Loss of Skeletal Muscle Inositol Polyphosphate Multikinase Disrupts Glucose Regulation and Limits Exercise Capacity
Abstract
1. Introduction
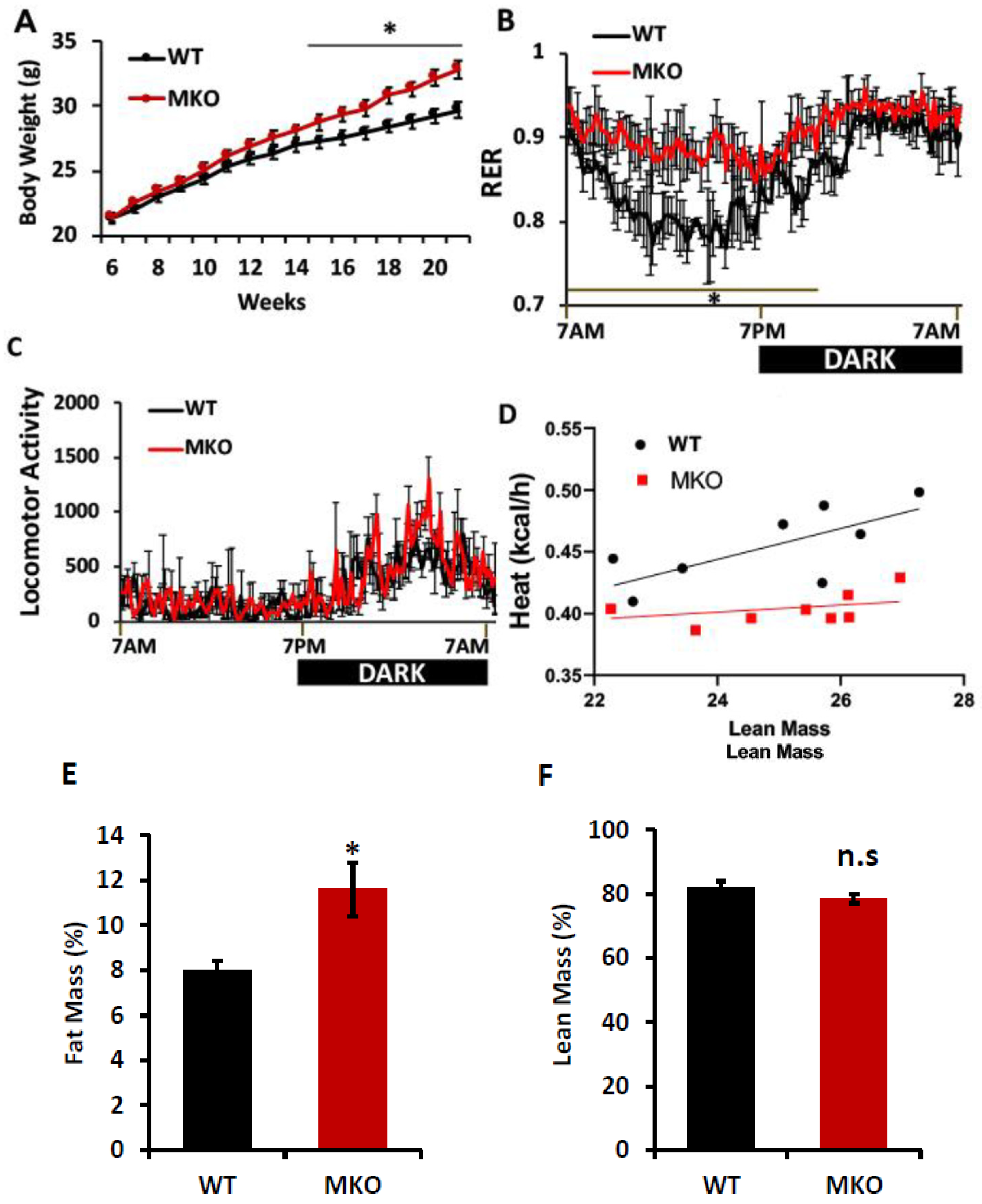
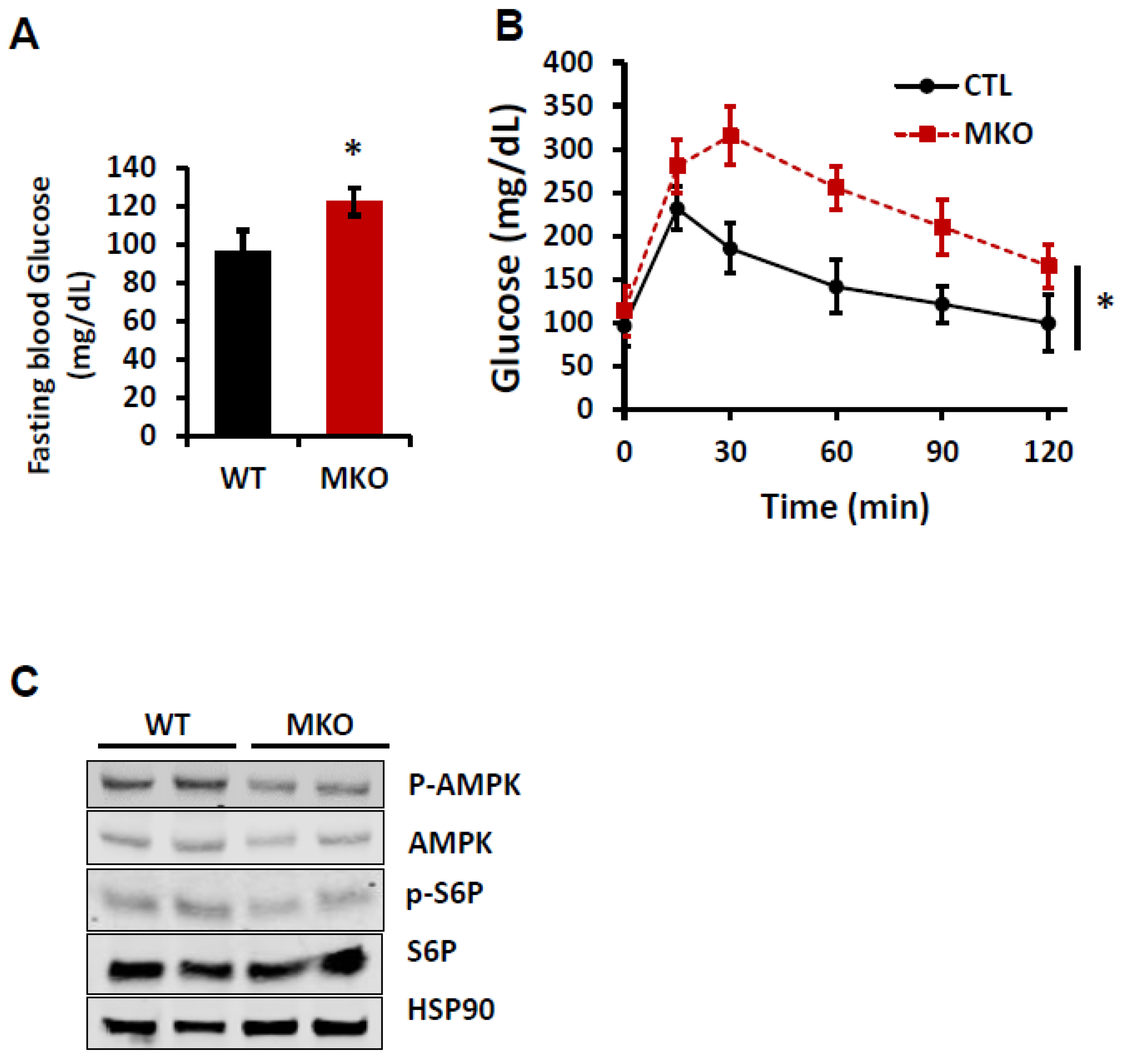
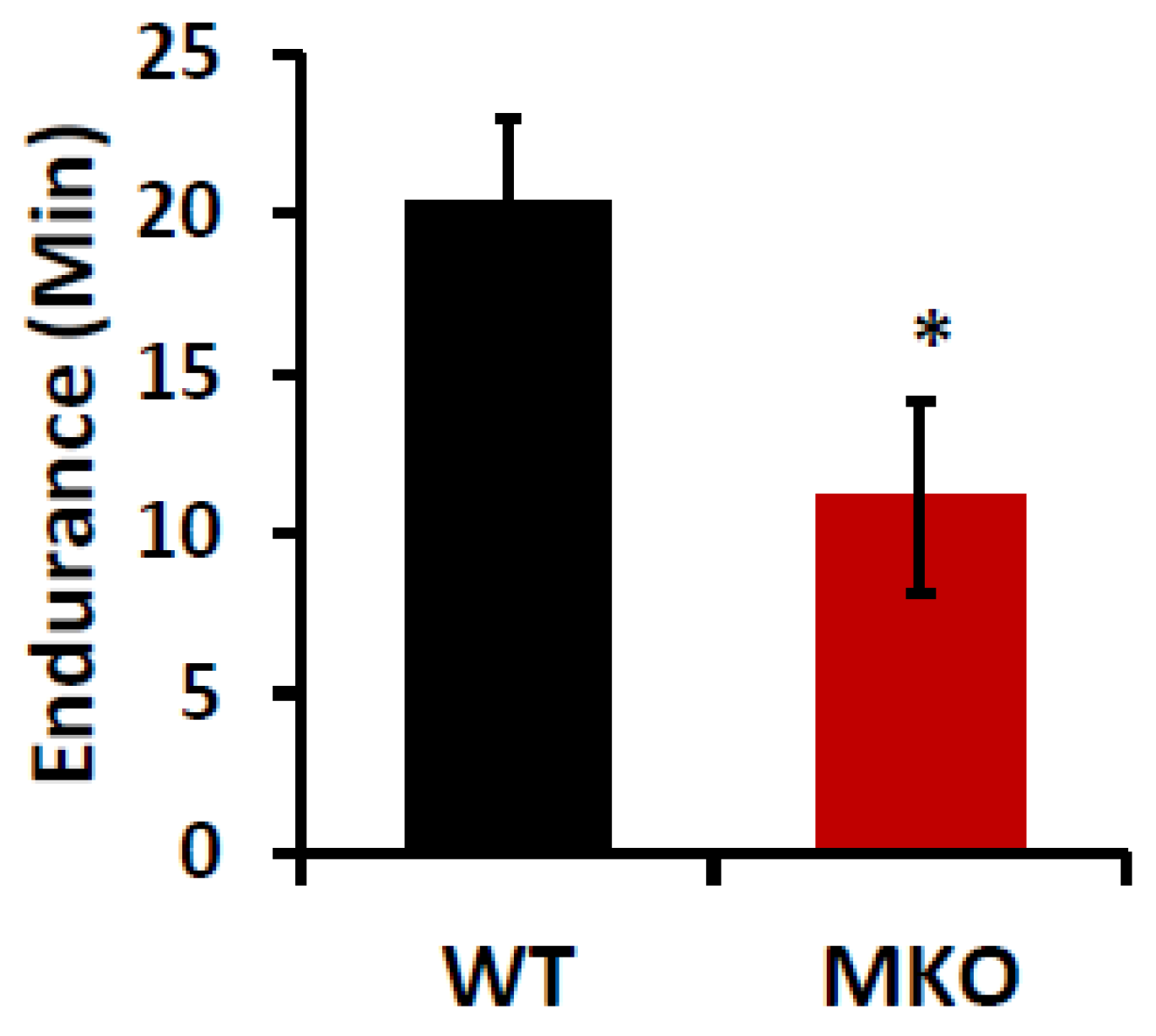
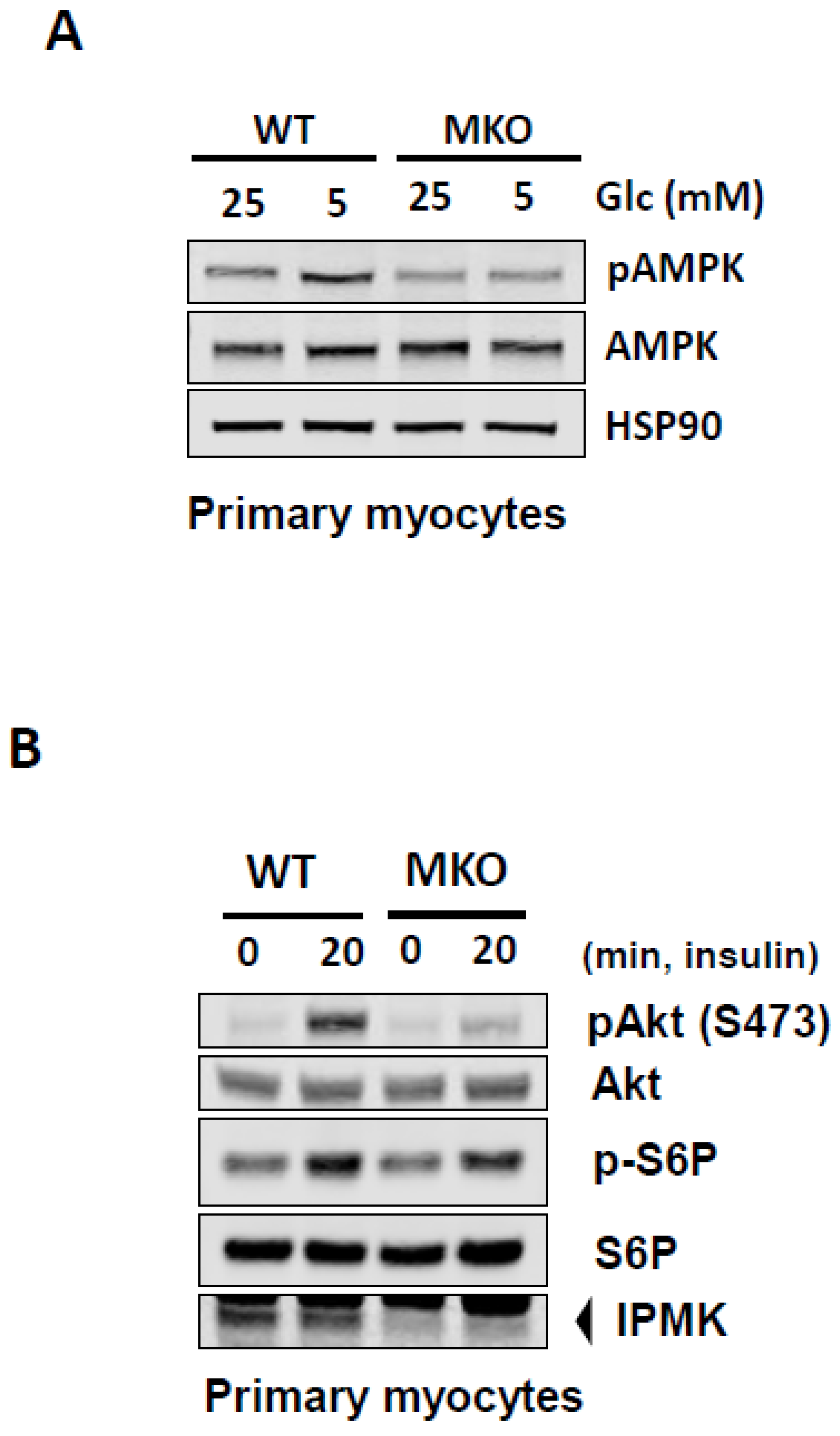
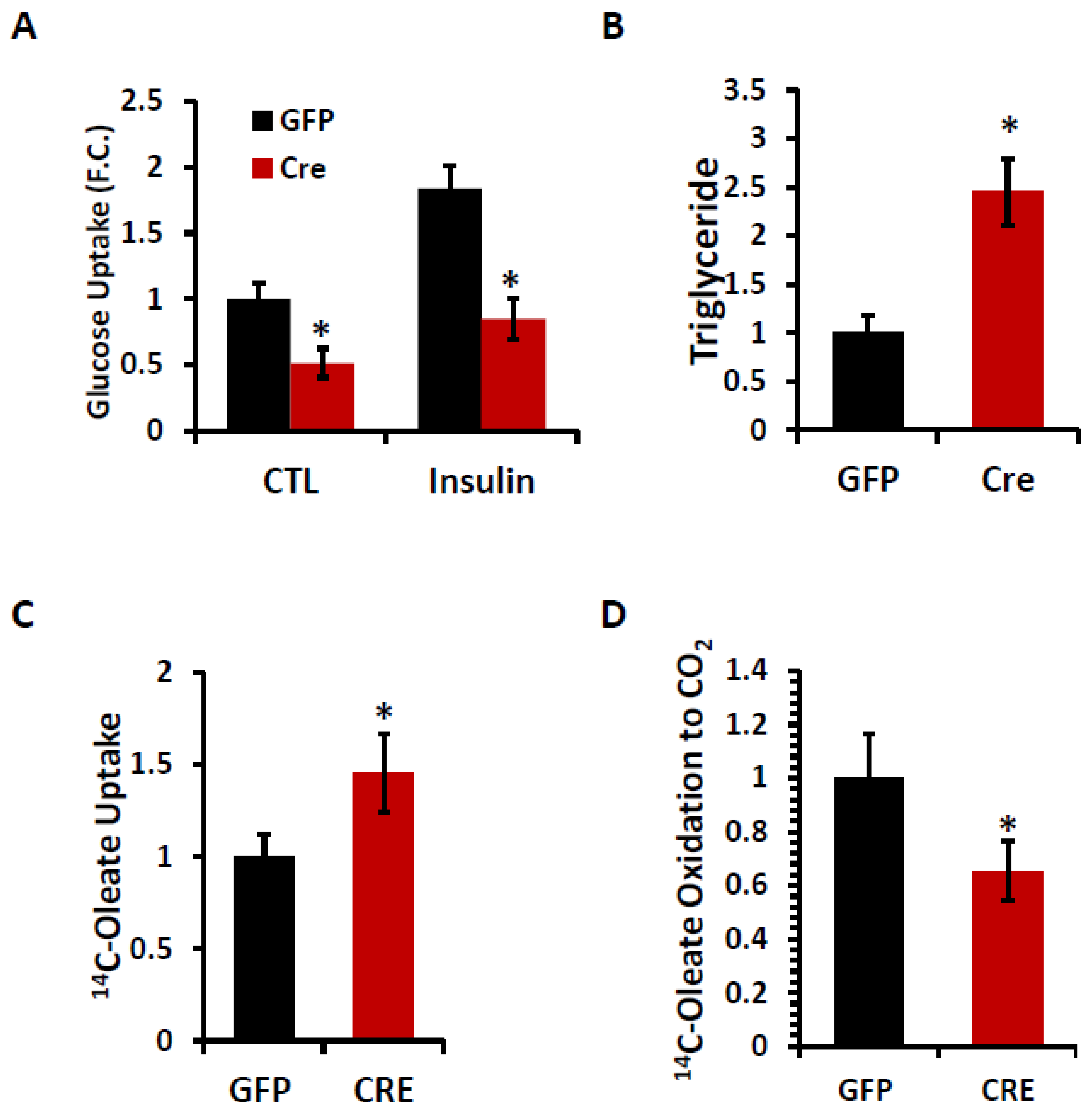
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatch, A.J.; York, J.D. SnapShot: Inositol phosphates. Cell 2010, 143, 1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tyagi, R.; Lee, J.Y.; Park, J.; Kim, Y.R.; Beon, J.; Chen, P.Y.; Cha, J.Y.; Snyder, S.H.; Kim, S. Inositol polyphosphate multikinase is a coactivator for serum response factor-dependent induction of immediate early genes. Proc. Natl. Acad. Sci. USA 2013, 110, 19938–19943. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xu, J.; Cheng, W.; Rojas, T.; Chin, A.C.; Snowman, A.M.; Harraz, M.M.; Snyder, S.H. Neuronal migration is mediated by inositol hexakisphosphate kinase 1 via alpha-actinin and focal adhesion kinase. Proc. Natl. Acad. Sci. USA 2017, 114, 2036–2041. [Google Scholar] [CrossRef]
- Maag, D.; Maxwell, M.J.; Hardesty, D.A.; Boucher, K.L.; Choudhari, N.; Hanno, A.G.; Ma, J.F.; Snowman, A.S.; Pietropaoli, J.W.; Xu, R.; et al. Inositol polyphosphate multikinase is a physiologic PI3-kinase that activates Akt/PKB. Proc. Natl. Acad. Sci. USA 2011, 108, 1391–1396. [Google Scholar] [CrossRef]
- Irvine, R.F.; Schell, M.J. Back in the water: The return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef]
- Kim, E.; Ahn, H.; Kim, M.G.; Lee, H.; Kim, S. The Expanding Significance of Inositol Polyphosphate Multikinase as a Signaling Hub. Mol. Cells 2017, 40, 315–321. [Google Scholar] [CrossRef]
- Bang, S.; Kim, S.; Dailey, M.J.; Chen, Y.; Moran, T.H.; Snyder, S.H.; Kim, S.F. AMP-activated protein kinase is physiologically regulated by inositol polyphosphate multikinase. Proc. Natl. Acad. Sci. USA 2012, 109, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Tu-Sekine, B.; Padhi, A.; Jin, S.; Kalyan, S.; Singh, K.; Apperson, M.; Kapania, R.; Hur, S.C.; Nain, A.; Kim, S.F. Inositol polyphosphate multikinase is a metformin target that regulates cell migration. FASEB J. 2019, 33, 14137–14146. [Google Scholar] [CrossRef]
- Illies, C.; Gromada, J.; Fiume, R.; Leibiger, B.; Yu, J.; Juhl, K.; Yang, S.N.; Barma, D.K.; Falck, J.R.; Saiardi, A.; et al. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science 2007, 318, 1299–1302. [Google Scholar] [CrossRef]
- Ren, L.; Hong, E.S.; Mendoza, A.; Issaq, S.; Tran Hoang, C.; Lizardo, M.; LeBlanc, A.; Khanna, C. Metabolomics uncovers a link between inositol metabolism and osteosarcoma metastasis. Oncotarget 2017, 8, 38541–38553. [Google Scholar] [CrossRef]
- Rao, F.; Xu, J.; Fu, C.; Cha, J.Y.; Gadalla, M.M.; Xu, R.; Barrow, J.C.; Snyder, S.H. Inositol pyrophosphates promote tumor growth and metastasis by antagonizing liver kinase B1. Proc. Natl. Acad. Sci. USA 2015, 112, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.I.; Louie, S.M.; Mulvihill, M.M.; Kohnz, R.A.; Li, D.S.; Chan, L.G.; Sorrentino, A.; Bandyopadhyay, S.; Cozzo, A.; Ohiri, A.; et al. Inositol phosphate recycling regulates glycolytic and lipid metabolism that drives cancer aggressiveness. ACS Chem. Biol. 2014, 9, 1340–1350. [Google Scholar] [CrossRef]
- El, A.M.; Messenguy, F.; Scherens, B.; Dubois, E. Arg82p is a bifunctional protein whose inositol polyphosphate kinase activity is essential for nitrogen and PHO gene expression but not for Mcm1p chaperoning in yeast. Mol. Microbiol. 2003, 49, 457–468. [Google Scholar]
- Dubois, E.; Dewaste, V.; Erneux, C.; Messenguy, F. Inositol polyphosphate kinase activity of Arg82/ArgRIII is not required for the regulation of the arginine metabolism in yeast. FEBS Lett. 2000, 486, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.J.; Enerback, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 2018, 14, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Suchacki, K.J.; Roberts, F.; Lovdel, A.; Farquharson, C.; Morton, N.M.; MacRae, V.E.; Cawthorn, W.P. Skeletal energy homeostasis: A paradigm of endocrine discovery. J. Endocrinol. 2017, 234, R67–R79. [Google Scholar] [CrossRef]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Ahima, R.S.; Park, H.K. Connecting Myokines and Metabolism. Endocrinol. Metab. 2015, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Kjobsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmoller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Parker, B.L.; Chaudhuri, R.; Fazakerley, D.J.; Serup, A.; Thomas, K.C.; Krycer, J.R.; Sylow, L.; Fritzen, A.M.; Hoffman, N.J.; et al. mTORC2 and AMPK differentially regulate muscle triglyceride content via Perilipin 3. Mol. Metab. 2016, 5, 646–655. [Google Scholar] [CrossRef]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Carling, D.; Viollet, B. Beyond energy homeostasis: The expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab. 2015, 21, 799–804. [Google Scholar] [CrossRef]
- Hoppeler, H. Molecular networks in skeletal muscle plasticity. J. Exp. Biol. 2016, 219 Pt 2, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found. Symp. 2007, 287, 60–63; discussion 63–69. [Google Scholar] [PubMed]
- Jung, I.; Anokye-Danso, F.; Jin, S.; Ahima, R.S.; Kim, S.F. IPMK modulates hepatic glucose production and insulin signaling. J. Cell Physiol. 2022, 237, 3421. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J., 3rd; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Perreault, L.; Newsom, S.A.; Strauss, A.; Kerege, A.; Kahn, D.E.; Harrison, K.A.; Snell-Bergeon, J.K.; Nemkov, T.; D’Alessandro, A.; Jackman, M.R.; et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight 2018, 3, e96805. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef]
- Moro, C.; Galgani, J.E.; Luu, L.; Pasarica, M.; Mairal, A.; Bajpeyi, S.; Schmitz, G.; Langin, D.; Liebisch, G.; Smith, S.R. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J. Clin. Endocrinol. Metab. 2009, 94, 3440–3447. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Kraus, R.M.; Hickner, R.C.; Howell, A.W.; Wolfe, R.R.; Dohm, G.L. Impaired plasma fatty acid oxidation in extremely obese women. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1076–E1081. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Fujimoto, B.A.; Young, M.; Nakamura, N.; Ha, H.; Carter, L.; Pitts, M.W.; Torres, D.; Noh, H.L.; Suk, S.; Kim, J.K.; et al. Disrupted glucose homeostasis and skeletal-muscle-specific glucose uptake in an exocyst knockout mouse model. J. Biol. Chem. 2021, 296, 100482. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T.; Dvorak, R.V.; DeNino, W.F.; Brochu, M.; Ades, P.A. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: A controlled randomized trial. J. Clin. Endocrinol. Metab. 2000, 85, 2463–2468. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.H.; Martin, B.C.; Krolewski, A.S.; Soeldner, J.S.; Kahn, C.R. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann. Intern. Med. 1990, 113, 909–915. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.T.; Lauritzen, H.P.; Hirshman, M.F.; Smyth, G.; Goodyear, L.J.; Kahn, C.R. Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis. Cell Rep. 2015, 11, 1220–1235. [Google Scholar] [CrossRef]
- Baudry, A.; Leroux, L.; Jackerott, M.; Joshi, R.L. Genetic manipulation of insulin signaling, action and secretion in mice. Insights into glucose homeostasis and pathogenesis of type 2 diabetes. EMBO Rep. 2002, 3, 323–328. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Kim, J.K.; Yakar, S.; Dupont, J.; Hernandez-Sanchez, C.; Castle, A.L.; Filmore, J.; Shulman, G.I.; Le Roith, D. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001, 15, 1926–1934. [Google Scholar] [CrossRef]
- Moller, D.E.; Chang, P.Y.; Yaspelkis, B.B., 3rd; Flier, J.S.; Wallberg-Henriksson, H.; Ivy, J.L. Transgenic mice with muscle-specific insulin resistance develop increased adiposity, impaired glucose tolerance, and dyslipidemia. Endocrinology 1996, 137, 2397–2405. [Google Scholar] [CrossRef]
- Jung, I.R.; Ahima, R.S.; Kim, S.F. Inositol polyphosphate multikinase modulates free fatty acids-induced insulin resistance in primary mouse hepatocytes. J. Cell. Biochem. 2023, 124, 1695–1704. [Google Scholar] [CrossRef]
- Bang, S.; Chen, Y.; Ahima, R.S.; Kim, S.F. Convergence of IPMK and LKB1-AMPK signaling pathways on metformin action. Mol. Endocrinol. 2014, 28, 1186–1193. [Google Scholar] [CrossRef]
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Ho, R.C.; Manabe, Y.; Jessen, N.; Toyoda, T.; Holland, W.L.; Summers, S.A.; Hirshman, M.F.; Goodyear, L.J. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes 2008, 57, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Sen, N.; Paul, B.D.; Snowman, A.M.; Rao, F.; Vandiver, M.S.; Xu, J.; Snyder, S.H. Inositol polyphosphate multikinase is a coactivator of p53-mediated transcription and cell death. Sci.Signal. 2013, 6, ra22. [Google Scholar] [CrossRef]
- Xu, R.; Paul, B.D.; Smith, D.R.; Tyagi, R.; Rao, F.; Khan, A.B.; Blech, D.J.; Vandiver, M.S.; Harraz, M.M.; Guha, P.; et al. Inositol polyphosphate multikinase is a transcriptional coactivator required for immediate early gene induction. Proc. Natl. Acad. Sci. USA 2013, 110, 16181–16186. [Google Scholar] [CrossRef]
- Li, S.; Czubryt, M.P.; McAnally, J.; Bassel-Duby, R.; Richardson, J.A.; Wiebel, F.F.; Nordheim, A.; Olson, E.N. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1082–1087. [Google Scholar] [CrossRef]
- Tyagi, R.; Chakraborty, S.; Tripathi, S.J.; Jung, I.R.; Kim, S.F.; Snyder, S.H.; Paul, B.D. Inositol polyphosphate multikinase modulates redox signaling through nuclear factor erythroid 2-related factor 2 and glutathione metabolism. iScience 2023, 26, 107199. [Google Scholar] [CrossRef]
- He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 2001, 50, 817–823. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Kelley, D.E. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J. Appl. Physiol. 1997, 83, 166–171. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Colberg, S.R.; Thaete, F.L.; Kelley, D.E. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995, 9, 273–278. [Google Scholar] [CrossRef]
- McPherron, A.C.; Huynh, T.V.; Lee, S.J. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev. Biol. 2009, 9, 24. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Jung, I.-R.; Tu-Sekine, B.; Jin, S.; Anokye-Danso, F.; Ahima, R.S.; Kim, S.F. Loss of Skeletal Muscle Inositol Polyphosphate Multikinase Disrupts Glucose Regulation and Limits Exercise Capacity. Int. J. Mol. Sci. 2025, 26, 2395. https://doi.org/10.3390/ijms26062395
Lee J-H, Jung I-R, Tu-Sekine B, Jin S, Anokye-Danso F, Ahima RS, Kim SF. Loss of Skeletal Muscle Inositol Polyphosphate Multikinase Disrupts Glucose Regulation and Limits Exercise Capacity. International Journal of Molecular Sciences. 2025; 26(6):2395. https://doi.org/10.3390/ijms26062395
Chicago/Turabian StyleLee, Ji-Hyun, Ik-Rak Jung, Becky Tu-Sekine, Sunghee Jin, Frederick Anokye-Danso, Rexford S. Ahima, and Sangwon F. Kim. 2025. "Loss of Skeletal Muscle Inositol Polyphosphate Multikinase Disrupts Glucose Regulation and Limits Exercise Capacity" International Journal of Molecular Sciences 26, no. 6: 2395. https://doi.org/10.3390/ijms26062395
APA StyleLee, J.-H., Jung, I.-R., Tu-Sekine, B., Jin, S., Anokye-Danso, F., Ahima, R. S., & Kim, S. F. (2025). Loss of Skeletal Muscle Inositol Polyphosphate Multikinase Disrupts Glucose Regulation and Limits Exercise Capacity. International Journal of Molecular Sciences, 26(6), 2395. https://doi.org/10.3390/ijms26062395





