Impact of Tick-Borne Orthoflaviviruses Infection on Compact Human Brain Endothelial Barrier
Abstract
1. Introduction
2. Results
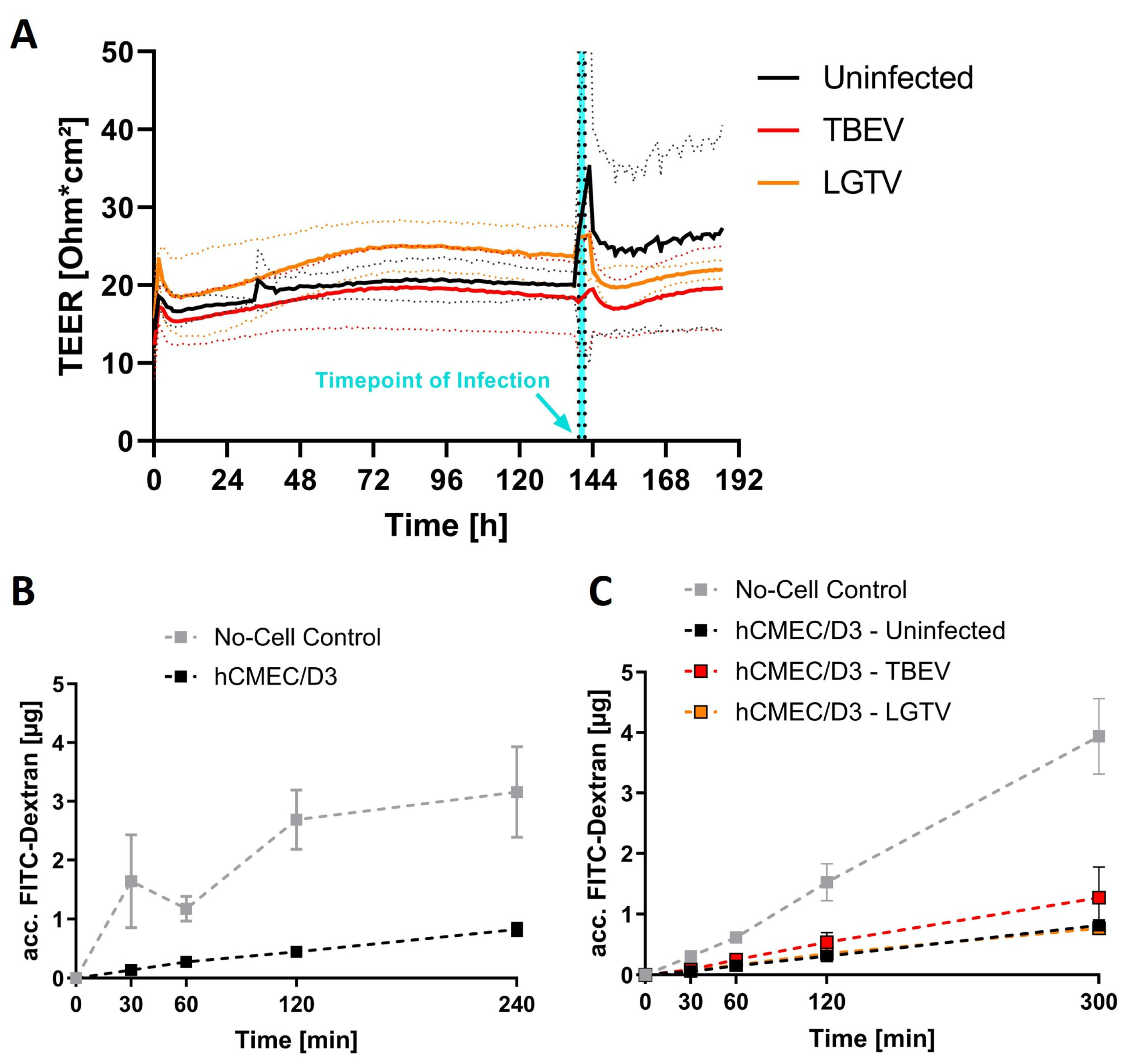
2.1. Barrier Integrity Is Not Affected by Virus Inoculation
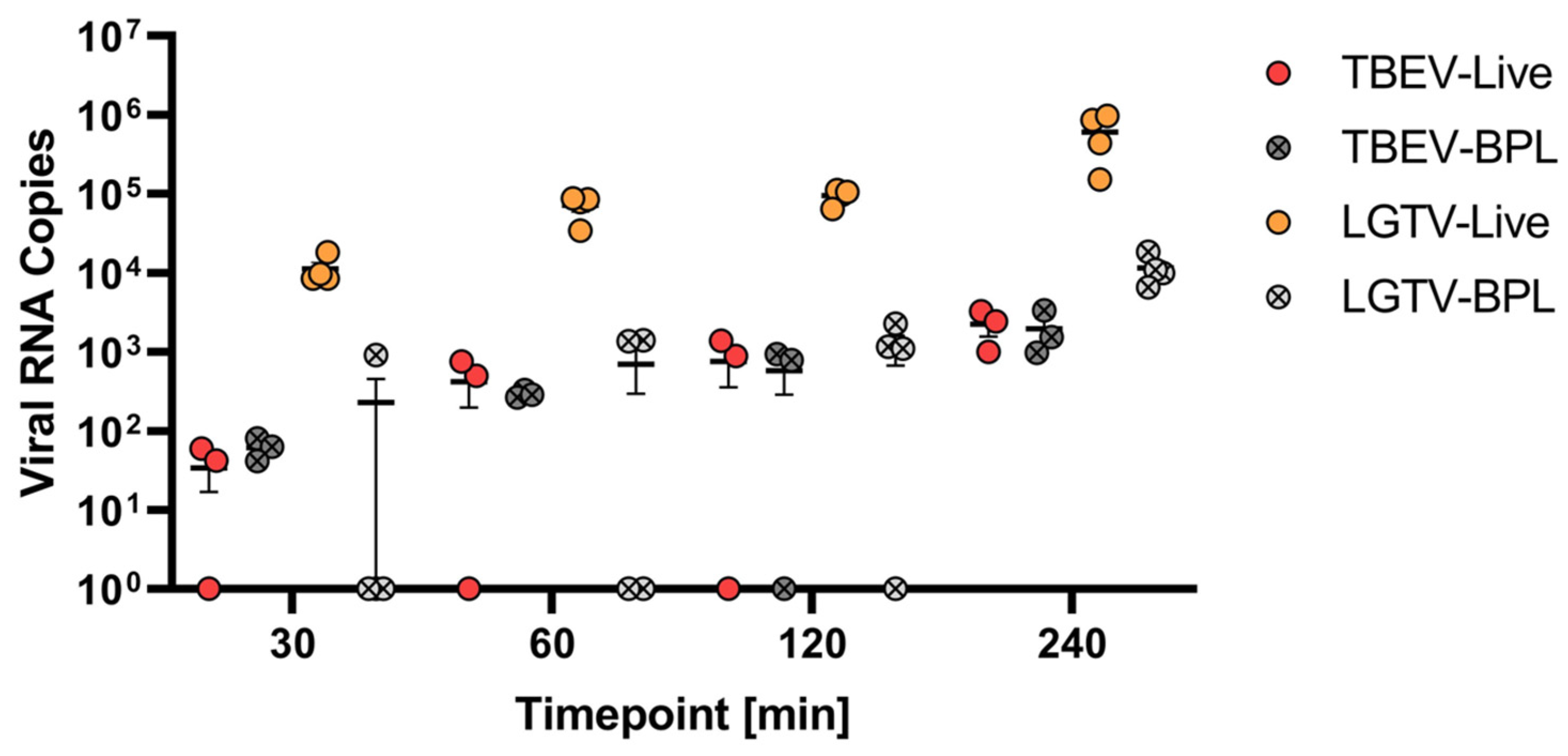
2.2. Virus Translocation Across Compact hCMEC/D3 Barrier
2.3. Influence of Modulators of Endocytosis on LGTV Translocation
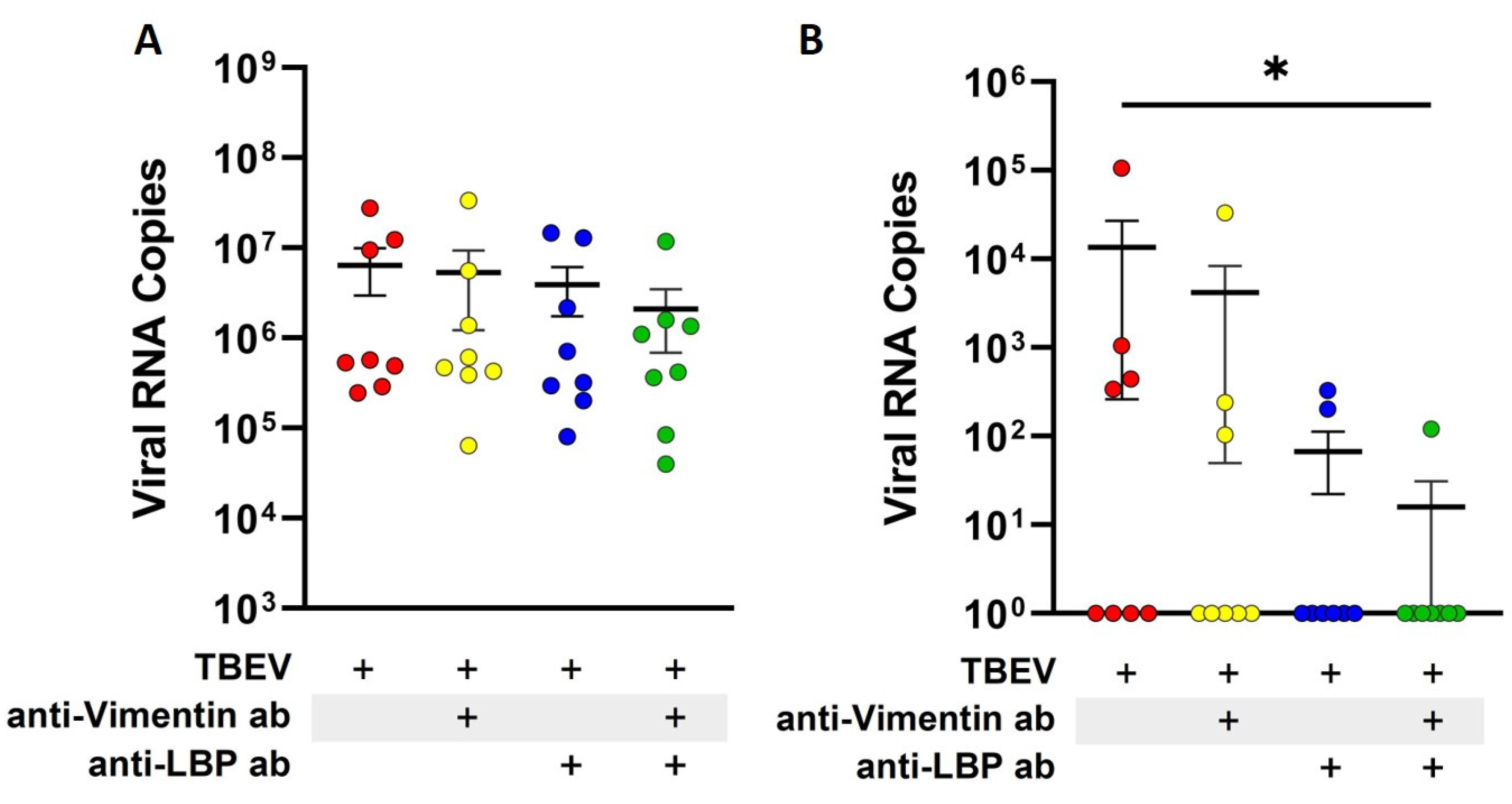
2.4. Blocking of Laminin-Binding Protein and Vimentin Reduces Translocation of TBEV Across Endothelial Barrier
3. Discussion
4. Materials and Methods
4.1. Viruses
4.2. Human Cerebral Microvascular Endothelial Cells
4.3. In Vitro Transwell Barrier Model
4.4. Barrier Permeability Assay
4.5. Virus Translocation Across hCMEC/D3 Monolayer Cultured on Transwell Inserts
4.6. RNA Extraction
4.7. Virus Quantification RT-qPCR
4.8. Immunocytochemistry of hCMEC/D3 Inoculated with Virus
| Primary Antibody | Secondary Antibody | ||||
|---|---|---|---|---|---|
| Antigen/Target | Origin | Clonality | Dilution | Provider | |
| Tick-borne encephalitis virus E-protein | Mouse | Monoclonal (Clone 1786) | 1:100 | Matthias Niedrig [56] | Goat-anti-mouse IgG Alexa Fluor® 568 (abcam, Cambridge, UK) |
| Human laminin-binding protein | Rabbit | Polyclonal | 1:500 | Merck, Darmstadt, Germany | Goat-anti-rabbit IgG Alexa Fluor® 488 (Thermo Scientific invitrogen, Carlsbad, CA, USA) |
| Human Vimentin | Goat | Polyclonal (antiserum) | 1:50 | Merck, Darmstadt, Germany | Donkey-anti-goat IgG Alexa Fluor® 488 (Thermo Scientific invitrogen, Carlsbad, CA, USA) |
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BFA | Brefeldin A |
| BHQ1 | Black hole quencher 1 |
| BPL | β-propiolactone |
| CCD | Cytochalasin D |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| DENV | Dengue virus |
| DMSO | Dimethyl sulfoxide |
| dpi | Days post inoculation |
| EGM+ | EndoGRO®-MV complete culture medium supplemented with hFGF-2 |
| E-protein | Envelope protein |
| FAM | Carboxy fluorescein |
| FITC | Fluorescein isothiocyanate |
| hCMEC/D3 | Human Cerebral Microvascular Endothelial Cell Line (D3) |
| hFGF-2 | Human fibroblast growth factor 2 |
| hpi | Hours post inoculation |
| ICC | Immunocytochemistry |
| JEV | Japanese encephalitis virus |
| LBP | Laminin-binding protein |
| LGTV | Langat virus |
| PBS | Phosphate-buffered saline |
| PBS-T | Phosphate-buffered saline supplemented with 0.05% Tween20 reagent |
| RNA | Ribonucleic acid |
| RTC | Rat tail collagen |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RT-qPCR | Reverse transcription real-time polymerase chain reaction |
| TBE | Tick-borne encephalitis |
| TBEV | Tick-borne encephalitis virus |
| TCID50 | Tissue culture infectious dose |
| TEER | Transendothelial electrical resistance |
| WNV | West Nile virus |
| ZIKV | Zika virus |
| ZO-1 | Zonula occludens protein 1 |
References
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-Borne Encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Lindquist, L.; Vapalahti, O. Tick-Borne Encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Pradier, S.; Lecollinet, S.; Leblond, A. West Nile Virus Epidemiology and Factors Triggering Change in Its Distribution in Europe. Rev. Sci. Tech. Int. Off. Epizoot. 2012, 31, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. The Clinical and Epidemiological Profile of Tick-Borne Encephalitis in Southern Germany 1994–98: A Prospective Study of 656 Patients. Brain 1999, 122, 2067–2078. [Google Scholar] [CrossRef]
- Cornelius, A.D.A.; Hosseini, S.; Schreier, S.; Fritzsch, D.; Weichert, L.; Michaelsen-Preusse, K.; Fendt, M.; Kröger, A. Langat Virus Infection Affects Hippocampal Neuron Morphology and Function in Mice without Disease Signs. J. Neuroinflam. 2020, 17, 278. [Google Scholar] [CrossRef]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-Borne Encephalitis in Europe and Russia: Review of Pathogenesis, Clinical Features, Therapy, and Vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Kubinski, M.; Beicht, J.; Gerlach, T.; Volz, A.; Sutter, G.; Rimmelzwaan, G.F. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines 2020, 8, 451. [Google Scholar] [CrossRef]
- Petry, M.; Palus, M.; Leitzen, E.; Mitterreiter, J.G.; Huang, B.; Kröger, A.; Verjans, G.M.G.M.; Baumgärtner, W.; Rimmelzwaan, G.F.; Růžek, D.; et al. Immunity to TBEV Related Flaviviruses with Reduced Pathogenicity Protects Mice from Disease but Not from TBEV Entry into the CNS. Vaccines 2021, 9, 196. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 Cell Line as a Model of the Human Blood Brain Barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Dunton, A.D.; Göpel, T.; Ho, D.H.; Burggren, W. Form and Function of the Vertebrate and Invertebrate Blood-Brain Barriers. Int. J. Mol. Sci. 2021, 22, 12111. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef]
- Verma, S.; Lo, Y.; Chapagain, M.; Lum, S.; Kumar, M.; Gurjav, U.; Luo, H.; Nakatsuka, A.; Nerurkar, V.R. West Nile Virus Infection Modulates Human Brain Microvascular Endothelial Cells Tight Junction Proteins and Cell Adhesion Molecules: Transmigration across the in Vitro Blood-Brain Barrier. Virology 2009, 385, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.T.; John, A.L.S. Japanese Encephalitis Virus and Its Mechanisms of Neuroinvasion. PLoS Pathog. 2020, 16, e1008260. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.D.; Salimi, H.; Diamond, M.S.; Klein, R.S. Mechanisms of Pathogen Invasion into the Central Nervous System. Neuron 2019, 103, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Mustafá, Y.M.; Meuren, L.M.; Coelho, S.V.A.; de Arruda, L.B. Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System. Front. Microbiol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Marshall, E.M.; Koopmans, M.P.G.; Rockx, B. A Journey to the Central Nervous System: Routes of Flaviviral Neuroinvasion in Human Disease. Viruses 2022, 14, 2096. [Google Scholar] [CrossRef]
- Růžek, D.; Dobler, G.; Mantke, O.D. Tick-Borne Encephalitis: Pathogenesis and Clinical Implications. Travel Med. Infect. Dis. 2010, 8, 223–232. [Google Scholar] [CrossRef]
- Ruzek, D.; Salát, J.; Singh, S.K.; Kopecký, J. Breakdown of the Blood-Brain Barrier during Tick-Borne Encephalitis in Mice Is Not Dependent on CD8+ T-Cells. PLoS ONE 2011, 6, e20472. [Google Scholar] [CrossRef]
- Weber, E.; Finsterbusch, K.; Lindquist, R.; Nair, S.; Lienenklaus, S.; Gekara, N.O.; Janik, D.; Weiss, S.; Kalinke, U.; Överby, A.K.; et al. Type I Interferon Protects Mice from Fatal Neurotropic Infection with Langat Virus by Systemic and Local Antiviral Responses. J. Virol. 2014, 88, 12202–12212. [Google Scholar] [CrossRef]
- Palus, M.; Vancova, M.; Sirmarova, J.; Elsterova, J.; Perner, J.; Ruzek, D. Tick-Borne Encephalitis Virus Infects Human Brain Microvascular Endothelial Cells without Compromising Blood-Brain Barrier Integrity. Virology 2017, 507, 110–122. [Google Scholar] [CrossRef]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-Brain Barrier-Specific Properties of a Human Adult Brain Endothelial Cell Line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Gutmann, H.; Krähenbühl, S.; Weksler, B.; Romero, I.; Couraud, P.-O.; Tuffin, G.; Drewe, J.; Huwyler, J. The Human Brain Endothelial Cell Line hCMEC/D3 as a Human Blood-Brain Barrier Model for Drug Transport Studies. J. Neurochem. 2008, 107, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative Study of Four Immortalized Human Brain Capillary Endothelial Cell Lines, hCMEC/D3, hBMEC, TY10, and BB19, and Optimization of Culture Conditions, for an in Vitro Blood–Brain Barrier Model for Drug Permeability Studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Sade, H.; Baumgartner, C.; Hugenmatter, A.; Moessner, E.; Freskgård, P.-O.; Niewoehner, J. A Human Blood-Brain Barrier Transcytosis Assay Reveals Antibody Transcytosis Influenced by pH-Dependent Receptor Binding. PLoS ONE 2014, 9, e96340. [Google Scholar] [CrossRef]
- van der Helm, M.W.; Odijk, M.; Frimat, J.-P.; van der Meer, A.D.; Eijkel, J.C.T.; van den Berg, A.; Segerink, L.I. Direct Quantification of Transendothelial Electrical Resistance in Organs-on-Chips. Biosens. Bioelectron. 2016, 85, 924–929. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, C.; Wu, Y.; Chen, B.; Zhang, W.; Zhang, J. Autophagy Protects the Blood-Brain Barrier Through Regulating the Dynamic of Claudin-5 in Short-Term Starvation. Front. Physiol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Mutso, M.; St John, J.A.; Ling, Z.L.; Burt, F.J.; Poo, Y.S.; Liu, X.; Žusinaite, E.; Grau, G.E.; Hueston, L.; Merits, A.; et al. Basic Insights into Zika Virus Infection of Neuroglial and Brain Endothelial Cells. J. Gen. Virol. 2020, 101, 622–634. [Google Scholar] [CrossRef]
- Malygin, A.A.; Bondarenko, E.I.; Ivanisenko, V.A.; Protopopova, E.V.; Karpova, G.G.; Loktev, V.B. C-Terminal Fragment of Human Laminin-Binding Protein Contains a Receptor Domain for Venezuelan Equine Encephalitis and Tick-Borne Encephalitis Viruses. Biochem. Mosc. 2009, 74, 1328–1336. [Google Scholar] [CrossRef]
- Liang, J.-J.; Yu, C.-Y.; Liao, C.-L.; Lin, Y.-L. Vimentin Binding Is Critical for Infection by the Virulent Strain of Japanese Encephalitis Virus. Cell. Microbiol. 2011, 13, 1358–1370. [Google Scholar] [CrossRef]
- Raub, T.J.; Newton, C.R. Recycling Kinetics and Transcytosis of Transferrin in Primary Cultures of Bovine Brain Microvessel Endothelial Cells. J. Cell. Physiol. 1991, 149, 141–151. [Google Scholar] [CrossRef]
- Hasebe, R.; Suzuki, T.; Makino, Y.; Igarashi, M.; Yamanouchi, S.; Maeda, A.; Horiuchi, M.; Sawa, H.; Kimura, T. Transcellular Transport of West Nile Virus-like Particles across Human Endothelial Cells Depends on Residues 156 and 159 of Envelope Protein. BMC Microbiol. 2010, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Papa, M.P.; Meuren, L.M.; Coelho, S.V.A.; de Oliveira Lucas, C.G.; Mustafá, Y.M.; Lemos Matassoli, F.; Silveira, P.P.; Frost, P.S.; Pezzuto, P.; Ribeiro, M.R.; et al. Zika Virus Infects, Activates, and Crosses Brain Microvascular Endothelial Cells, without Barrier Disruption. Front. Microbiol. 2017, 8, 2557. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Moureau, G.; Charrel, R.N.; Lemasson, J.-J.; Gonzalez, J.-P.; Gallian, P.; Gritsun, T.S.; Holmes, E.C.; Gould, E.A.; de Lamballerie, X. Genetic Characterization of Tick-Borne Flaviviruses: New Insights into Evolution, Pathogenetic Determinants and Taxonomy. Virology 2007, 361, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Iacono-Connors, L.C.; Schmaljohn, C.S. Cloning and Sequence Analysis of the Genes Encoding the Nonstructural Proteins of Langat Virus and Comparative Analysis with Other Flaviviruses. Virology 1992, 188, 875–880. [Google Scholar] [CrossRef]
- Griffin, J.A.; Compans, R.W. Effect of Cytochalasin B on the Maturation of Enveloped Viruses. J. Exp. Med. 1979, 150, 379–391. [Google Scholar] [CrossRef]
- Bohn, W.; Rutter, G.; Hohenberg, H.; Mannweiler, K.; Nobis, P. Involvement of Actin Filaments in Budding of Measles Virus: Studies on Cytoskeletons of Infected Cells. Virology 1986, 149, 91–106. [Google Scholar] [CrossRef]
- Calvert, B.A.; Quiroz, E.J.; Lorenzana, Z.; Doan, N.; Kim, S.; Senger, C.N.; Anders, J.J.; Wallace, W.D.; Salomon, M.P.; Henley, J.; et al. Neutrophilic Inflammation Promotes SARS-CoV-2 Infectivity and Augments the Inflammatory Responses in Airway Epithelial Cells. Front. Immunol. 2023, 14, 1112870. [Google Scholar] [CrossRef]
- Raekiansyah, M.; Mori, M.; Nonaka, K.; Agoh, M.; Shiomi, K.; Matsumoto, A.; Morita, K. Identification of Novel Antiviral of Fungus-Derived Brefeldin A against Dengue Viruses. Trop. Med. Health 2017, 45, 32. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Li, J.; Wu, W.; Jiu, Y. The Role of Host Cytoskeleton in Flavivirus Infection. Virol. Sin. 2019, 34, 30–41. [Google Scholar] [CrossRef]
- Farias, K.J.S.; Machado, P.R.L.; de Almeida Júnior, R.F.; Lopes da Fonseca, B.A. Brefeldin A and Cytochalasin B Reduce Dengue Virus Replication in Cell Cultures but Do Not Protect Mice against Viral Challenge. Access Microbiol. 2019, 1, e000041. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes Serve as Novel Modes of Tick-Borne Flavivirus Transmission from Arthropod to Human Cells and Facilitates Dissemination of Viral RNA and Proteins to the Vertebrate Neuronal Cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Gao, N.; Chen, Z.; Tian, Y.; An, J. Up-Regulated Expression of Β3 Integrin Induced by Dengue Virus Serotype 2 Infection Associated with Virus Entry into Human Dermal Microvascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2007, 356, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Keller, M.; Bader, B.L.; Korytář, T.; Finke, S.; Ziegler, U.; Groschup, M.H. Integrins Modulate the Infection Efficiency of West Nile Virus into Cells. J. Gen. Virol. 2013, 94, 1723–1733. [Google Scholar] [CrossRef]
- Zaitsev, B.N.; Benedetti, F.; Mikhaylov, A.G.; Korneev, D.V.; Sekatskii, S.K.; Karakouz, T.; Belavin, P.A.; Netesova, N.A.; Protopopova, E.V.; Konovalova, S.N.; et al. Force-Induced Globule–Coil Transition in Laminin Binding Protein and Its Role for Viral–Cell Membrane Fusion. J. Mol. Recognit. 2014, 27, 727–738. [Google Scholar] [CrossRef]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Wettersten, H.I.; Beck, S.; Clark, A.E.; Wu, Q.; et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin Avβ5 Axis. Cell Stem Cell 2020, 26, 187–204.e10. [Google Scholar] [CrossRef] [PubMed]
- Protopopova, E.V.; Sorokin, A.V.; Konovalova, S.N.; Kachko, A.V.; Netesov, S.V.; Loktev, V.B. Human Laminin Binding Protein as a Cell Receptor for the Tick-Borne Encephalitis Virus. Zentralblatt Für Bakteriol. 1999, 289, 632–638. [Google Scholar] [CrossRef]
- Zou, Y.; He, L.; Huang, S.-H. Identification of a Surface Protein on Human Brain Microvascular Endothelial Cells as Vimentin Interacting with Escherichia Coli Invasion Protein IbeA. Biochem. Biophys. Res. Commun. 2006, 351, 625–630. [Google Scholar] [CrossRef]
- Yang, J.; Zou, L.; Yang, Y.; Yuan, J.; Hu, Z.; Liu, H.; Peng, H.; Shang, W.; Zhang, X.; Zhu, J.; et al. Superficial Vimentin Mediates DENV-2 Infection of Vascular Endothelial Cells. Sci. Rep. 2016, 6, 38372. [Google Scholar] [CrossRef]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef]
- Mäger, I.; Meyer, A.H.; Li, J.; Lenter, M.; Hildebrandt, T.; Leparc, G.; Wood, M.J.A. Targeting Blood-Brain-Barrier Transcytosis—Perspectives for Drug Delivery. Neuropharmacology 2017, 120, 4–7. [Google Scholar] [CrossRef]
- Kubinski, M.; Beicht, J.; Zdora, I.; Saletti, G.; Kircher, M.; Petry-Gusmag, M.; Steffen, I.; Puff, C.; Jung, K.; Baumgärtner, W.; et al. Cross-Reactive Antibodies against Langat Virus Protect Mice from Lethal Tick-Borne Encephalitis Virus Infection. Front. Immunol. 2023, 14, 1134371. [Google Scholar] [CrossRef] [PubMed]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial Electrical Resistance Measurement across the Blood–Brain Barrier: A Critical Review of Methods. Micromachines 2021, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.R.; Begg, D.A. Concentration-Dependent Effects of Cytochalasin D on Tight Junctions and Actin Filaments in MDCK Epithelial Cells. J. Cell Sci. 1994, 107, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, M.; Cassinotti, P. Development of a Quantitative Real-Time RT-PCR Assay with Internal Control for the Laboratory Detection of Tick Borne Encephalitis Virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef]
- Kurhade, C.; Zegenhagen, L.; Weber, E.; Nair, S.; Michaelsen-Preusse, K.; Spanier, J.; Gekara, N.O.; Kröger, A.; Överby, A.K. Type I Interferon Response in Olfactory Bulb, the Site of Tick-Borne Flavivirus Accumulation, Is Primarily Regulated by IPS-1. J. Neuroinflam. 2016, 13, 22. [Google Scholar] [CrossRef]
- Niedrig, M.; Klockmann, U.; Lang, W.; Roeder, J.; Burk, S.; Modrow, S.; Pauli, G. Monoclonal Antibodies Directed against Tick-Borne Encephalitis Virus with Neutralizing Activity In Vivo. Acta Virol. 1994, 38, 141–149. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweitzer, F.; Letoha, T.; Osterhaus, A.; Prajeeth, C.K. Impact of Tick-Borne Orthoflaviviruses Infection on Compact Human Brain Endothelial Barrier. Int. J. Mol. Sci. 2025, 26, 2342. https://doi.org/10.3390/ijms26052342
Schweitzer F, Letoha T, Osterhaus A, Prajeeth CK. Impact of Tick-Borne Orthoflaviviruses Infection on Compact Human Brain Endothelial Barrier. International Journal of Molecular Sciences. 2025; 26(5):2342. https://doi.org/10.3390/ijms26052342
Chicago/Turabian StyleSchweitzer, Felix, Tamás Letoha, Albert Osterhaus, and Chittappen Kandiyil Prajeeth. 2025. "Impact of Tick-Borne Orthoflaviviruses Infection on Compact Human Brain Endothelial Barrier" International Journal of Molecular Sciences 26, no. 5: 2342. https://doi.org/10.3390/ijms26052342
APA StyleSchweitzer, F., Letoha, T., Osterhaus, A., & Prajeeth, C. K. (2025). Impact of Tick-Borne Orthoflaviviruses Infection on Compact Human Brain Endothelial Barrier. International Journal of Molecular Sciences, 26(5), 2342. https://doi.org/10.3390/ijms26052342







