Resveratrol Upregulates Antioxidant Factors Expression and Downmodulates Interferon-Inducible Antiviral Factors in Aging
Abstract
1. Introduction
2. Results
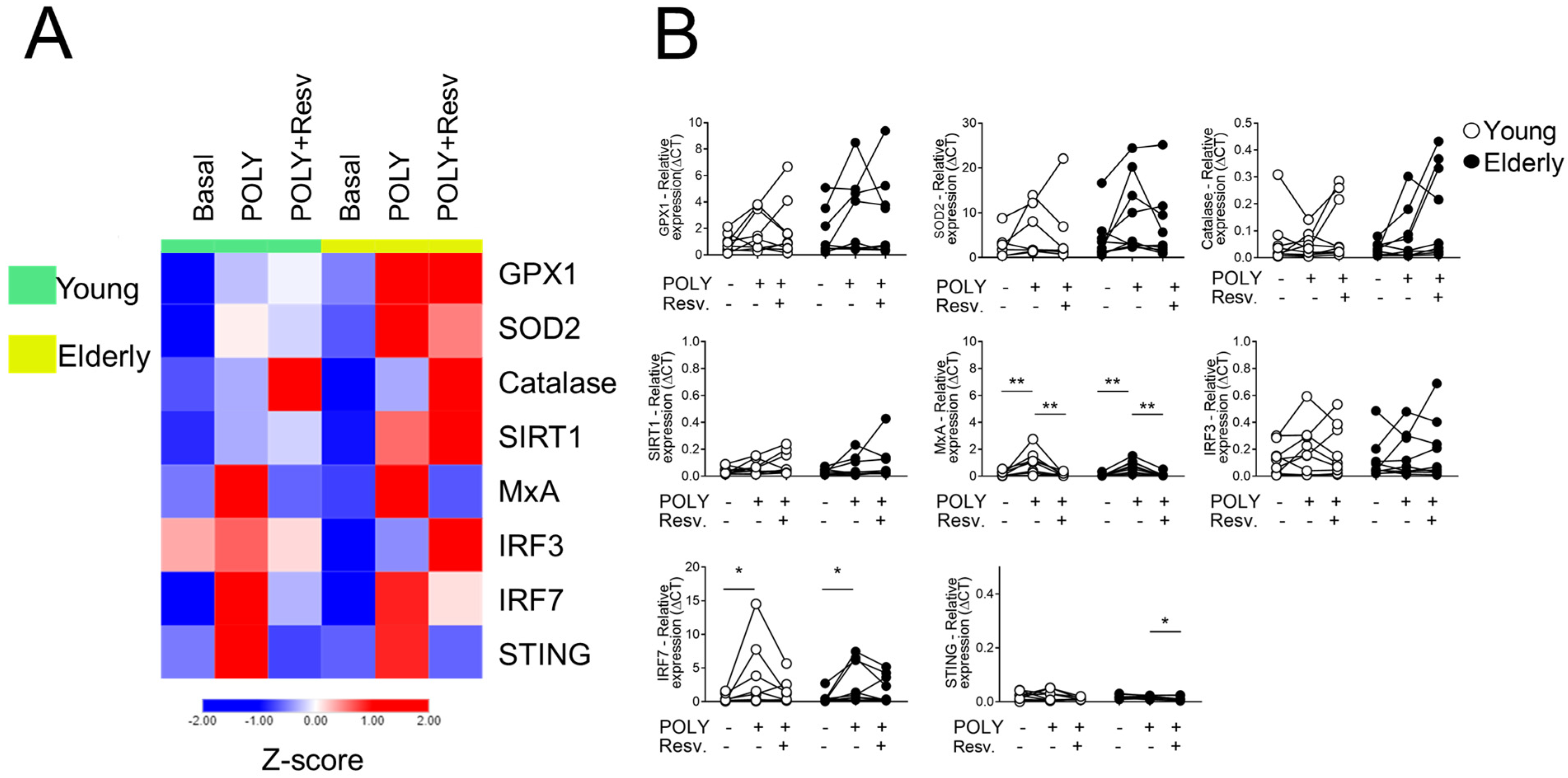
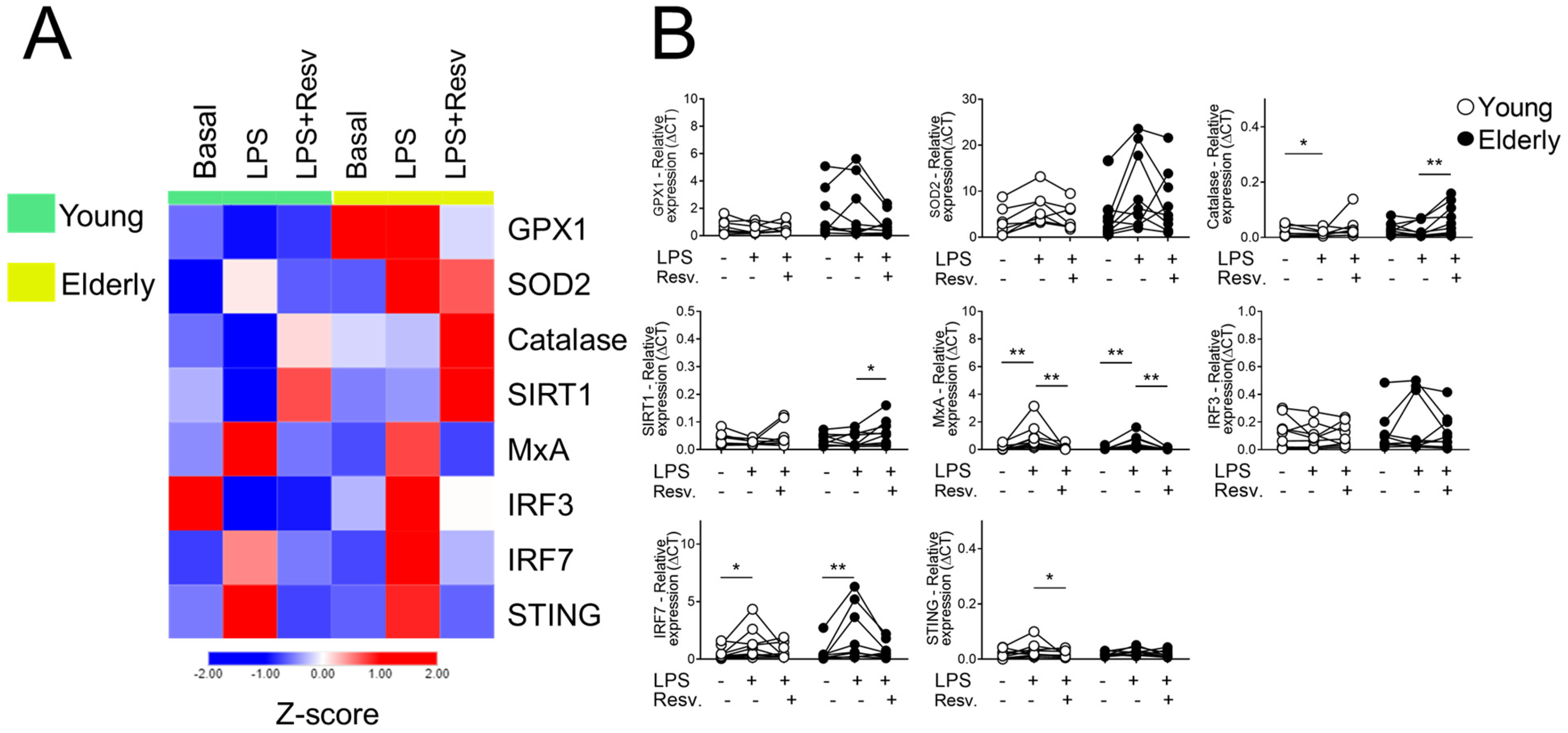
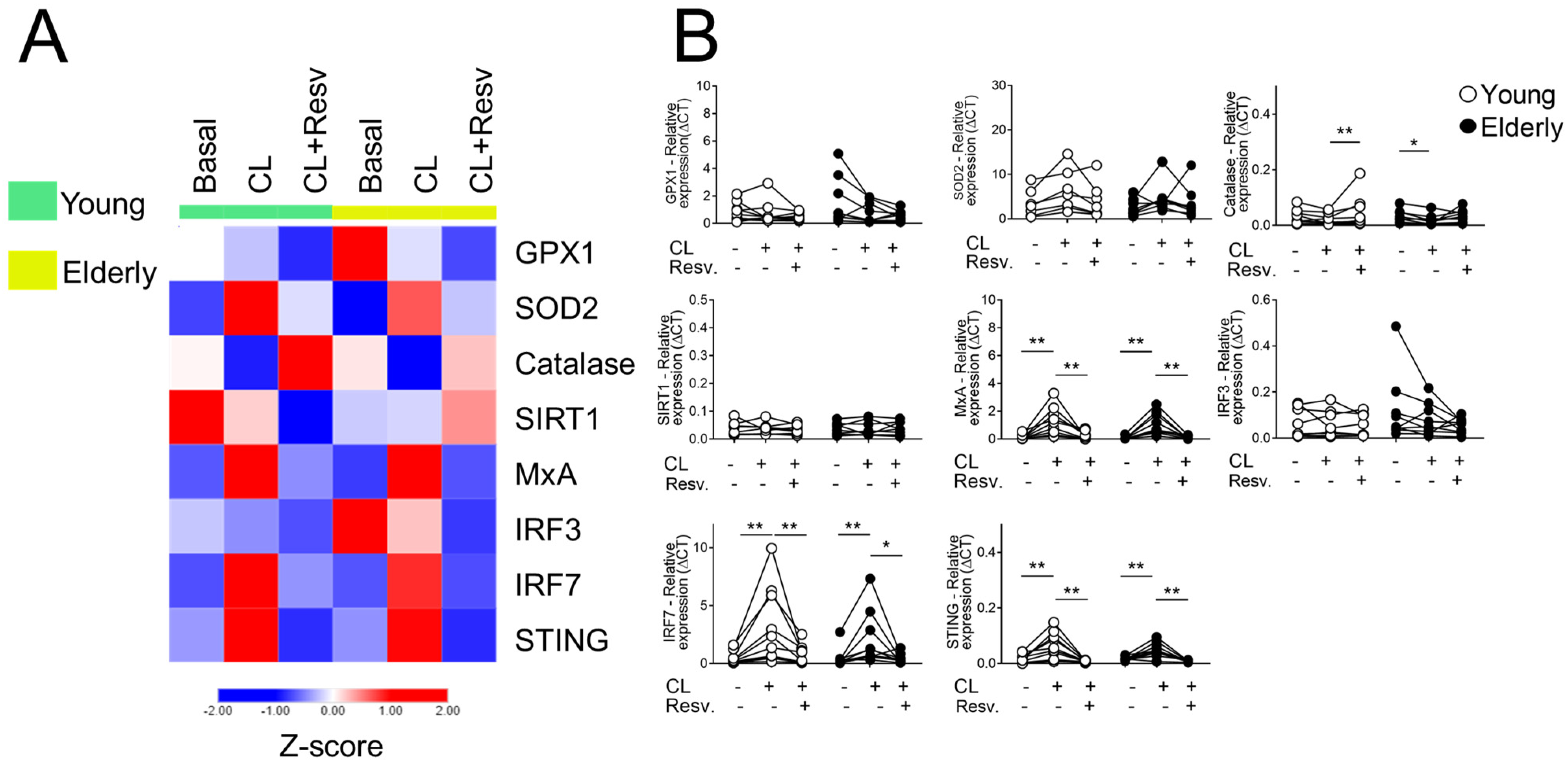
2.1. Effect of Resv on the Antioxidant and Antiviral Transcriptional Expression Induced by TLR Agonists Stimulation
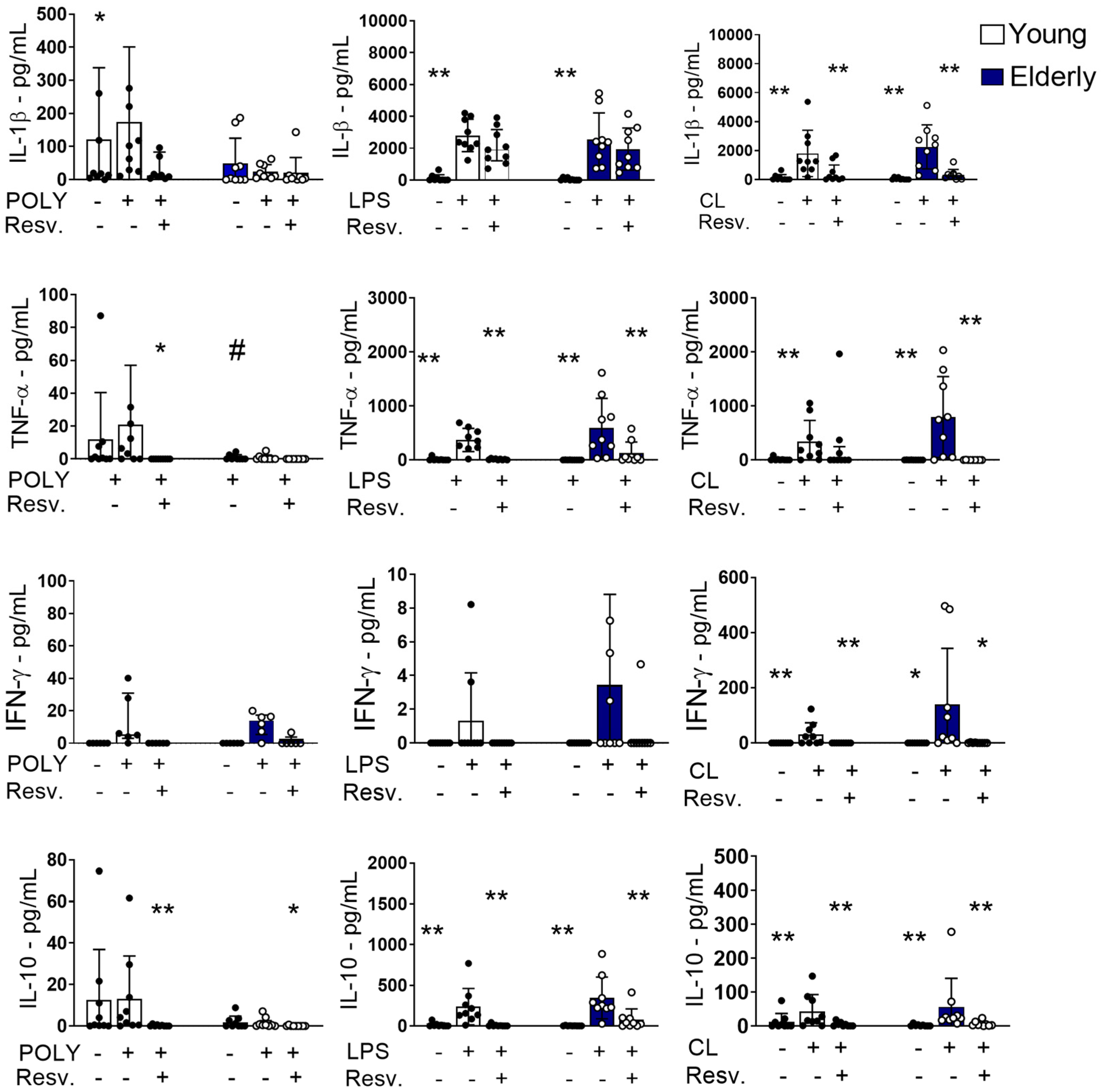
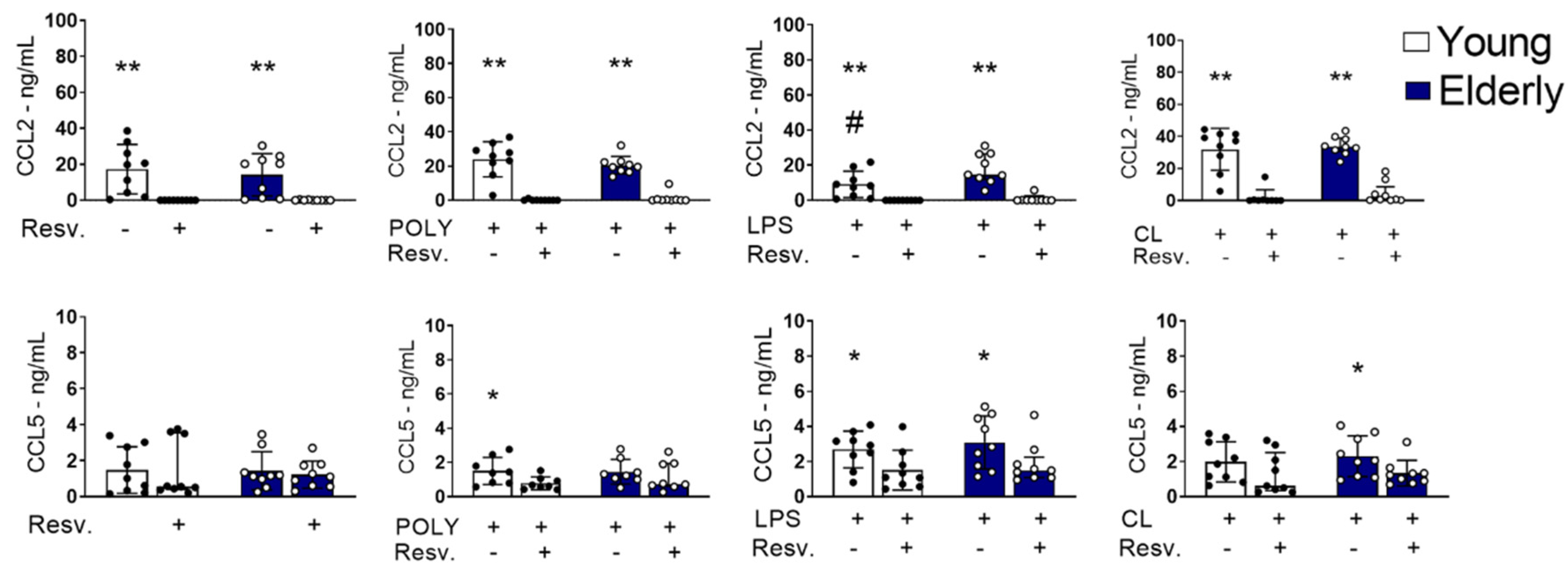
2.2. Resv Cytokines/Chemokines Induced by Innate Response Stimuli Through TLR Activation
3. Discussion
4. Materials and Methods
4.1. Study Design and Casuistic
4.2. Cultures of PBMCs with TLR Agonists
4.3. Cell Viability
4.4. Real-Time PCR (qPCR)
4.5. Cytokine Measurement
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Ageing and health World Health Organization 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 8 June 2023).
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, Z.; Mo, P.; Li, X.; Ma, Z.; Song, S.; Chen, X.; Luo, M.; Liang, K.; Gao, S.; et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: A single-centered, retrospective study. J. Gerontol. Ser. A 2020, 75, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Yan, L.; Wang, P.; Zhao, F.; Hu, S. The role of toll-like receptors in immune tolerance induced by Helicobacter pylori infection. Helicobacter. 2023, 28, e13020. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 12–14. [Google Scholar] [CrossRef]
- Branco, A.C.C.C.; Pereira, N.Z.; Yoshikawa, F.S.Y.; Oliveira, L.M.D.S.; Teixeira, F.M.E.; Oliveira, L.D.M.; Pietrobon, A.J.; Torrealba, M.P.; de Lima, J.F.; Duarte, A.J.D.S.; et al. Proinflammatory profile of neonatal monocytes induced by microbial ligands is downmodulated by histamine. Sci. Rep. 2019, 9, 13721. [Google Scholar] [CrossRef]
- Pietrobon, A.J.; Yoshikawa, F.S.; Oliveira, L.M.; Pereira, N.Z.; Matozo, T.; de Alencar, B.C.; Duarte, A.J.; Sato, M.N. Antiviral Response Induced by Toll-Like Receptor (TLR) 7/TLR8 Activation Inhibits Human Immunodeficiency Virus Type 1 Infection in Cord Blood Macrophages. J. Infect. Dis. 2022, 225, 510–519. [Google Scholar] [CrossRef]
- Cardoso, E.C.; Pereira, N.Z.; Mitsunari, G.E.; Oliveira, L.M.D.S.; Ruocco, R.M.S.; Francisco, R.P.V.; Zugaib, M.; da Silva Duarte, A.J.; Sato, M.N. TLR7/TLR8 activation restores defective cytokine secretion by myeloid dendritic cells but not by plasmacytoid dendritic cells in HIV-infected pregnant women and newborns. PLoS ONE 2013, 8, e67036. [Google Scholar] [CrossRef]
- de Lollo, C.; de Moraes Vasconcelos, D.; da Silva Oliveira, L.M.; Domingues, R.; de Carvalho, G.C.; da Silva Duarte, A.J.; Zugaib, M.; da Silva Duarte, A.J.; Sato, M.N. Chemokine, cytokine and type I interferon production induced by Toll-like receptor activation in common variable immune deficiency. Clin. Immunol. 2016, 169, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Manfrere, K.C.; Torrealba, M.P.; Miyashiro, D.R.; Oliveira, L.M.; de Carvalho, G.C.; Lima, J.F.; Branco, A.C.C.C.; Pereira, N.Z.; Pereira, J.; Sanches, J.J.A.; et al. Toll-like receptor agonists partially restore the production of pro-inflammatory cytokines and type I interferon in Sézary syndrome. Oncotarget 2016, 7, 74592. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.; de Carvalho, G.C.; Aoki, V.; da Silva Duarte, A.J.; Sato, M.N. Activation of myeloid dendritic cells, effector cells and regulatory T cells in lichen planus. J. Transl. Med. 2016, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.F.; Oliveira, L.; Pereira, N.Z.; Duarte, A.J.; Sato, M.N. Polyfunctional natural killer cells with a low activation profile in response to Toll-like receptor 3 activation in HIV-1-exposed seronegative subjects. Sci. Rep. 2017, 7, 524. [Google Scholar] [CrossRef]
- Shaw, A.C.; Panda, A.; Joshi, S.R.; Qian, F.; Allore, H.G.; Montgomery, R.R. Dysregulation of human Toll-like receptor function in aging. Ageing Res. Rev. 2011, 10, 346–353. [Google Scholar] [CrossRef]
- Connors, J.; Taramangalam, B.; Cusimano, G.; Bell, M.R.; Matt, S.M.; Runner, K.; Gaskill, P.J.; DeFilippis, V.; Nikolich-Žugich, J.; Kutzler, M.A.; et al. Aging alters antiviral signaling pathways resulting in functional impairment in innate immunity in response to pattern recognition receptor agonists. GeroScience 2022, 44, 2555–2572. [Google Scholar] [CrossRef]
- Gatouillat, G.; Balasse, E.; Joseph-Pietras, D.; Morjani, H.; Madoulet, C. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J. Cell Biochem. 2010, 110, 893–902. [Google Scholar] [CrossRef]
- Lu, F.; Zahid, M.; Wang, C.; Saeed, M.; Cavalieri, E.L.; Rogan, E.G. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008, 1, 135–145. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Mou, S.F.; Chen, X.Q.; Gong, L.L.; Ge, W.S. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NF-κB in animal models of acute pharyngitis. Mol. Med. Rep. 2018, 17, 1269–1274. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1202–1208. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.-P.; Fu, J.-S.; Bai, L.; Guo, L. Resveratrol augments therapeutic efficiency of mouse bone marrow mesenchymal stem cell-based therapy in experimental autoimmune encephalomyelitis. Int. J. Dev. Neurosci. 2016, 49, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of resveratrol and other polyphenols on Sirt1: Relevance to brain function during aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxidative Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Petrella, C.; Francati, S.; Lucarelli, M.; Barbato, C.; Minni, A.; Ralli, M.; Greco, A.; Tarani, L.; Fiore, M.; et al. Antioxidant intervention to improve cognition in the aging brain: The example of hydroxytyrosol and resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef]
- de Souza Andrade, M.M.; Leal, V.N.; Fernandes, I.G.; Gozzi-Silva, S.C.; Beserra, D.R.; Oliveira, E.A.; Teixeira, F.M.E.; Yendo, T.M.; Sousa, M.d.G.T.; Teodoro, W.R.; et al. Resveratrol Downmodulates Neutrophil Extracellular Trap (NET) Generation by Neutrophils in Patients with Severe COVID-19. Antioxidants 2022, 11, 1690. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Persaud, A.T.; Bennett, S.A.; Thaya, L.; Burnie, J.; Guzzo, C. Human monocytes store and secrete preformed CCL5, independent of de novo protein synthesis. J. Leukoc. Biol. 2022, 111, 573–583. [Google Scholar] [CrossRef]
- Forsyth, K.S.; Jiwrajka, N.; Lovell, C.D.; Toothacre, N.E.; Anguera, M.C. The conneXion between sex and immune responses. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; McCabe, E.J.; Lieberman, S.M. Mechanisms underlying sex differences in autoimmunity. J. Clin. Investig. 2024, 134, e180076. [Google Scholar] [CrossRef]
- Moulton, V. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Khaksari, M.; Pourali, M.; Talabon, S.R.; Navashenaq, J.G.; Bashiri, H.; Amiresmaili, S. Protective effects of 17-β-estradiol on liver injury: The role of TLR4 signaling pathway and inflammatory response. Cytokine 2024, 181, 156686. [Google Scholar] [CrossRef]
- Boehmer, E.D.; Goral, J.; Faunce, D.E.; Kovacs, E.J. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leucoc. Biol. 2004, 75, 342–349. [Google Scholar] [CrossRef]
- Echem, C.; Akamine, E.H. Toll-like receptors represent an important link for sex differences in cardiovascular aging and diseases. Front. Aging 2021, 2, 709914. [Google Scholar] [CrossRef]
- Bupp, M.R.G. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Y.; Xiong, T.; Nie, X.; Zhang, H.; Zhu, C. Effect of dietary resveratrol supplementation on growth performance, antioxidant capacity, intestinal immunity and gut microbiota in yellow-feathered broilers challenged with lipopolysaccharide. Front. Microbiol. 2022, 13, 977087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Luo, X.-F.; Li, M.-T.; Xu, D.; Zhou, S.; Chen, H.-Z.; Gao, N.; Chen, Z.; Zhang, L.-L.; Zeng, X.-F. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS ONE 2014, 9, e114792. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Tran, H.B.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther. Adv. Respir. Dis. 2020, 14, 1753466620905280. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant effect of Resveratrol: Change in MAPK cell signaling pathway during the aging process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef]
- Franco, F.N.; de Cassia Cardoso, L.; Silva, B.N.M.; de Araújo, G.R.; Chaves, M.M. Aging: Silencing the PKA and AkT/PKB signaling pathways alters the antioxidant capacity of resveratrol. Biogerontology 2023, 24, 913–923. [Google Scholar] [CrossRef]
- Yang, X.; Dong, W.-B.; Lei, X.-P.; Li, Q.-P.; Zhang, L.-Y.; Zhang, L.-P. Resveratrol suppresses hyperoxia-induced nucleocytoplasmic shuttling of SIRT1 and ROS production in PBMC from preterm infants in vitro. J. Matern.-Fetal Neonatal Med. 2018, 31, 1142–1150. [Google Scholar] [CrossRef]
- Chung, E.Y.; Kim, B.H.; Hong, J.-T.; Lee, C.-K.; Ahn, B.; Nam, S.-Y.; Han, S.-B.; Kim, Y. Resveratrol down-regulates interferon-γ-inducible inflammatory genes in macrophages: Molecular mechanism via decreased STAT-1 activation. J. Nutr. Biochem. 2011, 22, 902–909. [Google Scholar] [CrossRef]
- Kang, N.; Shi, Y.; Song, J.; Gao, F.; Fan, M.; Jin, W.; Gao, Y.; Lv, P. Resveratrol reduces inflammatory response and detrimental effects in chronic cerebral hypoperfusion by down-regulating stimulator of interferon genes/TANK-binding kinase 1/interferon regulatory factor 3 signaling. Front. Aging Neurosci. 2022, 14, 868484. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, P.; del Signore, A.; Sabbioni, E.; Di Gioacchino, M.; Di Giampaolo, L.; Reale, M.; Conti, P.; Paganelli, R.; Giaccio, M. Effects of resveratrol on lymphocyte proliferation and cytokine release. Ann. Clin. Lab. Sci. 2003, 33, 226–231. [Google Scholar] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Faith, S.A.; Sweet, T.J.; Bailey, E.; Booth, T.; Docherty, J.J. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antivir. Res. 2006, 72, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zang, N.; Zhou, N.; Li, W.; Xie, X.; Deng, Y.; Ren, L.; Long, X.; Li, S.; Zhou, L.; et al. Resveratrol inhibits the TRIF-dependent pathway by upregulating sterile alpha and armadillo motif protein, contributing to anti-inflammatory effects after respiratory syncytial virus infection. J. Virol. 2014, 88, 4229–4236. [Google Scholar] [CrossRef]
- Mohd, A.; Zainal, N.; Tan, K.-K.; AbuBakar, S. Resveratrol affects Zika virus replication in vitro. Sci. Rep. 2019, 9, 14336. [Google Scholar] [CrossRef]
- Yang, M.; Wei, J.; Huang, T.; Lei, L.; Shen, C.; Lai, J.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.; et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother. Res. 2020, 35, 1127. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the Anti-inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022, 2022, 7138756. [Google Scholar] [CrossRef]
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res. 2000, 60, 3477–3483. [Google Scholar]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Zou, Y.; Li, L.; Yin, Z. Beneficial effects of resveratrol administration—Focus on potential biochemical mechanisms in cardiovascular conditions. Nutrients 2018, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Ka, S.-M.; Hsu, W.-H.; Chen, A.; Chao, L.K.; Lin, C.-C.; Hsieh, C.-C.; Chen, M.-C.; Chiu, H.-W.; Ho, C.-L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2020, 19, 1824–1834. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Zheng, F.; Liu, H.; Qiu, F.; Chen, Y.; Liang, C.L.; Dai, Z. Resveratrol exerts antitumor effects by downregulating CD8+ CD122+ Tregs in murine hepatocellular carcinoma. Oncoimmunology 2020, 9, 1829346. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Regler, M.; Urschel, K.; Goppelt-Struebe, M.; Daniel, W.G.; Garlichs, C.D. Resveratrol inhibits monocytic cell chemotaxis to MCP-1 and prevents spontaneous endothelial cell migration through Rho kinase-dependent mechanism. J. Atheroscler. Thromb. 2011, 18, 1031–1042. [Google Scholar] [CrossRef][Green Version]
- Xie, X.-H.; Zang, N.; Li, S.-M.; Wang, L.-J.; Deng, Y.; He, Y.; Yang, X.-Q.; Liu, E.-M. Resveratrol Inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation 2012, 35, 1392–1401. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Morpheus. Available online: https://software.broadinstitute.org/morpheus (accessed on 24 May 2023).
| Volunteer | Age | Medication Use | Illness | IMC |
|---|---|---|---|---|
| Young—1 | 25 | No | No | 24.78 |
| Young—2 | 26 | No | No | 21.09 |
| Young—3 | 27 | No | No | 27.24 |
| Young—4 | 22 | No | No | 21.63 |
| Young—5 | 21 | No | No | 29.03 |
| Young—6 | 26 | No | No | 24.73 |
| Young—7 | 26 | Anticoncepcional Diclin | No | 21.51 |
| Young—8 | 21 | No | No | 24.61 |
| Young—9 | 29 | No | No | 19.63 |
| Young—10 | 30 | No | No | 22.23 |
| Volunteer | Age | Medication Use | Illness | IMC |
|---|---|---|---|---|
| Elderly—1 | 67 | Simvastatin | Controlled hypertension | 29.62 |
| Elderly—2 | 65 | No | No | 25.50 |
| Elderly—3 | 73 | Carvedilol | Controlled hypertension | 23.24 |
| Elderly—4 | 63 | Rosuvastatin | Labyrinthitis | 34.60 |
| Elderly—5 | 64 | No | No | 27.48 |
| Elderly—6 | 72 | No | No | 25.04 |
| Elderly—7 | 60 | No | No | 29.05 |
| Elderly—8 | 71 | No | No | 25.71 |
| Elderly—9 | 63 | No | No | 23.56 |
| Elderly—10 | 77 | Ablok, corus | Controlled hypertension | 33.67 |
| Primer | Sequence |
|---|---|
| SOD2 | F: GCCCTGGAACCTCACATCAA R: TCAGGTTGTTCACGTAGGCC |
| GPX-1 | F: TTGAGAAGTTCCTGGTGGGC R: CGATGTCAGGCTCGATGTCA |
| GAPDH | F: GAAGGTGAAGGTCGGAGT R: GAAGATGGTGATGGGATTTC |
| IRF3 | F: AGAGGCTCGTGATGGTCAAGGTT R: AGAGTGGGTGGCTGTTGGAAATG |
| IRF7 | F: TGGTCCTGGTGAAGCTGGAA R: GATGTCGTCATAGAGGCTGTTG |
| MxA | F: AAGCTGATCCGCCTCCACTT R: TGCAATGCACCCCTGTATACC |
| STING | F: ATATCTGCGGCTGATCCTGC R: GGTCTGCTGGGGCAGTTTAT |
| CATALASE | F: CTCCGGAACAACAGCCTTCT R: GAATGCCCGCACCTGAGTAA |
| SIRT1 | F: TGAATATGCCAAACTTTGCTG R: GGGTGGCAACTCTGACAAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, I.G.; Oliveira, L.d.M.; Andrade, M.M.d.S.; Alberca, R.W.; Lima, J.C.; de Sousa, E.S.A.; Pietrobon, A.J.; Pereira, N.Z.; Castelo Branco, A.C.C.; Duarte, A.J.d.S.; et al. Resveratrol Upregulates Antioxidant Factors Expression and Downmodulates Interferon-Inducible Antiviral Factors in Aging. Int. J. Mol. Sci. 2025, 26, 2345. https://doi.org/10.3390/ijms26052345
Fernandes IG, Oliveira LdM, Andrade MMdS, Alberca RW, Lima JC, de Sousa ESA, Pietrobon AJ, Pereira NZ, Castelo Branco ACC, Duarte AJdS, et al. Resveratrol Upregulates Antioxidant Factors Expression and Downmodulates Interferon-Inducible Antiviral Factors in Aging. International Journal of Molecular Sciences. 2025; 26(5):2345. https://doi.org/10.3390/ijms26052345
Chicago/Turabian StyleFernandes, Iara Grigoletto, Luana de M. Oliveira, Milena M. de Souza Andrade, Ricardo W. Alberca, Júlia Cataldo Lima, Emanuella Sarmento Alho de Sousa, Anna Julia Pietrobon, Nátalli Zanete Pereira, Anna Cláudia Calvielli Castelo Branco, Alberto José da Silva Duarte, and et al. 2025. "Resveratrol Upregulates Antioxidant Factors Expression and Downmodulates Interferon-Inducible Antiviral Factors in Aging" International Journal of Molecular Sciences 26, no. 5: 2345. https://doi.org/10.3390/ijms26052345
APA StyleFernandes, I. G., Oliveira, L. d. M., Andrade, M. M. d. S., Alberca, R. W., Lima, J. C., de Sousa, E. S. A., Pietrobon, A. J., Pereira, N. Z., Castelo Branco, A. C. C., Duarte, A. J. d. S., & Sato, M. N. (2025). Resveratrol Upregulates Antioxidant Factors Expression and Downmodulates Interferon-Inducible Antiviral Factors in Aging. International Journal of Molecular Sciences, 26(5), 2345. https://doi.org/10.3390/ijms26052345






