A Simplified and Robust Model for the Study of Diabetic Nephropathy: Streptozotocin-Induced Diabetic Mice Fed a High-Protein Diet
Abstract
1. Introduction
2. Results
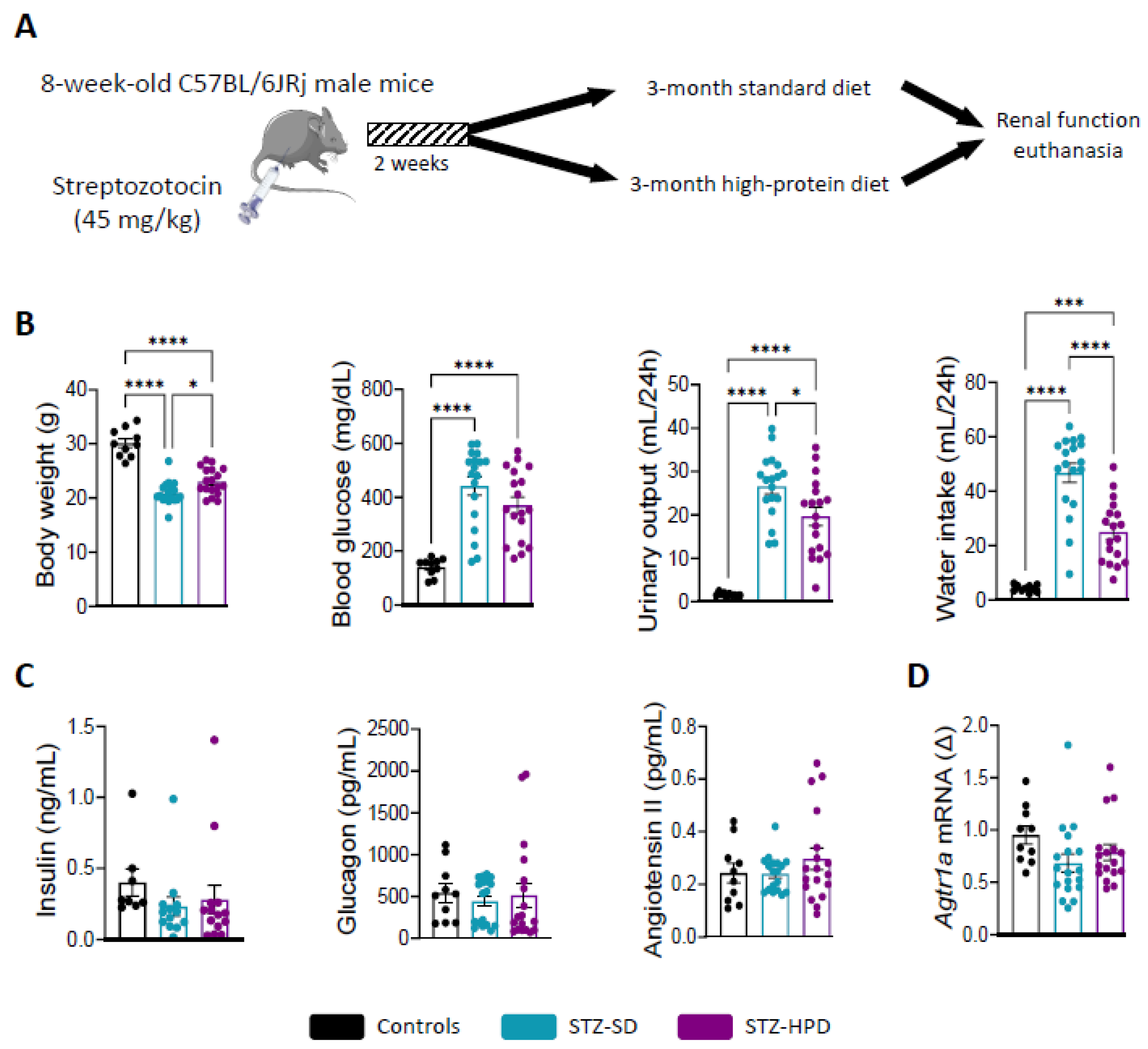
2.1. Impact of Treatments on Diabetes-Related Parameters
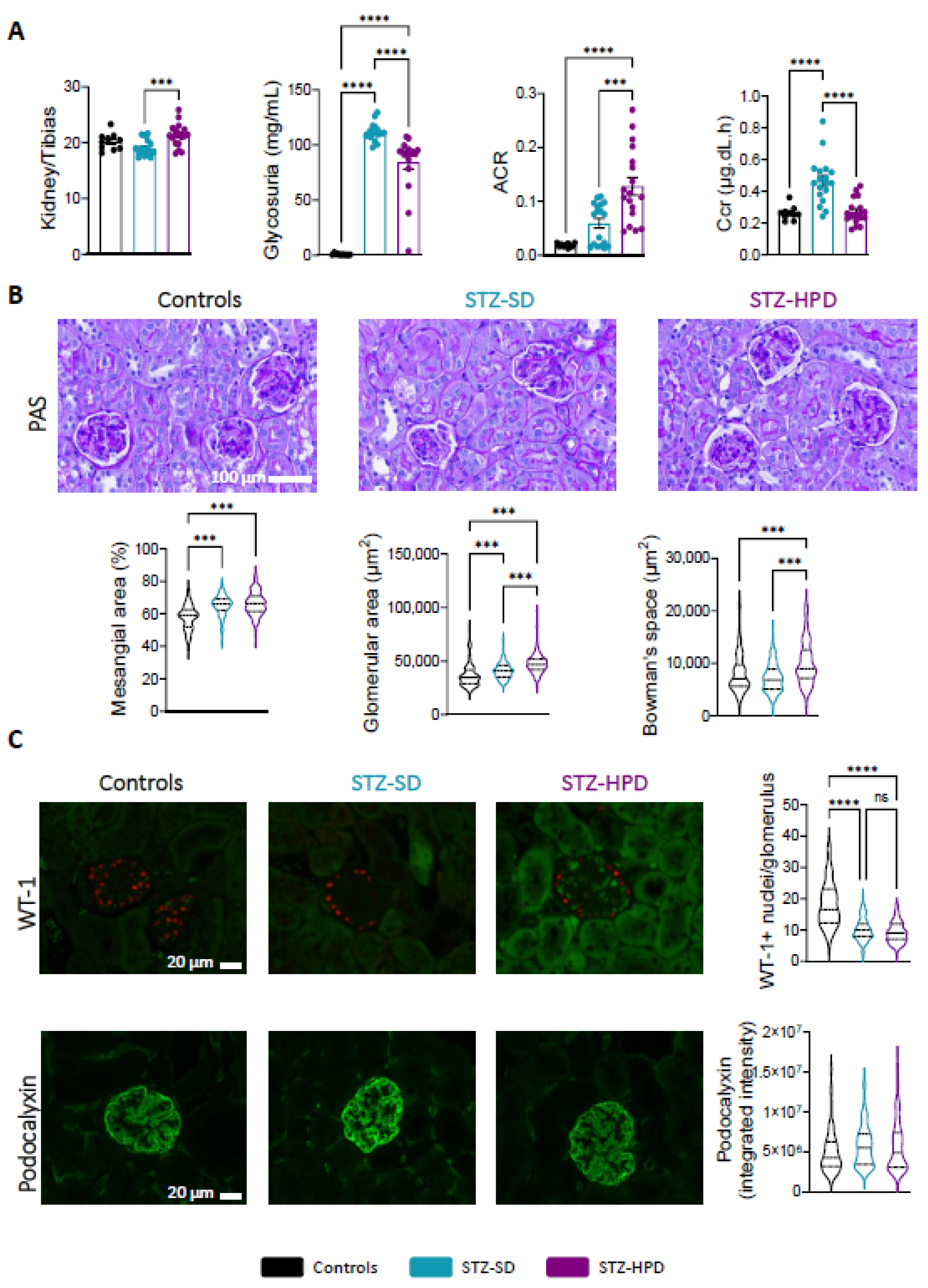
2.2. Impact of High-Protein Diet on Renal Glomeruli
2.3. Impact of High-Protein Diet on Renal Tubules
2.4. Impact of High-Protein Diet on Renal Fibrosis
3. Discussion
4. Materials and Methods
4.1. Animal Experimentation
4.2. Animals and Diet
4.3. Biochemical Markers
4.4. Histology
4.5. PCR
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | Albumin-to-Creatinine Ratio |
| AGEs | Advanced Glycation End-products |
| Ccr | Creatinine Clearance |
| CKD | Chronic Kidney Disease |
| DN | Diabetic Nephropathy |
| GFR | Glomerular Filtration Rate |
| HPD | High Protein Diet |
| PTEC | Proximal Tubular Epithelial Cells |
| ROS | Reactive Oxygen Species |
| SD | Standard Diet |
| STZ | Streptozotocin |
Appendix A
| Protein | Antigen Retrieval | Reference | Dilution | Incubation Time |
|---|---|---|---|---|
| Lipocalin-2 | EDTA pH 9.0 | Bio Techne–AF1857 (Minneapolis, MN, USA) | 1/100 | Overnight, 4 °C |
| LRP2 | EDTA pH 9.0 | BiCell Scientific-31012 (San Diego, CA, USA) | 1/300 | 1 h, RT |
| Podocalyxin | EDTA pH 9.0 | R&D Systems-AF1556 (Minneapolis, MN, USA) | 1/1000 | Overnight, 4 °C |
| SGLT2 | EDTA pH 9.0 | BiCell Scientific-20802 (San Diego, CA, USA) | 1/500 | 1 h, RT |
| WT-1 | Citrate pH 6.0 | BiCell Scientific-00180 (San Diego, CA, USA) | 1/400 | Overnight, 4 °C |
| Gene | Forward | Reverse |
|---|---|---|
| Agtr1a | AACAGCTTGGTGGTGATCGTC | CATAGCGGTATAGACAGCCCA |
| Ccl2 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| Dkk3 | CTCGGGGGTATTTTGCTGTGT | TCCTCCTGAGGGTAGTTGAGA |
| Hprt | AGTCCCAGCGTCGTGATTAG | TTTCCAAATCCTCGGCATAATGA |
| Il6 | GAACAACGATGATGCACTTGC | TCCAGGTAGCTATGGTATTCC |
| Lcn2 | GCAGGTGGTACGTTGTGGG | CTCTTGTAGCTCATAGATGGTGC |
| Nox2 | CCTCTACCAAAACCATTCGGAG | CTGTCCACGTACAATTCGTTCA |
| Nox4 | GAAGGGGTTAAACACCTCTGC | ATGCTCTGCTTAAACACAATCCT |
| Rpl19 | ATGAGTATGCTCAGGCTACAGA | GCATTGGCGATTTCATTGGTC |
| Rpl32 | TTAAGCGAAACTGGCGGAAAC | TTGTTGCTCCCATAACCGATG |
| Rplp0 | AGATTCGGGATATGCTGTTGGC | TCGGGTCCTAGACCAGTGTTC |
| Tim1 | ACATATCGTGGAATCACAACGAC | GCTACGACGTGGGCTACAG |
| Tnf | CCCTCACACTCAGATCATCTTCT | CCTGGACGTTAAAGGTCGTCA |
References
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Collaboration, G.C.K.D. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.P.; Tauber-Lassen, E.; Jensen, B.R.; Parving, H.-H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002, 62, 220–228. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef]
- Pedrini, M.T.; Levey, A.S.; Lau, J.; Chalmers, T.C.; Wang, P.H. The Effect of Dietary Protein Restriction on the Progression of Diabetic and Nondiabetic Renal Diseases. Ann. Intern. Med. 1996, 124, 627–632. [Google Scholar] [CrossRef]
- Walker, J.D.; Dodds, R.A.; Murrells, T.J.; Bending, J.J.; Mattock, M.B.; Keen, H.; Viberti, G.C. Restriction of dietary protein and progression of renal failure in diabetic nephropathy. Lancet 1989, 334, 1411–1415. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M.; Avesani, C.M.; D’Alessandro, C.; Carrero, J.J.; Piccoli, G.B. Medical Nutritional Therapy for Patients with Chronic Kidney Disease not on Dialysis: The Low Protein Diet as a Medication. J. Clin. Med. 2020, 9, 3644. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Paller, M.S.; Hostetter, T.H. Dietary protein increases plasma renin and reduces pressor reactivity to angiotensin II. Am. J. Physiol. Ren. Physiol. 1986, 251, F34–F39. [Google Scholar] [CrossRef]
- Correa-Rotter, R.; Hostetter, T.H.; Rosenberg, M.E. Effect of dietary protein on renin and angiotensinogen gene expression after renal ablation. Am. J. Physiol. 1992, 262, F631–F638. [Google Scholar] [CrossRef]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary protein intake and renal function. Nutr. Metab. 2005, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.R.; Tappenden, K.A.; Erdman, J.W., Jr. Altering Dietary Protein Type and Quantity Reduces Urinary Albumin Excretion without Affecting Plasma Glucose Concentrations in BKS.cg-m +Leprdb/+Leprdb (db/db) Mice. J. Nutr. 2003, 133, 673–678. [Google Scholar] [CrossRef]
- Brouhard, B.H.; Rajaraman, S.; LaGrone, L.F. Effect of varied protein intake on the nephropathy of the diabetic mouse (C57BL/s J db/db). Int. J. Pediatr. Nephrol. 1987, 8, 59–68. [Google Scholar]
- Nørgaard, S.A.; Briand, F.; Sand, F.W.; Galsgaard, E.D.; Søndergaard, H.; Sørensen, D.B.; Sulpice, T. Nephropathy in diabetic db/db mice is accelerated by high protein diet and improved by the SGLT2 inhibitor dapagliflozin. Eur. J. Pharmacol. 2019, 860, 172537. [Google Scholar] [CrossRef]
- Rossert, J.; Terraz-Durasnel, C.; Brideau, G. Growth factors, cytokines, and renal fibrosis during the course of diabetic nephropathy. Diabetes Metab. 2000, 26 (Suppl. S4), 16–24. [Google Scholar]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2019, 67, 663–681. [Google Scholar] [CrossRef]
- Soler, M.J.; Riera, M.; Batlle, D. New Experimental Models of Diabetic Nephropathy in Mice Models of Type 2 Diabetes: Efforts to Replicate Human Nephropathy. J. Diabetes Res. 2012, 2012, 616313. [Google Scholar] [CrossRef]
- Luo, W.; Tang, S.; Xiao, X.; Luo, S.; Yang, Z.; Huang, W.; Tang, S. Translation Animal Models of Diabetic Kidney Disease: Biochemical and Histological Phenotypes, Advantages and Limitations. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 1297–1321. [Google Scholar] [CrossRef] [PubMed]
- Viau, A.; El Karoui, K.; Laouari, D.; Burtin, M.; Nguyen, C.; Mori, K.; Pillebout, E.; Berger, T.; Mak, T.W.; Knebelmann, B.; et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Investig. 2010, 120, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Arimura, E.; Horiuchi, M.; Kawaguchi, H.; Miyoshi, N.; Aoyama, K.; Takeuchi, T. Low-protein diet improves blood and urinary glucose levels and renal manifestations of diabetes in C57BLKS-db/db mice. Eur. J. Nutr. 2013, 52, 813–824. [Google Scholar] [CrossRef]
- Meek, R.L.; LeBoeuf, R.C.; Saha, S.A.; Alpers, C.E.; Hudkins, K.L.; Cooney, S.K.; Anderberg, R.J.; Tuttle, K.R. Glomerular cell death and inflammation with high-protein diet and diabetes. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2013, 28, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Rocha, S.; Valente, M.J.; Catarino, C.; Bronze-da-Rocha, E.; Belo, L.; Santos-Silva, A. New Insights into Adiponectin and Leptin Roles in Chronic Kidney Disease. Biomedicines 2022, 10, 2642. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N. Leptin in chronic kidney disease: A link between hematopoiesis, bone metabolism, and nutrition. Int. Urol. Nephrol. 2014, 46, 1169–1174. [Google Scholar] [CrossRef]
- Wolf, G.; Ziyadeh, F.N. Leptin and renal fibrosis. Contrib. Nephrol. 2006, 151, 175–183. [Google Scholar] [CrossRef]
- Dessie, G.; Ayelign, B.; Akalu, Y.; Shibabaw, T.; Molla, M.D. Effect of Leptin on Chronic Inflammatory Disorders: Insights to Therapeutic Target to Prevent Further Cardiovascular Complication. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3307–3322. [Google Scholar] [CrossRef]
- He, W.H.; Li, B.; Zhu, X.; Zhang, K.H.; Li, B.M.; Liu, Z.J.; Liu, G.Y.; Wang, J. The role and mechanism of NADPH oxidase in leptin-induced reactive oxygen species production in hepatic stellate cells. Zhonghua Gan Zang Bing Za Zhi 2010, 18, 849–854. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Björnson Granqvist, A.; Ericsson, A.; Sanchez, J.; Tonelius, P.; William-Olsson, L.; Dahlqvist, U.; Andersson, A.K.; Tesan Tomic, T.; Hudkins, K.; Alpers, C.E.; et al. High-protein diet accelerates diabetes and kidney disease in the BTBRob/ob mouse. Am. J. Physiol. Ren. Physiol. 2020, 318, F763–F771. [Google Scholar] [CrossRef]
- Tanaka, S.; Wakui, H.; Azushima, K.; Tsukamoto, S.; Yamaji, T.; Urate, S.; Suzuki, T.; Abe, E.; Taguchi, S.; Yamada, T.; et al. Effects of a High-Protein Diet on Kidney Injury under Conditions of Non-CKD or CKD in Mice. Int. J. Mol. Sci. 2023, 24, 7778. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.W. Effect of salt intake on progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2006, 15, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Pari, L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem. Biol. Interact. 2016, 245, 1–11. [Google Scholar] [CrossRef]
- Tesch, G.H.; Allen, T.J. Rodent models of streptozotocin-induced diabetic nephropathy (Methods in Renal Research). Nephrology 2007, 12, 261–266. [Google Scholar] [CrossRef]
- Noshahr, Z.S.; Salmani, H.; Khajavi Rad, A.; Sahebkar, A. Animal Models of Diabetes-Associated Renal Injury. J. Diabetes Res. 2020, 2020, 9416419. [Google Scholar] [CrossRef]
- Jacquot, L.; Pointeau, O.; Roger-Villeboeuf, C.; Passilly-Degrace, P.; Belkaid, R.; Regazzoni, I.; Leemput, J.; Buch, C.; Demizieux, L.; Vergès, B.; et al. Therapeutic potential of a novel peripherally restricted CB1R inverse agonist on the progression of diabetic nephropathy. Front. Nephrol. 2023, 3, 1138416. [Google Scholar] [CrossRef]
- Olivo-Marin, J.-C. Extraction of spots in biological images using multiscale products. Pattern Recognit. 2002, 35, 1989–1996. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pointeau, O.; Barbosa, R.; Loriot, M.; Leemput, J.; Dubus, E.; Causse, S.Z.; Demizieux, L.; Passilly-Degrace, P.; Degrace, P.; Vergès, B.; et al. A Simplified and Robust Model for the Study of Diabetic Nephropathy: Streptozotocin-Induced Diabetic Mice Fed a High-Protein Diet. Int. J. Mol. Sci. 2025, 26, 2477. https://doi.org/10.3390/ijms26062477
Pointeau O, Barbosa R, Loriot M, Leemput J, Dubus E, Causse SZ, Demizieux L, Passilly-Degrace P, Degrace P, Vergès B, et al. A Simplified and Robust Model for the Study of Diabetic Nephropathy: Streptozotocin-Induced Diabetic Mice Fed a High-Protein Diet. International Journal of Molecular Sciences. 2025; 26(6):2477. https://doi.org/10.3390/ijms26062477
Chicago/Turabian StylePointeau, Océane, Romain Barbosa, Maéva Loriot, Julia Leemput, Elisabeth Dubus, Sébastien Zwe Causse, Laurent Demizieux, Patricia Passilly-Degrace, Pascal Degrace, Bruno Vergès, and et al. 2025. "A Simplified and Robust Model for the Study of Diabetic Nephropathy: Streptozotocin-Induced Diabetic Mice Fed a High-Protein Diet" International Journal of Molecular Sciences 26, no. 6: 2477. https://doi.org/10.3390/ijms26062477
APA StylePointeau, O., Barbosa, R., Loriot, M., Leemput, J., Dubus, E., Causse, S. Z., Demizieux, L., Passilly-Degrace, P., Degrace, P., Vergès, B., & Jourdan, T. (2025). A Simplified and Robust Model for the Study of Diabetic Nephropathy: Streptozotocin-Induced Diabetic Mice Fed a High-Protein Diet. International Journal of Molecular Sciences, 26(6), 2477. https://doi.org/10.3390/ijms26062477






