |||||||
| 1P | 14.07 | 112-39-0 | Methyl palmitate | 0.80 | 0.54 | 270.3 | 74.0 |
| 2P | 16.21 | 301-00-8 | Methyl linolenate | 1.32 | 0.36 | 292.2 | 79.1 |
| 3P | 21.64 | 7683-64-9 | Squalene | 10.83 | 13.27 | 410.4 | 69.1 |
| Secondary Metabolites | |||||||
| 1S | 3.62 | 5989-27-5 | D-Limonene | 9.76 | 0.96 | 136.1 | 68.1 |
| 2S | 3.92 | 470-82-6 | Eucalyptol | 1.28 | 4.65 | 154.1 | 43.0 |
| 3S | 7.30 | 3242-08-8 | γ-Elemene | np | 0.56 | 204.2 | 121.1 |
| 4S | 7.68 | 17699-14-8 | α-Cubebene | 0.92 | 1.07 | 204.2 | 119.1 |
| 5S | 8.16 | 1461-03-6 | β-Himachalene | 1.4 | np | 204.2 | 119.1 |
| 6S | 8.31 | 13877-93-5 | Isocaryophyllene | np | 0.24 | 93.1 | 93.1 |
| 7S | 8.50 | 87-44-5 | β-Caryophyllene | 16.46 | 23.06 | 204.2 | 93.1 |
| 8S | 8.67 | 72747-25-2 | (+)-Aromadendrene | np | 6.66 | 204.2 | 161.1 |
| 9S | 9.02 | 6753-98-6 | α-Humulene | 10.28 | 2.67 | 204.2 | 93.1 |
| 10S | 9.09 | 6831-16-9 | (-)-Aristolene | np | 1.17 | 204.2 | 91.1 |
| 11S | 9.33 | 39029-41-9 | γ-Cadinene | np | 0.89 | 204.2 | 161.1 |
| 12S | 9.49 | 17066-67-0 | β-Selinene | 5.20 | 6.85 | 204.2 | 105.1 |
| 13S | 9.59 | 22567-17-5 | γ-Gurjunene | 10.45 | np | 204.2 | 189.2 |
| 14S | 9.66 | 495-61-4 | β-Bisabolene | np | 9.26 | 204.2 | 93.1 |
| 15S | 9.79 | 495-60-3 | α-Zingiberene | 3.84 | np | 204.2 | 119.1 |
| 16S | 10.07 | 483-76-1 | δ-Cadinene | 1.99 | 1.58 | 204.2 | 161.1 |
| 17S | 10.77 | 142-50-7 | Nerolidol | 1.33 | 0.58 | 222.2 | 69.1 |
| 18S | 11.10 | 489-41-8 | (-)-Globulol | 2.09 | 2.21 | 222.2 | 43.1 |
| 19S | 11.26 | 1139-30-6 | Caryophyllene oxide | 1.66 | 2.22 | 220.2 | 79.1 |
| 20S | 12.25 | 473-04-1 | Juniper camphor | np | 4.69 | 222.2 | 43.0 |
| 21S | 12.42 | 150-86-7 | Phytol | 7.50 | 2.59 | 296.3 | 68.1 |
| 22S | 17.77 | 1120-07-6 | Nonanamide | 0.94 | 0.62 | 157.1 | 59.0 |
| 23S | 20.70 | 88-24-4 | Antioxidant 425 | 0.35 | 1.83 | 368.3 | 191.1 |
| 24S | 22.18 | 77898-97-6 | α-Springene | 0.37 | 2.59 | 418.6 | 149.1 |
| 25S | 28.71 | 59-02-9 | Vitamin E | 11.24 | 8.88 | 430.4 | 165.1 |
| CB2 Protein | ||||||
|---|---|---|---|---|---|---|
| Compound (Ligand) | PubChem ID | Origin | Binding Affinity (kcal/mol) | Cavity Size | Aminoacidic Interaction | Reference |
| β-caryophyllene | 5281515 | Natural | −8.6 | 1654 | 87–288 | |
| Quercetin | 5280343 | Natural | −8.8 | 1654 | 24–288 | [39] |
| Codeine | 5284371 | Natural | −9.1 | 1654 | 110–288 | [40] |
| Cannabidiol | 644019 | Natural | −9.3 | 1654 | 87–288 | [41] |
| HU-308 | 5311172 | Synthetic | −9.5 | 1654 | 87–288 | [41] |
| JWH-015 | 4273754 | Synthetic | −10.6 | 1654 | 87–285 | [41] |
| Levonantradol | 5361881 | Synthetic | −11.0 | 1654 | 87–288 | [40] |
| PPARα protein | ||||||
| β-caryophyllene | 5281515 | Natural | −7.2 | 3640 | 272–464 | |
| Arachidonic Acid | 444899 | Natural | −7.0 | 3640 | 220–464 | [42] |
| Resveratrol | 445154 | Natural | −8.4 | 3640 | 269–464 | [43] |
| Fenofibric acid | 64929 | Synthetic | −7.8 | 3640 | 269–464 | [44] |
| Bezafibrate | 39042 | Synthetic | −8.8 | 3640 | 272–464 | [44] |
| Pemafibrate | 11526038 | Synthetic | −9.2 | 3640 | 218–355 | [44] |
| BAX protein | ||||||
| β-caryophyllene | 5281515 | Natural | −5.5 | 183 | 13–159 | |
| Thymoquinone | 10281 | Natural | −5.3 | 148 | 13–159 | [45] |
| Kaempferol | 5280863 | Natural | −6.3 | 326 | 13–159 | [46] |
| Quercetin | 5280343 | Natural | −6.4 | 180 | 13–159 | [45] |
| BAM7 | 3101542 | Synthetic | −7.2 | 148 | 28–61 | [47] |
| SMBA1 | 6070109 | Synthetic | −7.4 | 148 | 29–61 | [47] |
| BTSA1 | 3857348 | Synthetic | −7.8 | 183 | 29–61 | [47] |
| BCL2 protein | ||||||
| β-caryophyllene | 5281515 | Natural | −7.1 | 124 | 60–167 | |
| Maytansine | 5281828 | Natural | −7.2 | 272 | 65–114 | [48] |
| Annocatalin | 44566987 | Natural | −7.3 | 272 | 69–114 | [48] |
| Ginsenoside Rg1 | 441923 | Natural | −8.4 | 272 | 60–167 | [49] |
| Obatoclax | 11404337 | Synthetic | −7.5 | 94 | 65–114 | [50] |
| Navitoclax | 24978538 | Synthetic | −9.2 | 272 | 65–114 | [50] |
| Sonrotoclax | 149553242 | Synthetic | −9.4 | 91 | 65–114 | [50] |
| AKT1 protein | ||||||
| β-caryophyllene | 5281515 | Natural | −7.1 | 1673 | 164–291 | |
| Tehranolide | 6711941 | Natural | −7.3 | 1673 | 156–438 | [51] |
| Artemisinin | 68827 | Natural | −7.8 | 1673 | 156–292 | [51] |
| Shogaol | 5281794 | Natural | −7.5 | 1673 | 156–438 | [51] |
| XM1 | 46870040 | Synthetic | −8.7 | 1668 | 156–442 | [52] |
| Ipatasertib | 24788740 | Synthetic | −8.7 | 1668 | 156–442 | [52] |
| Uprosertib | 51042438 | Synthetic | −9.4 | 1668 | 156–438 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivia-Padilla, A.V.; Sharma, A.; Zegbe, J.A.; Morales-Domínguez, J.F. Metabolomic Characterization and Bioinformatic Studies of Bioactive Compounds in Two Varieties of Psidium guajava L. Leaf by GC–MS Analysis. Int. J. Mol. Sci. 2025, 26, 2530. https://doi.org/10.3390/ijms26062530
Valdivia-Padilla AV, Sharma A, Zegbe JA, Morales-Domínguez JF. Metabolomic Characterization and Bioinformatic Studies of Bioactive Compounds in Two Varieties of Psidium guajava L. Leaf by GC–MS Analysis. International Journal of Molecular Sciences. 2025; 26(6):2530. https://doi.org/10.3390/ijms26062530
Chicago/Turabian StyleValdivia-Padilla, Ana Victoria, Ashutosh Sharma, Jorge A. Zegbe, and José Francisco Morales-Domínguez. 2025. "Metabolomic Characterization and Bioinformatic Studies of Bioactive Compounds in Two Varieties of Psidium guajava L. Leaf by GC–MS Analysis" International Journal of Molecular Sciences 26, no. 6: 2530. https://doi.org/10.3390/ijms26062530
APA StyleValdivia-Padilla, A. V., Sharma, A., Zegbe, J. A., & Morales-Domínguez, J. F. (2025). Metabolomic Characterization and Bioinformatic Studies of Bioactive Compounds in Two Varieties of Psidium guajava L. Leaf by GC–MS Analysis. International Journal of Molecular Sciences, 26(6), 2530. https://doi.org/10.3390/ijms26062530







