Proteomic Alterations and Oxidative Stress in Seminal Plasma of Nellore Bulls Under Sexual Rest
Abstract
1. Introduction
2. Results
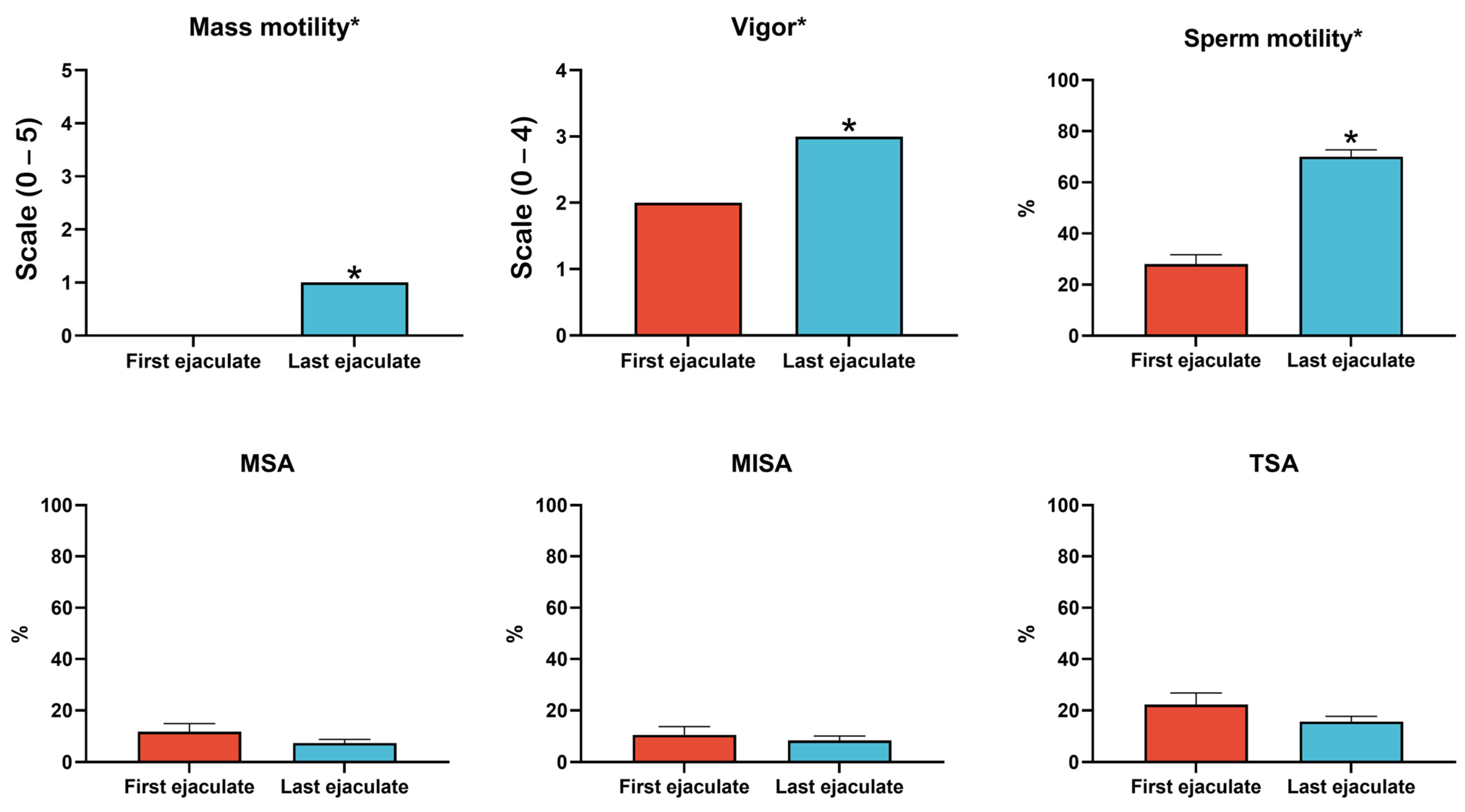
2.1. Sperm Quality Analysis
2.2. Oxidative Stress Analysis
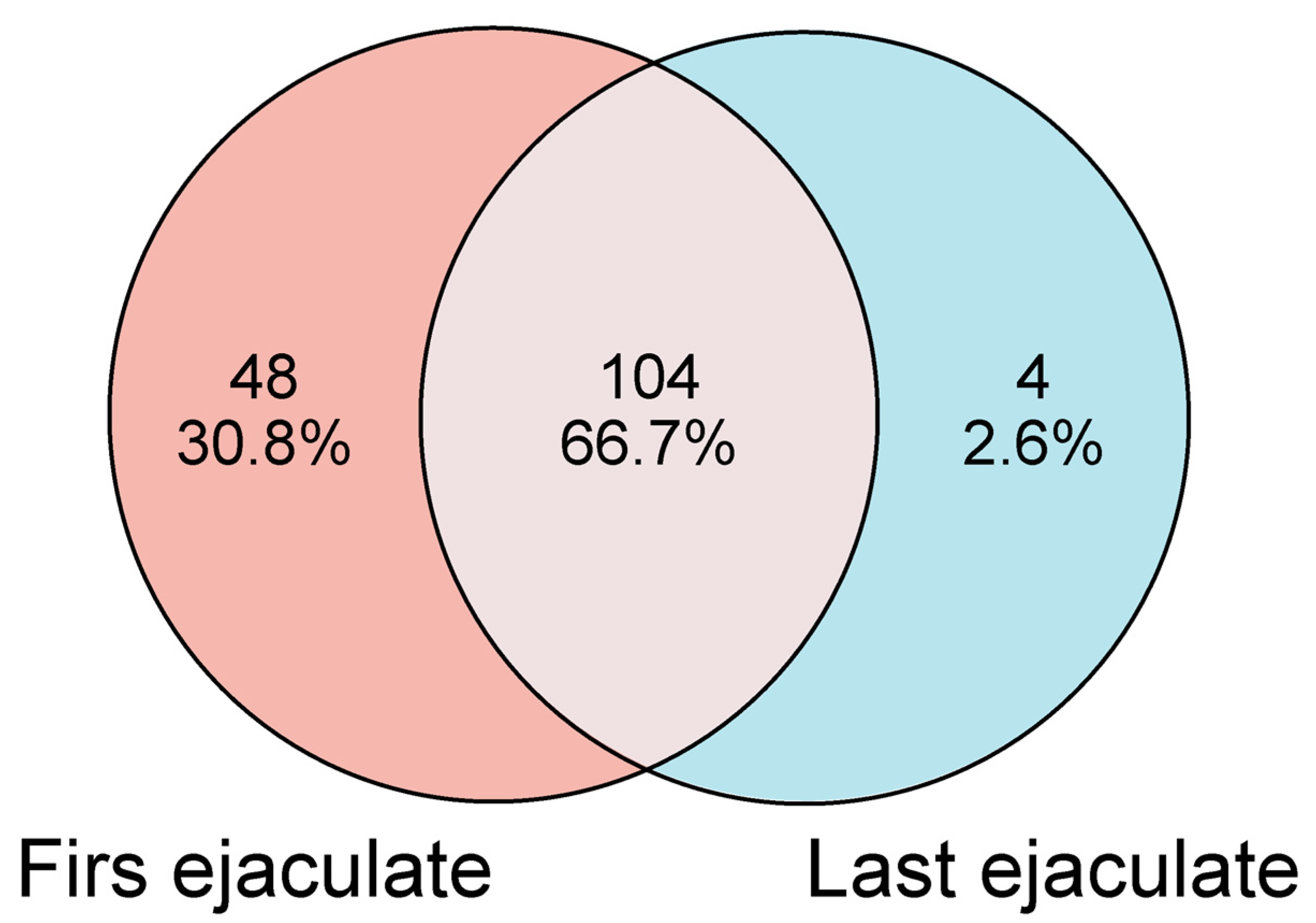
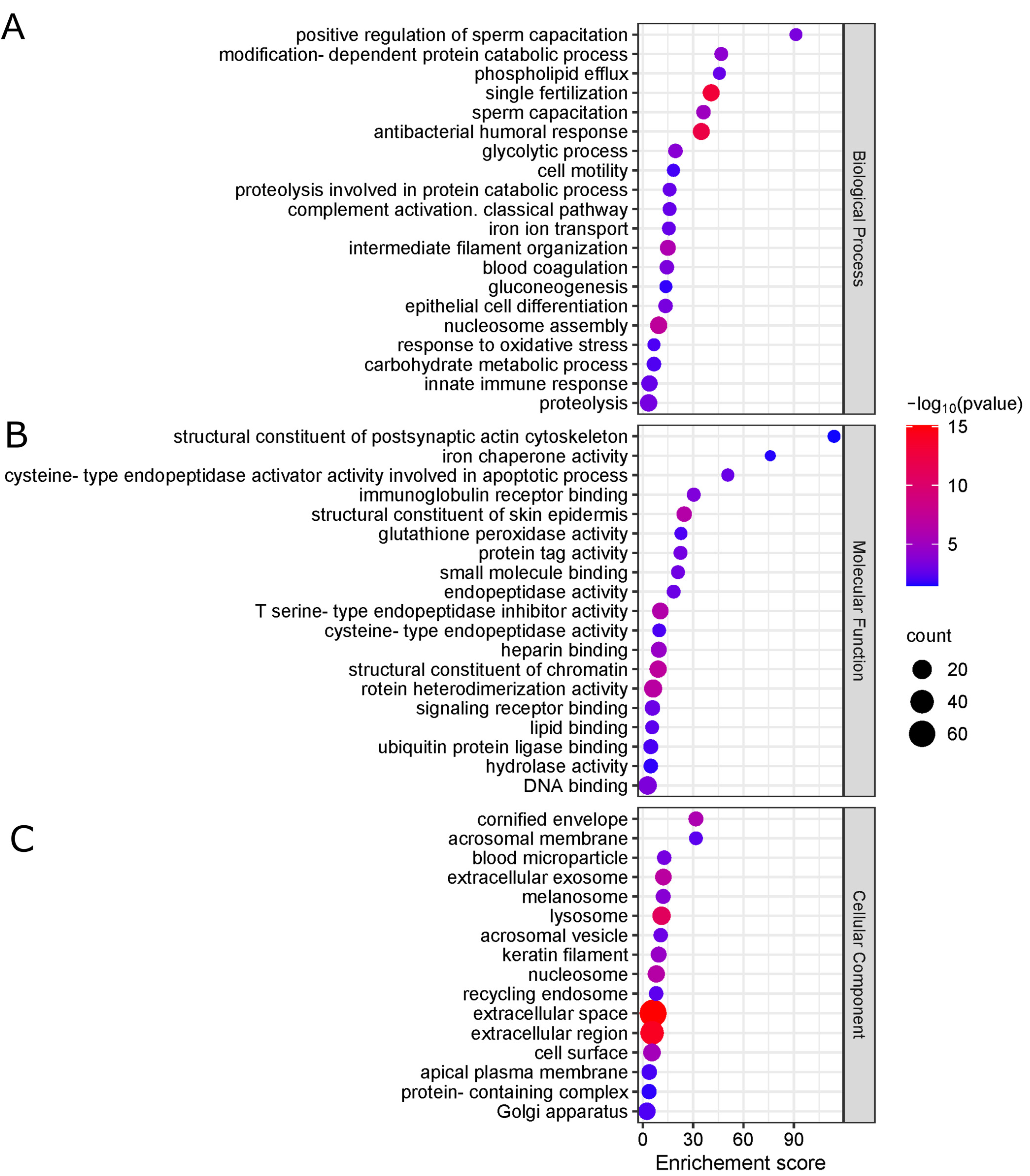
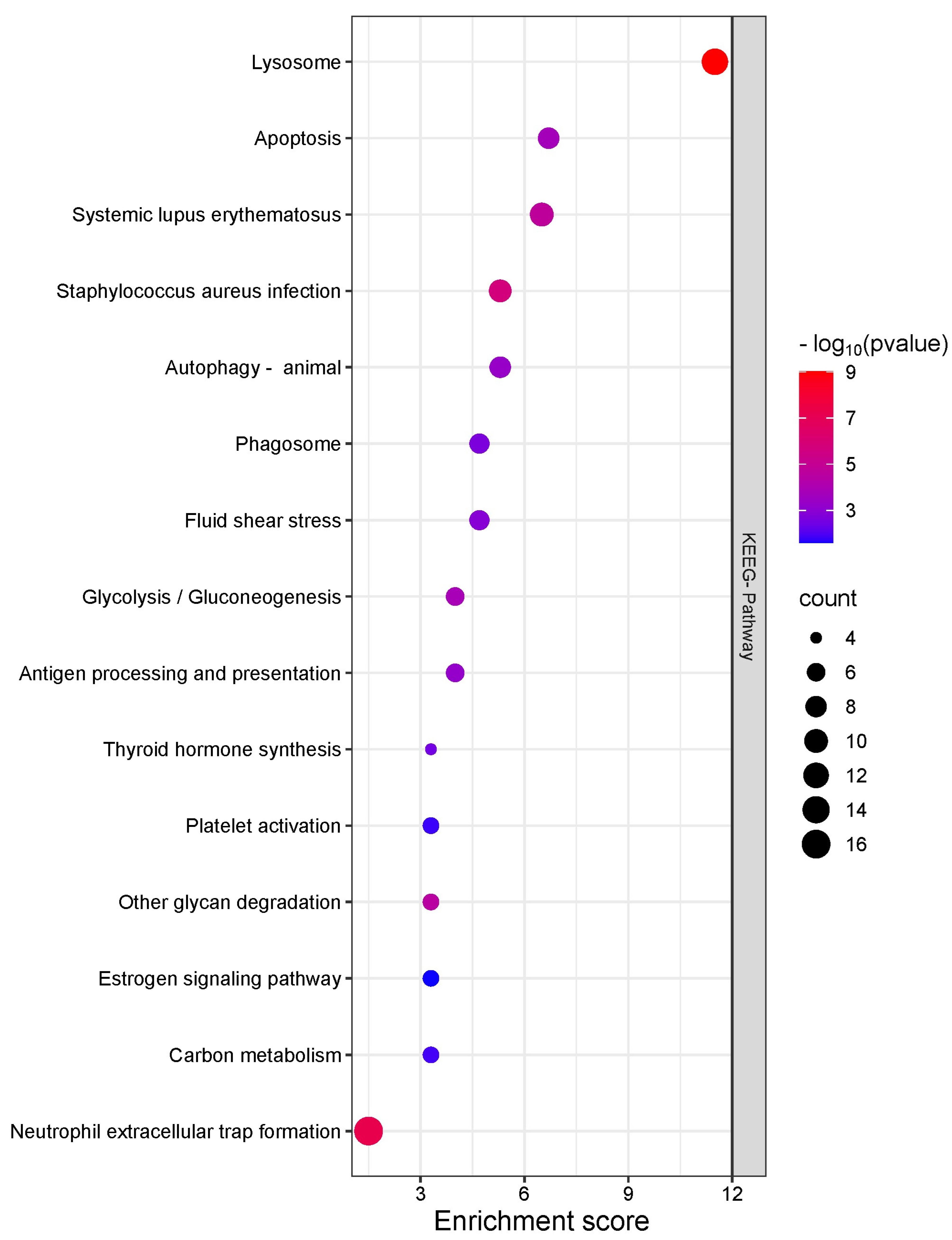
2.3. Qualitative Proteomics Analysis
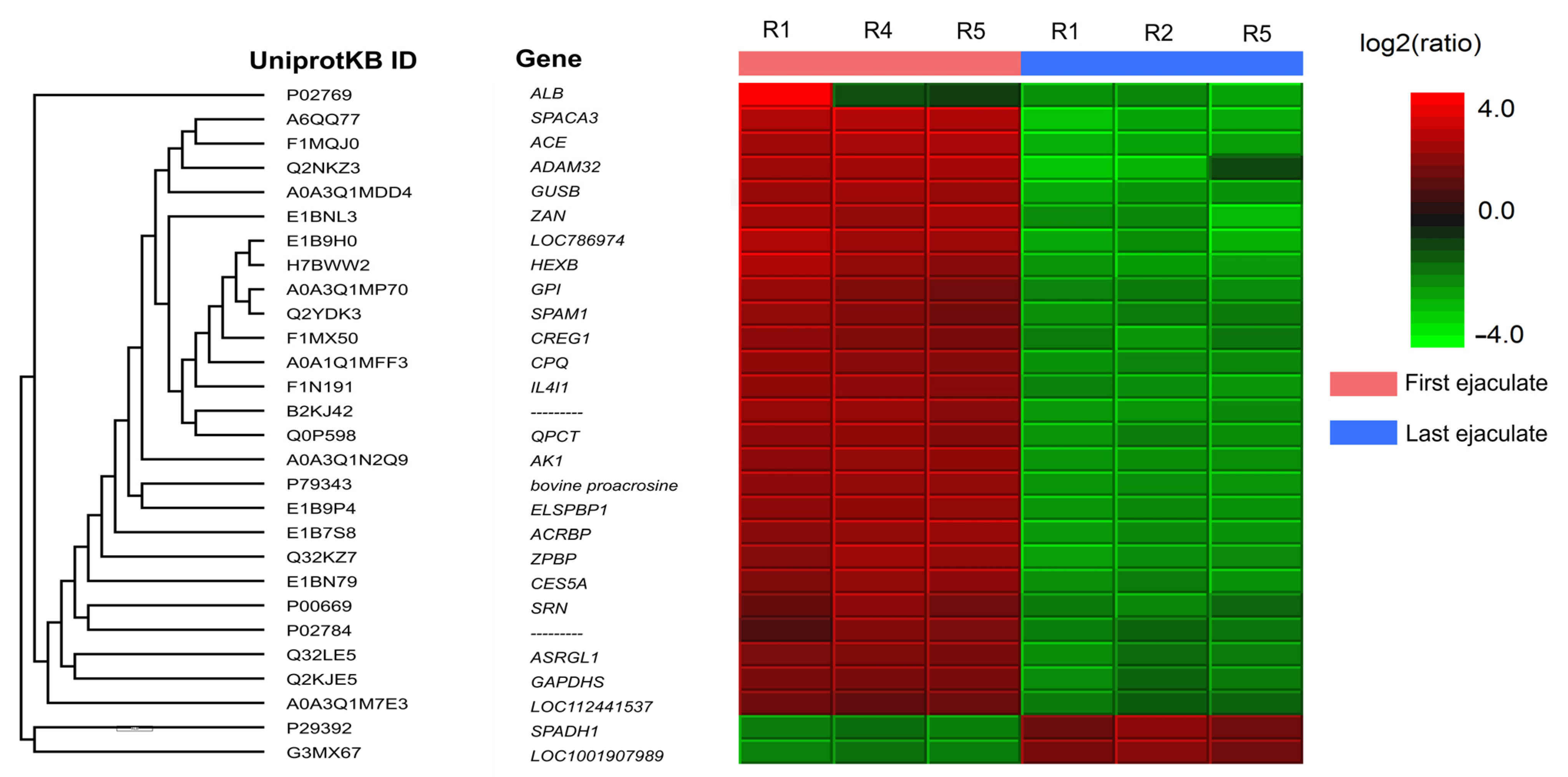
2.4. Quantitative Proteomics Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animal Selection
4.3. Oxidative Stress Sample Preparation
4.4. Extraction and Quantification of Soluble Proteins
4.5. Gel Protein Digestion
4.6. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
4.7. Protein Identification
4.8. Gene Ontology, Analysis of Functional Clusters, and Protein–Protein Interaction
4.9. Sperm Quality Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| CAT | Catalase |
| CP | Carbonyl protein |
| GDP | Gross Domestic Product |
| CBRA | Brazilian College of Animal Reproduction |
| DTT | Dithiothreitol |
| FDR | False Discovery Rate |
| GST | Glutathione S-transferase |
| LC-MS | Liquid chromatography-mass spectrometry |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| SR | Sexual rest |
| TFA | Trifluoroacetic acid |
References
- Valdes, C. Brazil’s Momentum as a Global Agricultural Supplier Faces Headwinds. Economic Research Service. Available online: https://www.ers.usda.gov/amber-waves/2022/september/brazil-s-momentum-as-a-global-agricultural-supplier-faces-headwinds (accessed on 10 February 2025).
- Malafaia, G.C.; Mores, G.D.V.; Casagranda, Y.G.; Barcellos, J.O.J.; Costa, F.P. The Brazilian Beef Cattle Supply Chain in the next Decades. Livest. Sci. 2021, 253, 104704. [Google Scholar] [CrossRef]
- Casagranda, Y.G.; Wiśniewska-Paluszak, J.; Paluszak, G.; Mores, G.D.V.; Moro, L.D.; Malafaia, G.C.; Azevedo, D.B.D.; Zhang, D. Emergent Research Themes on Sustainability in the Beef Cattle Industry in Brazil: An Integrative Literature Review. Sustainability 2023, 15, 4670. [Google Scholar] [CrossRef]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen Evaluation: Methodological Advancements in Sperm Quality-Specific Fertility Assessment—A Review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef]
- Mikkola, M.; Desmet, K.L.J.; Kommisrud, E.; Riegler, M.A. Recent Advancements to Increase Success in Assisted Reproductive Technologies in Cattle. Anim. Reprod. 2024, 21, e20240031. [Google Scholar] [CrossRef]
- Butler, M.L.; Bormann, J.M.; Weaber, R.L.; Grieger, D.M.; Rolf, M.M. Selection for Bull Fertility: A Review. Transl. Anim. Sci. 2019, 4, 423–441. [Google Scholar] [CrossRef]
- Khan, M.Z.; Chen, W.; Naz, S.; Liu, X.; Liang, H.; Chen, Y.; Kou, X.; Liu, Y.; Ashraf, I.; Han, Y.; et al. Determinant Genetic Markers of Semen Quality in Livestock. Front. Endocrinol. 2024, 15, 1456305. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Abdoli, R.; Ferdosi, M.H.; Eghbalsaied, S. Editorial: Genetics of Reproduction for Livestock Species. Front. Genet. 2023, 14, 1210904. [Google Scholar] [CrossRef]
- Rivero, M.J.; Lopez-Villalobos, N.; Evans, A.; Berndt, A.; Cartmill, A.; Neal, A.L.; McLaren, A.; Farruggia, A.; Mignolet, C.; Chadwick, D.; et al. Key Traits for Ruminant Livestock across Diverse Production Systems in the Context of Climate Change: Perspectives from a Global Platform of Research Farms. Reprod. Fertil. Dev. 2021, 33, 1–19. [Google Scholar] [CrossRef]
- Wells, M.E.; Wondafrash, T.; Awa, O.A.; Stephens, D.F. Effect of Sexual Rest and Subsequent Regular Collection on Acrosome Characteristics of Bull Spermatozoa. J. Anim. Sci. 1970, 31, 67–71. [Google Scholar] [CrossRef]
- Barth, A.D. Sperm Accumulation in the Ampullae and Cauda Epididymides of Bulls. Anim. Reprod. Sci. 2007, 102, 238–246. [Google Scholar] [CrossRef]
- Firman, R.C.; Young, F.J.; Rowe, D.C.; Duong, H.T.; Gasparini, C. Sexual Rest and Post-meiotic Sperm Ageing in House Mice. J. Evol. Biol. 2015, 28, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, C.J.; Barros, E.; Vidigal, P.M.; Okano, D.S.; Gomes, L.L.; Carvalho, R.P.R.; de Castro, A.G.; Baracat-Pereira, M.C.; Guimarães, S.E.F.; Guimarães, J.D. Oxidative Stress Associated with Proteomic and Fatty Acid Profiles of Sperm from Nellore Bulls at Rest. Biol. Reprod. 2023, 109, 878–891. [Google Scholar] [CrossRef]
- Barth, A.D.; Oko, R.J. Abnormal Morphology of Bovine Spermatozoa, 1st ed.; Iowa State University Press: New York, NY, USA, 1989. [Google Scholar]
- Omu, A.E.; Al-Bader, A.A.; Dasht, H. Effect of extracellular mg concentration on electrically induced contractions of rat vas deferens in vitro. Arch. Androl. 2001, 46, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, S.; Gasparini, C. Male Sperm Storage Impairs Sperm Quality in the Zebrafish. Sci. Rep. 2021, 11, 16689. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New Insights into Epididymal Biology and Function. Hum. Reprod. Update 2008, 15, 213–227. [Google Scholar] [CrossRef]
- Meunier, L.; Sorci, G.; Abi Hussein, H.; Hingrat, Y.; Rehspringer, N.; Saint-Jalme, M.; Lesobre, L.; Torres Carreira, J. Pre-but Not Post-Meiotic Senescence Affects Sperm Quality and Reproductive Success in the North African Houbara Bustard. Front. Ecol. Evol. 2022, 10, 977184. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Ramírez-López, C.J.; Barros, E.; Vidigal, P.M.P.; Silva Okano, D.; Duarte Rodrigues, J.N.; Lopes Gomes, L.; Montes-Vergara, J.C.; Petro Hernandez, V.G.; Baracat-Pereira, M.C.; Guimarães, S.E.F.; et al. Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters. Vet. Sci. 2023, 10, 610. [Google Scholar] [CrossRef]
- Moura, A.A.; Memili, E.; Portela, A.M.R.; Viana, A.G.; Velho, A.L.C.; Bezerra, M.J.B.; Vasconselos, F.R. Seminal Plasma Proteins and Metabolites: Effects on Sperm Function and Potential as Fertility Markers. Anim. Reprod. 2018, 15, 691–702. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The Enigmatic Seminal Plasma: A Proteomics Insight from Ejaculation to Fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the Harmful Effect of Oxidative Stress on Male Fertility: A Mechanistic Insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef]
- Pizzari, T.; Dean, R.; Pacey, A.; Moore, H.; Bonsall, M.B. The Evolutionary Ecology of Pre- and Post-Meiotic Sperm Senescence. Trends Ecol. Evol. 2008, 23, 131–140. [Google Scholar] [CrossRef]
- Hettyey, A.; Vági, B.; Penn, D.J.; Hoi, H.; Wagner, R.H. Post-Meiotic Intra-Testicular Sperm Senescence in a Wild Vertebrate. PLoS ONE 2012, 7, e50820. [Google Scholar] [CrossRef] [PubMed]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus 2023, 15, e42583. [Google Scholar] [CrossRef]
- Baker, M.A.; Aitken, R.J. The Importance of Redox Regulated Pathways in Sperm Cell Biology. Mol. Cell Endocrinol. 2004, 216, 47–54. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of Oxidative Stress on Male and Female Germ Cells: Implications for Fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Padilla, L.; Tvarijonaviciute, A.; Parrilla, I.; Martínez, E.A.; Rodriguez-Martinez, H.; Yeste, M.; Roca, J. Levels of Activity of Superoxide Dismutase in Seminal Plasma Do Not Predict Fertility of Pig AI-Semen Doses. Theriogenology 2019, 140, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Macanovic, B.; Vucetic, M.; Jankovic, A.; Stancic, A.; Buzadzic, B.; Garalejic, E.; Korac, A.; Korac, B.; Otasevic, V. Correlation between Sperm Parameters and Protein Expression of Antioxidative Defense Enzymes in Seminal Plasma: A Pilot Study. Dis. Markers 2015, 2015, 36236. [Google Scholar] [CrossRef]
- Almadaly, E.A.; Abdel-Salam, A.-B.S.; Sahwan, F.M.; Kahilo, K.A.; Abouzed, T.K.; El-Domany, W.B. Fertility-Associated Biochemical Components in Seminal Plasma and Serum of Buffalo (Bubalus bubalis) Bulls. Front. Vet. Sci. 2023, 9, 1043379. [Google Scholar] [CrossRef]
- Papas, M.; Arroyo, L.; Bassols, A.; Catalán, J.; Bonilla-Correal, S.; Gacem, S.; Yeste, M.; Miró, J. Activities of Antioxidant Seminal Plasma Enzymes (SOD, CAT, GPX and GSR) Are Higher in Jackasses than in Stallions and Are Correlated with Sperm Motility in Jackasses. Theriogenology 2019, 140, 180–187. [Google Scholar] [CrossRef]
- Wdowiak, A.; Bakalczuk, S.; Bakalczuk, G. Decreased Activity of Superoxide Dismutase in the Seminal Plasma of Infertile Men Correlates with Increased Sperm Deoxyribonucleic Acid Fragmentation during the First Hours after Sperm Donation. Andrology 2015, 3, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, J.T.; Carreira, J.T.; Carvalho, I.R.; Kipper, B.H.; Nagata, W.B.; Perri, S.H.V.; Franco Oliveira, M.E.; Pierucci, J.C.; Koivisto, M.B. de Does Lipid Peroxidation and Oxidative DNA Damage Differ in Cryopreserved Semen Samples from Young, Adult and Aged Nellore Bulls? Anim. Reprod. Sci. 2018, 195, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of Oxidative Stress-Induced Sperm Dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef] [PubMed]
- Žaja, I.Ž.; Samardžija, M.; Vince, S.; Vilić, M.; Majić-Balić, I.; Đuričić, D.; Milinković-Tur, S. Differences in Seminal Plasma and Spermatozoa Antioxidative Systems and Seminal Plasma Lipid and Protein Levels among Boar Breeds and Hybrid Genetic Traits. Anim. Reprod. Sci. 2016, 170, 75–82. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative Stress, Sperm Survival and Fertility Control. Mol. Cell Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Mao, H.-T.; Yang, W.-X. Modes of Acrosin Functioning during Fertilization. Gene 2013, 526, 75–79. [Google Scholar] [CrossRef]
- Hua, R.; Xue, R.; Liu, Y.; Li, Y.; Sha, X.; Li, K.; Gao, Y.; Shen, Q.; Lv, M.; Xu, Y.; et al. Acrosin Deficiency Causes Total Fertilization Failure in Humans by Preventing the Sperm from Penetrating the Zona Pellucida. Hum. Reprod. 2023, 38, 1213–1223. [Google Scholar] [CrossRef]
- Tardif, S.; Wilson, M.D.; Wagner, R.; Hunt, P.; Gertsenstein, M.; Nagy, A.; Lobe, C.; Koop, B.F.; Hardy, D.M. Zonadhesin Is Essential for Species Specificity of Sperm Adhesion to the Egg Zona Pellucida. J. Biol. Chem. 2010, 285, 24863–24870. [Google Scholar] [CrossRef]
- Breitbart, H.; Grinshtein, E. Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction. Int. J. Mol. Sci. 2023, 24, 17005. [Google Scholar] [CrossRef]
- Grunewald, S.; Fitzl, G.; Springsguth, C. Induction of Ultra-Morphological Features of Apoptosis in Mature and Immature Sperm. Asian J. Androl. 2017, 19, 533–537. [Google Scholar] [CrossRef]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative Stress and Autophagy in the Regulation of Lysosome-Dependent Neuron Death. Antioxid. Redox Signal 2009, 11, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Hartree, E.F. Lysosomal Enzymes in The Acrosome And Their Possible Role In Fertilization. Reproduction 1970, 21, 501–515. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Hughes, A.L.; Madeo, F.; Ruckenstuhl, C. The Crucial Impact of Lysosomes in Aging and Longevity. Ageing Res. Rev. 2016, 32, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The Potential Impacts of Micro-and-Nano Plastics on Various Organ Systems in Humans. eBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiolo Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Pérez, S.; Toledano, M.B.; Sastre, J. Mitochondrial Reactive Oxygen Species and Lytic Programmed Cell Death in Acute Inflammation. Antioxid. Redox Signal 2023, 39, 708–727. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.F.; Rodrigues, T.A.; Da Silveira, J.C.; Gonella-Diaza, A.M.; Binelli, M.; Lopes, J.V.; Moura, M.T.; Feitosa, W.B.; Paula-Lopes, F.F. Cellular Responses and microRNA Profiling in Bovine Spermatozoa under Heat Shock. Reproduction 2022, 164, 155–168. [Google Scholar] [CrossRef]
- Uribe, P.; Meriño, J.; Matus, C.E.; Schulz, M.; Zambrano, F.; Villegas, J.V.; Conejeros, I.; Taubert, A.; Hermosilla, C.; Sánchez, R. Autophagy Is Activated in Human Spermatozoa Subjected to Oxidative Stress and Its Inhibition Impairs Sperm Quality and Promotes Cell Death. Hum. Reprod. 2022, 37, 680–695. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Henry-Berger, J.; Vernet, P.; Cadet, R.; Saez, F.; Drevet, J.R. Mammalian Glutathione Peroxidases Control Acquisition and Maintenance of Spermatozoa Integrity. J. Anim. Sci. 2010, 88, 1321–1331. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Braga, P.C.; Martins, A.D.; Silva, B.M.; Alves, M.G.; Oliveira, P.F. Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility. Antioxidants 2021, 10, 1441. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Tvarijonaviciute, A.; González-Arostegui, L.G.; Rubio, C.P.; Yeste, M.; Miró, J.; Barranco, I. Impact of Seminal Plasma Antioxidants on Donkey Sperm Cryotolerance. Antioxidants 2022, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Crisol, L.; Matorras, R.; Aspichueta, F.; Expósito, A.; Hernández, M.L.; Ruiz-Larrea, M.B.; Mendoza, R.; Ruiz-Sanz, J.I. Glutathione Peroxidase Activity in Seminal Plasma and Its Relationship to Classical Sperm Parameters and in Vitro Fertilization-Intracytoplasmic Sperm Injection Outcome. Fertil. Steril. 2012, 97, 852–857.e1. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; García-Vicente, R.; Morales, M.L.; Ortiz-Ruiz, A.; Martínez-López, J.; Linares, M. Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies. Antioxidants 2020, 9, 1212. [Google Scholar] [CrossRef]
- Tola, A.J.; Jaballi, A.; Missihoun, T.D. Protein Carbonylation: Emerging Roles in Plant Redox Biology and Future Prospects. Plants 2021, 10, 1451. [Google Scholar] [CrossRef]
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm Protein Carbonylation. Andrologia 2019, 51, e13233. [Google Scholar] [CrossRef] [PubMed]
- Mostek, A.; Dietrich, M.A.; Słowińska, M.; Ciereszko, A. Cryopreservation of Bull Semen Is Associated with Carbonylation of Sperm Proteins. Theriogenology 2017, 92, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Rubio, C.P.; Tvarijonaviciute, A.; Rodriguez-Martinez, H.; Roca, J. Measurement of Oxidative Stress Index in Seminal Plasma Can Predict In Vivo Fertility of Liquid-Stored Porcine Artificial Insemination Semen Doses. Antioxidants 2021, 10, 1203. [Google Scholar] [CrossRef]
- DuTeaux, S.B.; Berger, T.; Hess, R.A.; Sartini, B.L.; Miller, M.G. Male Reproductive Toxicity of Trichloroethylene: Sperm Protein Oxidation and Decreased Fertilizing Ability. Biol. Reprod. 2004, 70, 1518–1526. [Google Scholar] [CrossRef]
- Mostek, A.; Westfalewicz, B.; Słowińska, M.; Dietrich, M.A.; Judycka, S.; Ciereszko, A. Differences in Sperm Protein Abundance and Carbonylation Level in Bull Ejaculates of Low and High Quality. PLoS ONE 2018, 13, e0206150. [Google Scholar] [CrossRef]
- Kuravsky, M.L.; Aleshin, V.V.; Frishman, D.; Muronetz, V.I. Testis-Specific Glyceraldehyde-3-Phosphate Dehydrogenase: Origin and Evolution. BMC Evol. Biol. 2011, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Krisfalusi, M.; Miki, K.; Magyar, P.L.; O’Brien, D.A. Multiple Glycolytic Enzymes Are Tightly Bound to the Fibrous Sheath of Mouse Spermatozoa1. Biol. Reprod. 2006, 75, 270–278. [Google Scholar] [CrossRef]
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’Brien, D.A. Glyceraldehyde 3-Phosphate Dehydrogenase-S, a Sperm-Specific Glycolytic Enzyme, Is Required for Sperm Motility and Male Fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 16501–16506. [Google Scholar] [CrossRef]
- Naletova, I.; Schmalhausen, E.; Tomasello, B.; Pozdyshev, D.; Attanasio, F.; Muronetz, V. The Role of Sperm-Specific Glyceraldehyde-3-Phosphate Dehydrogenase in the Development of Pathologies—From Asthenozoospermia to Carcinogenesis. Front. Mol. Biosci. 2023, 10, 1256963. [Google Scholar] [CrossRef] [PubMed]
- Klomsiri, C.; Karplus, P.A.; Poole, L.B. Cysteine-Based Redox Switches in Enzymes. Antioxid. Redox Signal 2011, 14, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.G.d.A.; Gomes, F.P.; Kaya, A.; Diedrich, J.; Topper, E.; Silvestre, D.D.; Machado-Neves, M.; Memili, E.; Moura, A.A.; Yates, J.R. Seminal Proteoforms from Bulls with Contrasting Semen Freezability: A Story Deciphered by Top-down Mass Spectrometry. Reproduction 2025, 169, e240051. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Gong, L.; Sun, C. Oxidation of Glyceraldehyde-3-Phosphate Dehydrogenase Decreases Sperm Motility in Diabetes Mellitus. Biochem. Biophys. Res. Commun. 2015, 465, 245–248. [Google Scholar] [CrossRef]
- Elkina, Y.L.; Atroshchenko, M.M.; Bragina, E.E.; Muronetz, V.I.; Schmalhausen, E.V. Oxidation of Glyceraldehyde-3-Phosphate Dehydrogenase Decreases Sperm Motility. Biochem. Mosc. 2011, 76, 268–272. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, H.; Haque, S.; Ahmad, S.; Srivastava, N.; Khan, A. Angiotensin-Converting Enzyme and Hypertension: A Systemic Analysis of Various ACE Inhibitors, Their Side Effects, and Bioactive Peptides as a Putative Therapy for Hypertension. J. Renin Angiotensin Aldosterone Syst. 2023, 2023, 7890188. [Google Scholar] [CrossRef]
- Heeneman, S.; Sluimer, J.C.; Daemen, M.J.A.P. Angiotensin-Converting Enzyme and Vascular Remodeling. Circ. Res. 2007, 101, 441–454. [Google Scholar] [CrossRef]
- Costa, D.S.; Thundathil, J.C. Characterization and Activity of Angiotensin-Converting Enzyme in Holstein Semen. Anim. Reprod. Sci. 2012, 133, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, H.; Kamata, M.; Hu, J.; Nakagawa, H.; Obara, H.; Kondoh, N.; Shima, H.; Sato, I. Activity of Testis Angiotensin Converting Enzyme (ACE) in Ejaculated Human Spermatozoa. Int. J. Androl. 2001, 24, 295–299. [Google Scholar] [CrossRef]
- Jia, B.; Liang, J.; Lv, C.; Memon, S.; Fang, Y.; Wu, G.; Quan, G. The Characteristics of Proteome and Metabolome Associated with Contrasting Sperm Motility in Goat Seminal Plasma. Sci. Rep. 2021, 11, 15562. [Google Scholar] [CrossRef] [PubMed]
- Ashwitha, A.; Ramesha, K.P.; Ramesh, P.; Kootimole, C.N.; Devadasan, M.J.; Ammankallu, S.; Jeyakumar, S.; Kumaresan, A.; Veerappa, V.G.; Das, D.N.; et al. Quantitative Proteomics Profiling of Spermatozoa and Seminal Plasma Reveals Proteins Associated with Semen Quality in Bos Indicus Bulls. J. Proteom. 2023, 273, 104794. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, P.; Lefebvre, J.; Jois, P.S.; Fan, J.; Wright, M.W. New Nomenclature for Mammalian BSP Genes1. Biol. Reprod. 2009, 80, 394–397. [Google Scholar] [CrossRef][Green Version]
- Sahin, E.; Petrunkina, A.M.; Ekhlasi-Hundrieser, M.; Hettel, C.; Waberski, D.; Harrison, R.A.P.; Töpfer-Petersen, E. Fibronectin Type II-Module Proteins in the Bovine Genital Tract and Their Putative Role in Cell Volume Control during Sperm Maturation. Reprod. Fertil. Dev. 2009, 21, 479. [Google Scholar] [CrossRef]
- Plante, G.; Prud’homme, B.; Fan, J.; Lafleur, M.; Manjunath, P. Evolution and Function of Mammalian Binder of Sperm Proteins. Cell Tissue Res. 2016, 363, 105–127. [Google Scholar] [CrossRef]
- Gwathmey, T.M.; Ignotz, G.G.; Mueller, J.L.; Manjunath, P.; Suarez, S.S. Bovine Seminal Plasma Proteins PDC-109, BSP-A3, and BSP-30-kDa Share Functional Roles in Storing Sperm in the Oviduct. Biol. Reprod. 2006, 75, 501–507. [Google Scholar] [CrossRef]
- Manjunath, P.; Bergeron, A.; Lefebvre, J.; Fan, J. Seminal Plasma Proteins: Functions and Interaction with Protective Agents during Semen Preservation. Soc. Reprod. Fertil. Suppl. 2007, 65, 217–228. [Google Scholar]
- Moura, A.A.; Koc, H.; Chapman, D.A.; Killian, G.J. Identification of Proteins in the Accessory Sex Gland Fluid Associated With Fertility Indexes of Dairy Bulls: A Proteomic Approach. J. Androl. 2006, 27, 201–211. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.K.; Sharma, V.K.; Pandey, S.; Kumaresan, A.; Srinivasan, A.; Datta, T.K.; Mohanty, T.K.; Yadav, S. Identification of Biomarker Candidates for Fertility in Spermatozoa of Crossbred Bulls through Comparative Proteomics. Theriogenology 2018, 119, 43–51. [Google Scholar] [CrossRef]
- Somashekar, L.; Selvaraju, S.; Parthipan, S.; Ravindra, J.P. Profiling of Sperm Proteins and Association of Sperm PDC-109 with Bull Fertility. Syst. Biol. Reprod. Med. 2015, 61, 376–387. [Google Scholar] [CrossRef]
- D’Amours, O.; Bordeleau, L.-J.; Frenette, G.; Blondin, P.; Leclerc, P.; Sullivan, R. Binder of Sperm 1 and Epididymal Sperm Binding Protein 1 Are Associated with Different Bull Sperm Subpopulations. Reproduction 2012, 143, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Pini, T.; Leahy, T.; Soleilhavoup, C.; Tsikis, G.; Labas, V.; Combes-Soia, L.; Harichaux, G.; Rickard, J.P.; Druart, X.; De Graaf, S.P. Proteomic Investigation of Ram Spermatozoa and the Proteins Conferred by Seminal Plasma. J. Proteome Res. 2016, 15, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Soleilhavoup, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.L.; Dacheux, J.L.; Guérin, Y.; Gatti, J.L.; De Graaf, S.P.; Druart, X. Ram Seminal Plasma Proteome and Its Impact on Liquid Preservation of Spermatozoa. J. Proteom. 2014, 109, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, D.; Singh, I.; Yadav, P.S. Seminal Plasma Proteome: Promising Biomarkers for Bull Fertility. Agric. Res. 2012, 1, 78–86. [Google Scholar] [CrossRef]
- Töpfer-Petersen, E.; Romero, A.; Varela, P.F.; Ekhlasi-Hundrieser, M.; Dostàlovà, Z.; Sanz, L.; Calvete, J.J. Spermadhesins: A New Protein Family. Facts, Hypotheses and Perspectives. Andrologia 2009, 30, 217–224. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal Plasma Proteins as Markers of Sperm Fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef]
- Codognoto, V.M.; Yamada, P.H.; Schmith, R.A.; de Ruediger, F.R.; Scott, C.; de Faria Lainetti, P.; Brochine, S.; de Paula Freitas-Dell’Aqua, C.; de Souza, F.F.; Oba, E. Functional Insights into the Role of Seminal Plasma Proteins on Sperm Motility of Buffalo. Anim. Reprod. Sci. 2018, 195, 251–258. [Google Scholar] [CrossRef]
- Colégio Brasileiro de Reprodução Animal. Manual Para Exame Andrológico e Avaliação de Sêmen Animal, 3rd ed.; Colégio Brasileiro de Reprodução Animal: Belo Horizonte, Brazil, 2013; pp. 15–30. [Google Scholar]
- Siqueira, J.; Oba, E.; Pinho, R.; Quintino, H.; Eler, J.; Miranda Neto, T.; Guimarães, S.; Guimarães, J. Heritability Estimate and Genetic Correlations of Reproductive Features in Nellore Bulls, Offspring of Super Precocious, Precocious and Normal Cows Under Extensive Farming Conditions. Reprod. Domest. Anim. 2012, 47, 313–318. [Google Scholar] [CrossRef]
- Okano, D.S.; Penitente-Filho, J.M.; Gomez León, V.E.; Maitan, P.P.; Silveira, C.O.; Waddington, B.; Díaz-Miranda, E.A.; Da Costa, E.P.; Guimarães, S.E.F.; Guimarães, J.D. In Vitro Evaluation of Cryopreserved Bovine Sperm and Its Relation to Field Fertility in Fixed-time Artificial Insemination. Reprod. Domest. Anima 2019, 54, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Blom, E. The ultrastructure of some characteristic sperm defects and a proposal for a new classification of the bull spermiogram. Nord. Vet. Med. 1973, 25, 383–391. [Google Scholar]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene Expression of Antioxidative Enzymes in the Human Heart: Increased Expression of Catalase in the End-Stage Failing Heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: New York, NY, USA, 1984; pp. 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Buege, J.; Aust, S. Microsomal Lipid Peroxidation. In Methods in Enzymology; Fleischer, S., Packer, L., Eds.; Academic Press: New York, NY, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Levine, R.; Garland, D.; Oliver, C.; Amici, A.; Climent, I.; Lenz, A.; Ahn, B.; Shaltiel, S.; Stadtman, E. Determination of Carbonyl Content in Oxidatively Modified Proteins. In Methods in Enzymology; Packer, L., Glazer, A., Eds.; Academic Press: New York, NY, USA, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Resjö, S.; Brus, M.; Ali, A.; Meijer, H.J.G.; Sandin, M.; Govers, F.; Levander, F.; Grenville-Briggs, L.; Andreasson, E. Proteomic Analysis of Phytophthora Infestans Reveals the Importance of Cell Wall Proteins in Pathogenicity. Mol. Cell Proteom. 2017, 16, 1958–1971. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-Gel Digestion for Mass Spectrometric Characterization of Proteins and Proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- McDonagh, B.; Sakellariou, G.K.; Smith, N.T.; Brownridge, P.; Jackson, M.J. Differential Cysteine Labeling and Global Label-Free Proteomics Reveals an Altered Metabolic State in Skeletal Muscle Aging. J. Proteome Res. 2014, 13, 5008–5021. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Proteomic Analysis of Whey Proteins in the Colostrum and Mature Milk of Xinong Saanen Goats. J. Dairy Sci. 2020, 103, 1164–1174. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udoekong, E.C.; Ramirez-Lopez, C.J.; Silva Okano, D.; Barros, E.; Pereira Vidigal, P.M.; Ribeiro, I.M.; Rodrigues Carvalho, R.P.; Machado-Neves, M.; Guimarães, J.D.; Facioni Guimarães, S.E. Proteomic Alterations and Oxidative Stress in Seminal Plasma of Nellore Bulls Under Sexual Rest. Int. J. Mol. Sci. 2025, 26, 2457. https://doi.org/10.3390/ijms26062457
Udoekong EC, Ramirez-Lopez CJ, Silva Okano D, Barros E, Pereira Vidigal PM, Ribeiro IM, Rodrigues Carvalho RP, Machado-Neves M, Guimarães JD, Facioni Guimarães SE. Proteomic Alterations and Oxidative Stress in Seminal Plasma of Nellore Bulls Under Sexual Rest. International Journal of Molecular Sciences. 2025; 26(6):2457. https://doi.org/10.3390/ijms26062457
Chicago/Turabian StyleUdoekong, Ekaette Chris, Camilo Jose Ramirez-Lopez, Denise Silva Okano, Edvaldo Barros, Pedro Marcus Pereira Vidigal, Iara Magalhães Ribeiro, Renner Philipe Rodrigues Carvalho, Mariana Machado-Neves, José Domingos Guimarães, and Simone Eliza Facioni Guimarães. 2025. "Proteomic Alterations and Oxidative Stress in Seminal Plasma of Nellore Bulls Under Sexual Rest" International Journal of Molecular Sciences 26, no. 6: 2457. https://doi.org/10.3390/ijms26062457
APA StyleUdoekong, E. C., Ramirez-Lopez, C. J., Silva Okano, D., Barros, E., Pereira Vidigal, P. M., Ribeiro, I. M., Rodrigues Carvalho, R. P., Machado-Neves, M., Guimarães, J. D., & Facioni Guimarães, S. E. (2025). Proteomic Alterations and Oxidative Stress in Seminal Plasma of Nellore Bulls Under Sexual Rest. International Journal of Molecular Sciences, 26(6), 2457. https://doi.org/10.3390/ijms26062457






