Targeting Lung Damage: Amniotic Mesenchymal Stem Cells Mitigate Lipopolysaccharide-Induced Acute Lung Injury via Multiple Signaling Pathways
Abstract
1. Introduction
2. Results
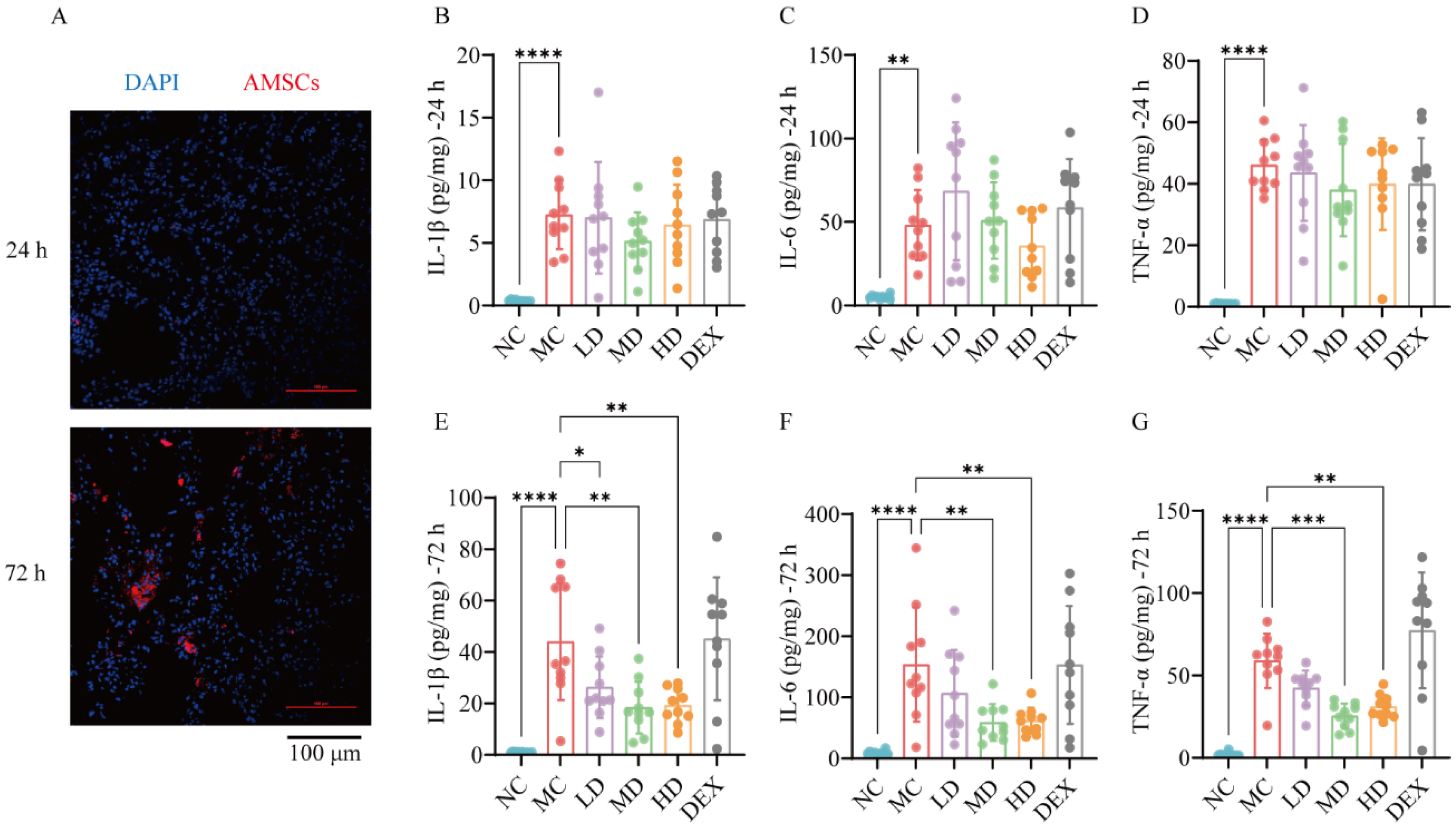
2.1. Homing Effect Directed AMSCs to ALI Mouse Lungs
2.2. AMSCs Reduced Inflammatory Factors in ALI
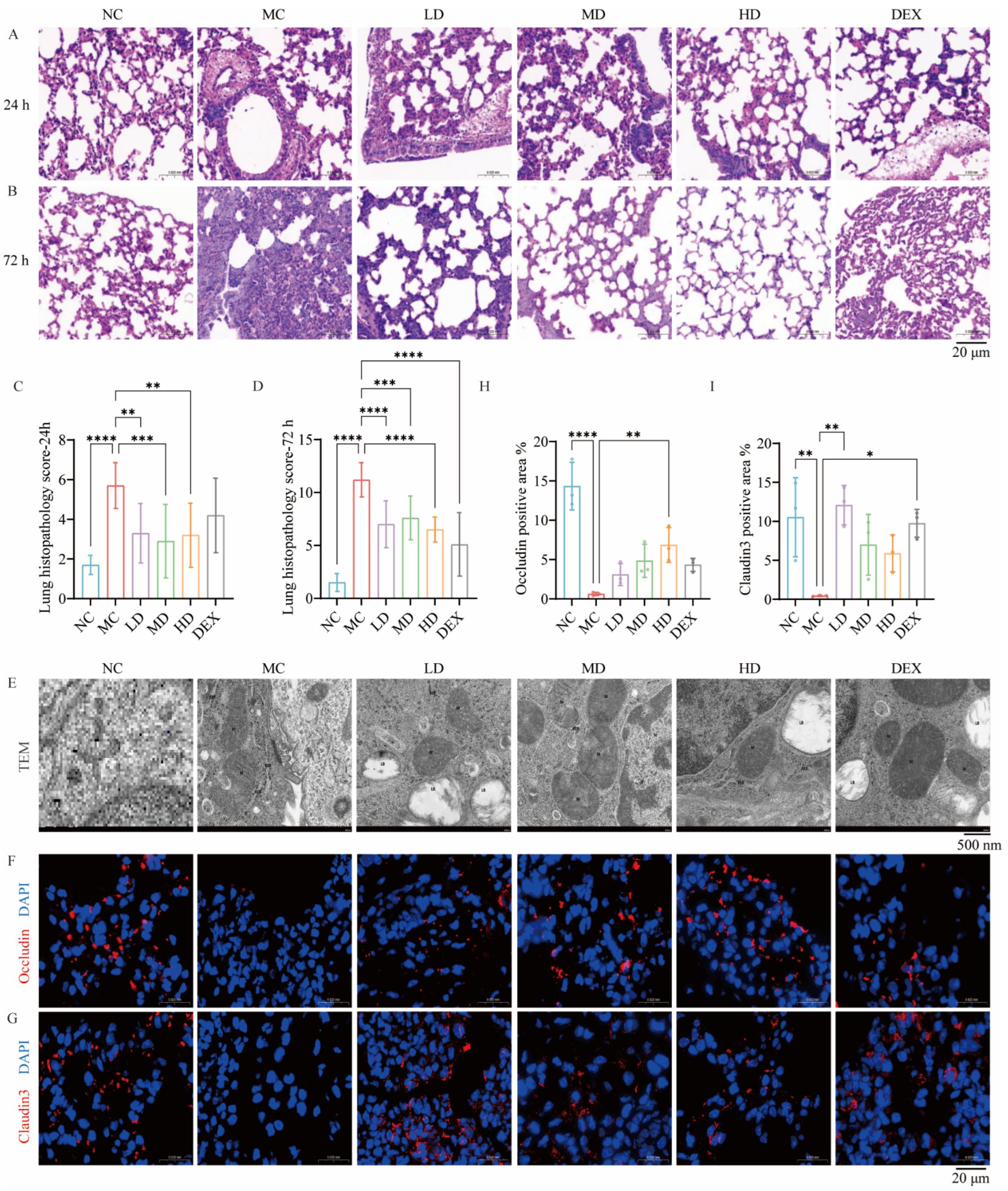
2.3. AMSCs Mitigated Pathological Lung Injury in a Murine Model of ALI
2.4. AMSCs Facilitated the Restoration of Organelle Architecture Following ALI
2.5. AMSCs Restored Tight Junction Integrity Compromised During ALI
2.6. AMSCs Reduced Collagen Deposition and Fibrosis Following ALI
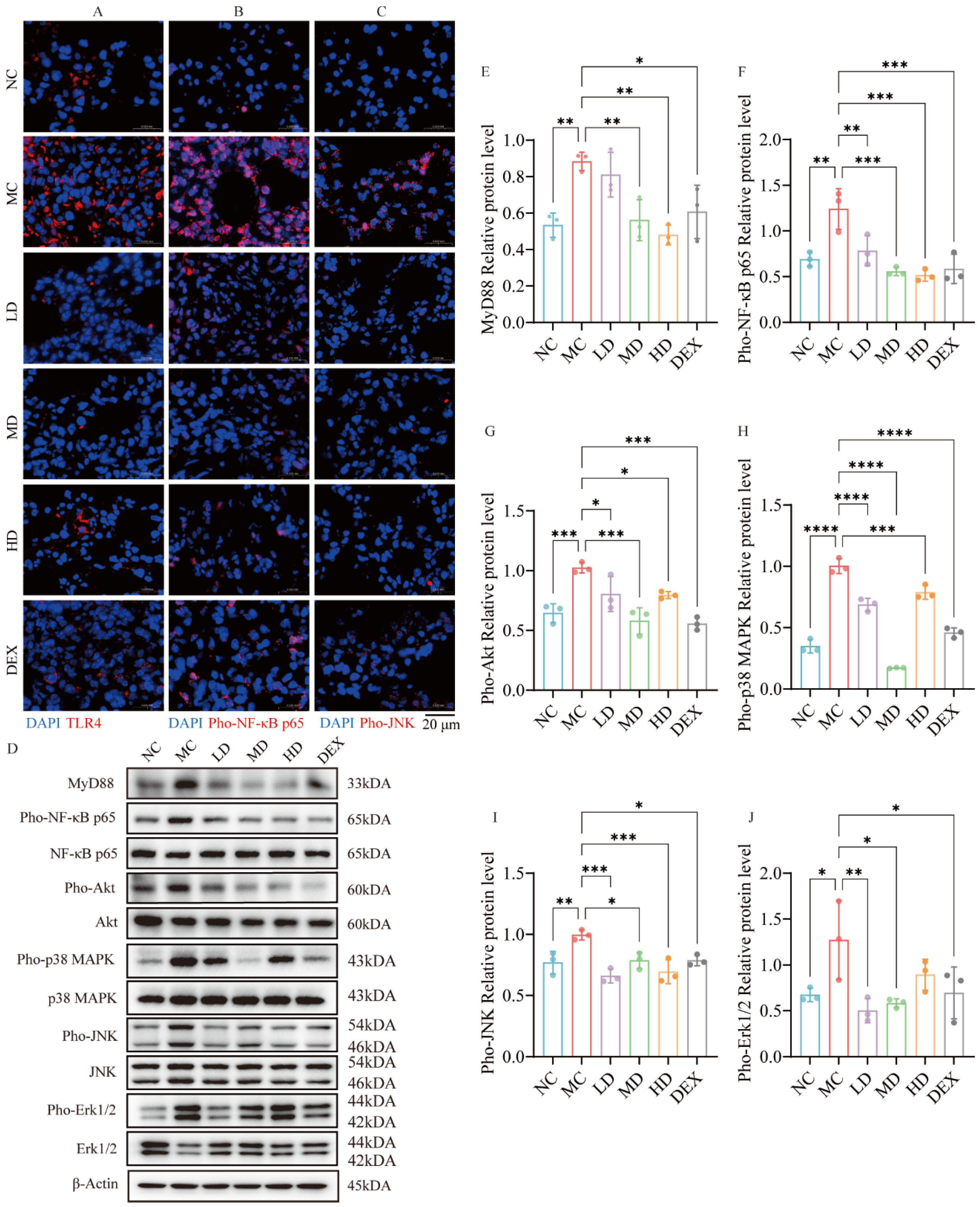
2.7. AMSCs Downregulated TLR4/NF-κB/MAPK Pathway Activation in ALI
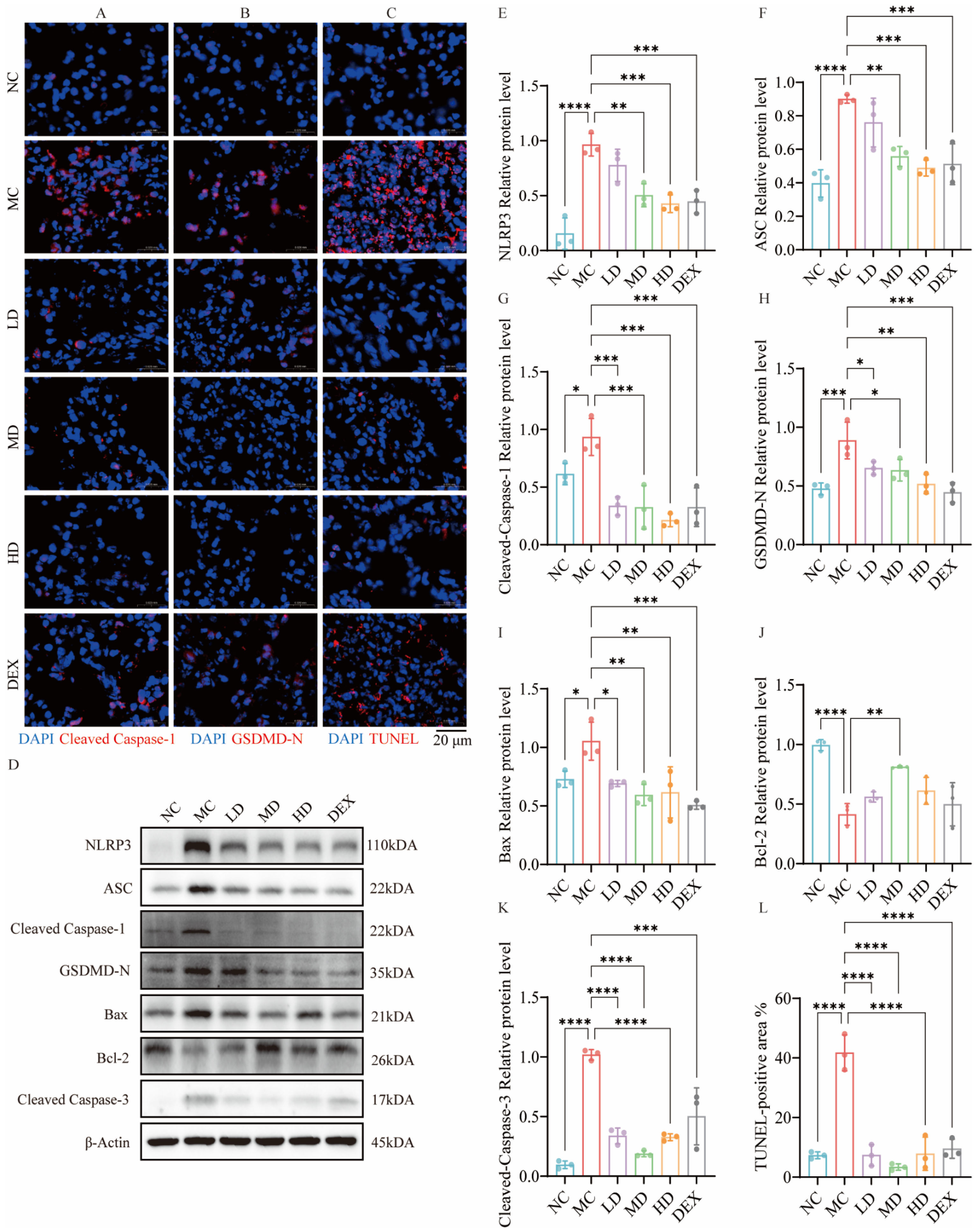
2.8. AMSCs Mitigated Pyroptosis and Apoptosis Triggered by ALI
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Experimental Animals
4.3. Creation of ALI Mouse Model
4.4. Grouping of Animals and Administration of Medication
4.5. Homing Detection of AMSCs
4.6. Histological Examination
4.7. TUNEL Analysis
4.8. Analysis of Levels of Inflammatory Factors
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-Ortíz, J.; Deal, V.; Reyes, F.; Maldonado-Martínez, G.; Ledesma, N.; Staback, F.; Croft, C.; Pacheco, A.; Ortiz-Zuazaga, H.; Yost, C.C.; et al. Platelet-derived TLT-1 is a prognostic indicator in ALI/ARDS and prevents tissue damage in the lungs in a mouse model. Blood 2018, 132, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, X.; Li, T.; Nyunoya, T.; Chen, K.; Kitsios, G.D.; Nouraie, S.M.; Zhang, Y.; McVerry, B.J.; Lee, J.S.; et al. Endotoxin stabilizes protein arginine methyltransferase 4 (PRMT4) protein triggering death of lung epithelia. Cell Death Dis. 2021, 12, 828. [Google Scholar] [CrossRef]
- Xu, M.M.; Kang, J.Y.; Ji, S.; Wei, Y.Y.; Wei, S.L.; Ye, J.J.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin Suppresses Macrophage M1 Polarization and ROS-Mediated Pyroptosis via Activating ApoE/LDLR Pathway in Influenza A-Induced Acute Lung Injury. Oxid. Med. Cell. Longev. 2022, 2022, 2520348. [Google Scholar] [CrossRef] [PubMed]
- Łowicka-Smolarek, M.; Kokoszka-Bargieł, I.; Knapik, M.; Śmietanka, K.; Dyrda, P.; Możdżeń, M.; Kurczab, M.; Borkowski, J.; Knapik, P. Analysis of Patients with Alcohol Dependence Treated in Silesian Intensive Care Units. Int. J. Environ. Res. Public Health 2022, 19, 5914. [Google Scholar] [CrossRef]
- Merenstein, C.; Bushman, F.D.; Collman, R.G. Alterations in the respiratory tract microbiome in COVID-19: Current observations and potential significance. Microbiome 2022, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Gul, L.; Modos, D.; Fonseca, S.; Madgwick, M.; Thomas, J.P.; Sudhakar, P.; Booth, C.; Stentz, R.; Carding, S.R.; Korcsmaros, T. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease. J. Extracell. Vesicles 2022, 11, e12189. [Google Scholar] [CrossRef]
- Kezic, J.; Taylor, S.; Gupta, S.; Planck, S.R.; Rosenzweig, H.L.; Rosenbaum, J.T. Endotoxin-induced uveitis is primarily dependent on radiation-resistant cells and on MyD88 but not TRIF. J. Leukoc. Biol. 2011, 90, 305–311. [Google Scholar] [CrossRef]
- Richard, K.; Piepenbrink, K.H.; Shirey, K.A.; Gopalakrishnan, A.; Nallar, S.; Prantner, D.J.; Perkins, D.J.; Lai, W.; Vlk, A.; Toshchakov, V.Y.; et al. A mouse model of human TLR4 D299G/T399I SNPs reveals mechanisms of altered LPS and pathogen responses. J. Exp. Med. 2021, 218, e20200675. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, J.; Guo, L.; Xu, Y.; Hu, L.; Mao, H.; Miao, L.; Zhang, H.; Chai, L. Neobavaisoflavone ameliorates LPS-induced RAW264.7 cell inflammations by suppressing the activation of NF-κB and MAPKs signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 1021–1027. [Google Scholar]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.F. Hyperoside decreases the apoptosis and autophagy rates of osteoblast MC3T3-E1 cells by regulating TNF-like weak inducer of apoptosis and the p38mitogen activated protein kinase pathway. Mol. Med. Rep. 2019, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Nakajima, S.; Hosojima, S.; Thi Nguyen, D.; Hattori, T.; Manh Le, T.; Hori, O.; Mahib, M.R.; Yamaguchi, Y.; Miura, M.; et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 2019, 10, 2091. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Jiang, X.; Wang, Y.; Yang, Z.; Mao, Y.; Wu, Z.; Li, G.; Chen, H. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3β pathway via c-MET in radiation-induced lung injury. J. Exp. Clin. Cancer Res. 2022, 41, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, H.; Xiang, F.; Song, L.J.; Zhou, L.L.; Cai, P.C.; Zhang, J.C.; Yu, F.; Shi, H.Z.; Su, Y.; et al. miR-18a-5p Inhibits Sub-pleural Pulmonary Fibrosis by Targeting TGF-β Receptor II. Mol. Ther. 2017, 25, 728–738. [Google Scholar] [CrossRef]
- Huang, S.; Lin, Y.; Deng, Q.; Zhang, Y.; Peng, S.; Qiu, Y.; Huang, W.; Wang, Z.; Lai, X. Suppression of OGN in lung myofibroblasts attenuates pulmonary fibrosis by inhibiting integrin αv-mediated TGF-β/Smad pathway activation. Matrix Biol. 2024, 132, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Chen, X.; Li, C.; Liu, C.; Huang, Y.; Wang, X.; Liang, H.; Yuan, X. Nintedanib Mitigates Radiation-Induced Pulmonary Fibrosis by Suppressing Epithelial Cell Inflammatory Response and Inhibiting Fibroblast-to-Myofibroblast Transition. Int. J. Biol. Sci. 2024, 20, 3353–3371. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, L.; Fan, Y.; Huselstein, C.; De Isla, N.; He, X.; Chen, Y.; Li, Y. Secretome of Mesenchymal Stem Cells from Consecutive Hypoxic Cultures Promotes Resolution of Lung Inflammation by Reprogramming Anti-Inflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4333. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Trohatou, O.; Anagnou, N.P. Amniotic fluid and amniotic membrane stem cells: Marker discovery. Stem Cells Int. 2012, 2012, 107836. [Google Scholar] [CrossRef]
- Onishi, R.; Ohnishi, S.; Higashi, R.; Watari, M.; Yamahara, K.; Okubo, N.; Nakagawa, K.; Katsurada, T.; Suda, G.; Natsuizaka, M.; et al. Human Amnion-Derived Mesenchymal Stem Cell Transplantation Ameliorates Dextran Sulfate Sodium-Induced Severe Colitis in Rats. Cell Transplant. 2015, 24, 2601–2614. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, Z.; Chen, L.; Zuo, B.; Liu, C.; Sun, D. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res. Ther. 2021, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Li, F.; Li, C.; Chen, S.; Gao, X.; Wang, X. The miR-124-3p regulates the allergic airway inflammation and remodeling in an ovalbumin-asthmatic mouse model by inhibiting S100A4. Immun. Inflamm. Dis. 2023, 11, e730. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Chai, Y.; Yu, R.; Liu, Y.; Wang, S.; Yuan, D.; Chen, J. Dexmedetomidine Attenuates Monocyte-Endothelial Adherence via Inhibiting Connexin43 on Vascular Endothelial Cells. Mediat. Inflamm. 2020, 2020, 7039854. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Wu, S.W.; Shi, Z.M.; Hu, B. Traditional Chinese medicine for colorectal cancer treatment: Potential targets and mechanisms of action. Chin. Med. 2023, 18, 14. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Lei, P.; Tang, X.; Huang, P. Exosomes Released by Bone Marrow Mesenchymal Stem Cells Attenuate Lung Injury Induced by Intestinal Ischemia Reperfusion via the TLR4/NF-κB Pathway. Int. J. Med. Sci. 2019, 16, 1238–1244. [Google Scholar] [CrossRef]
- Ji, S.; Peng, Y.; Liu, J.; Xu, P.; Tang, S. Human adipose tissue-derived stem cell extracellular vesicles attenuate ocular hypertension-induced retinal ganglion cell damage by inhibiting microglia- TLR4/MAPK/NF-κB proinflammatory cascade signaling. Acta Neuropathol. Commun. 2024, 12, 44. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Yang, Z.; Wang, M.; Zhou, Z.; Zhang, W.; Ren, Y.; Han, X.; Wei, M.; Sun, Z.; et al. Arctigenin Suppressed Epithelial-Mesenchymal Transition Through Wnt3a/β-Catenin Pathway in PQ-Induced Pulmonary Fibrosis. Front. Pharmacol. 2020, 11, 584098. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Yang, Y.; Feng, Y. Shen Qi Wan-Containing Serum Alleviates Renal Interstitial Fibrosis via Restraining Notch1-Mediated Epithelial-Mesenchymal Transition. Evid. Based Complement. Altern. Med. 2023, 2023, 3352353. [Google Scholar] [CrossRef]
- Du, T.; Zou, X.; Cheng, J.; Wu, S.; Zhong, L.; Ju, G.; Zhu, J.; Liu, G.; Zhu, Y.; Xia, S. Human Wharton’s jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res. Ther. 2013, 4, 59. [Google Scholar] [CrossRef]
- Ali, E.N.; Mansour, S.Z. Boswellic acids extract attenuates pulmonary fibrosis induced by bleomycin and oxidative stress from gamma irradiation in rats. Chin. Med. 2011, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Fritzsche, S.; Cording, J.; Richter, S.; Hartwig, J.; Walter, M.; Yu, D.; Turner, J.R.; Gehring, C.; Rahn, H.-P.; et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell. Mol. Life Sci. 2011, 68, 3903–3918. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, K.; Nishina, K.; Takao, Y.; Obara, H. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth. Analg. 2003, 97, 1751–1755. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Zhang, L.; Xing, S.; Liu, J.; Li, D.; Wang, Y.; Wang, Y.; Su, M. Targeting Lung Damage: Amniotic Mesenchymal Stem Cells Mitigate Lipopolysaccharide-Induced Acute Lung Injury via Multiple Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 2314. https://doi.org/10.3390/ijms26052314
Niu X, Zhang L, Xing S, Liu J, Li D, Wang Y, Wang Y, Su M. Targeting Lung Damage: Amniotic Mesenchymal Stem Cells Mitigate Lipopolysaccharide-Induced Acute Lung Injury via Multiple Signaling Pathways. International Journal of Molecular Sciences. 2025; 26(5):2314. https://doi.org/10.3390/ijms26052314
Chicago/Turabian StyleNiu, Xinhui, Lina Zhang, Shaoliang Xing, Jinrui Liu, Deming Li, Yating Wang, Yi Wang, and Manman Su. 2025. "Targeting Lung Damage: Amniotic Mesenchymal Stem Cells Mitigate Lipopolysaccharide-Induced Acute Lung Injury via Multiple Signaling Pathways" International Journal of Molecular Sciences 26, no. 5: 2314. https://doi.org/10.3390/ijms26052314
APA StyleNiu, X., Zhang, L., Xing, S., Liu, J., Li, D., Wang, Y., Wang, Y., & Su, M. (2025). Targeting Lung Damage: Amniotic Mesenchymal Stem Cells Mitigate Lipopolysaccharide-Induced Acute Lung Injury via Multiple Signaling Pathways. International Journal of Molecular Sciences, 26(5), 2314. https://doi.org/10.3390/ijms26052314





