Characterizing the Ovarian Cytogenetic Dynamics of Sichuan Bream (Sinibrama taeniatus) During Vitellogenesis at a Single-Cell Resolution
Abstract
1. Introduction
2. Results
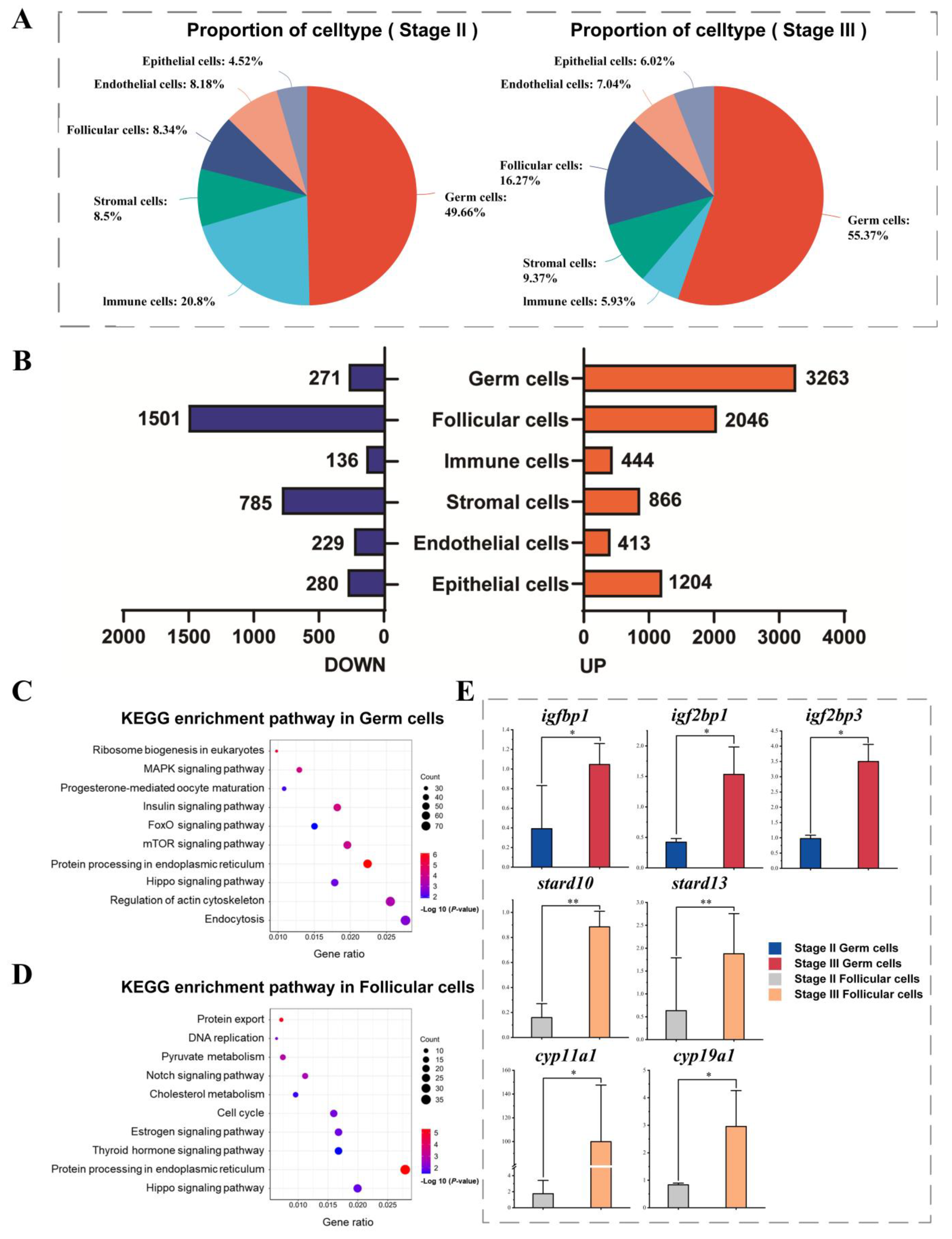
2.1. Single-Cell Transcriptome Atlas of the Sichuan Bream Ovary
2.2. Dynamics of Gene Expression in Germ Cells During Development
2.3. Effect of Vitellogenesis on the Gene Expression Profile of the Follicle Cell Population
2.4. Cell Communication Analysis Based on Ligand–Receptor Pairs
3. Discussion
3.1. Cell Composition of the Sichuan Bream Ovary
3.2. Genetic Dynamics of Germ Cell Development
3.3. DEGs’ Function in Follicles Before and After Vitellogenesis
3.4. Cellular Interaction Networks During Vitellogenesis
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Preparation and Animal Tissue Collection
4.3. snRNA-Seq Library Construction and Sequencing
4.4. Data Processing and Downstream Analysis
4.5. Pesudotemperal Trajectory Analysis
4.6. Transcriptional Regulation Analysis of Germ Cell Subtypes
4.7. Cell Communication Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Hiramatsu, N.; Todo, T.; Sullivan, C.V.; Schilling, J.; Reading, B.J.; Matsubara, T.; Ryu, Y.W.; Mizuta, H.; Luo, W.S.; Nishimiya, O.; et al. Ovarian yolk formation in fishes: Molecular mechanisms underlying formation of lipid droplets and vitellogenin-derived yolk proteins. Gen. Comp. Endocrinol. 2015, 221, 9–15. [Google Scholar] [CrossRef]
- Babin, P.J.; Carnevali, O.; Lubzens, E.; Schneider, W.J. Molecular Aspects of Oocyte Vitellogenesis in Fish; Springer: Dordrecht, The Netherlands, 2007; pp. 39–76. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerda, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Q.; Wang, H.Y.; Wei, L.; Wang, S.Y.; Li, S.L.; Yuan, D.Y.; Wang, Z.J. Integrated transcriptomic and metabolomic analyses identify key factors in the vitellogenesis of juvenile Sichuan bream (Sinibrama taeniatus). Front. Mar. Sci. 2023, 10, 1243767. [Google Scholar] [CrossRef]
- Guzman, J.M.; Luckenbach, J.A.; Yamamoto, Y.; Swanson, P. Expression Profiles of Fsh-Regulated Ovarian Genes during Oogenesis in Coho Salmon. PLoS ONE 2014, 9, e114176. [Google Scholar] [CrossRef]
- Wylie, M.J.; Symonds, J.E.; Setiawan, A.N.; Irvine, G.W.; Liu, H.; Elizur, A.; Lokman, P.M. Transcriptomic Changes during Previtellogenic and Vitellogenic Stages of Ovarian Development in Wreckfish (Hapuku), Polyprion oxygeneios (Perciformes). Fishes 2019, 4, 16. [Google Scholar] [CrossRef]
- Cowan, R.G.; Quirk, S.M. Cells responding to hedgehog signaling contribute to the theca of ovarian follicles. Reproduction 2021, 161, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.; Egbert, J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 2016, 79, 237–260. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Gao, X.Y.; Liu, Y.; Yan, X.Q.; Shi, H.C.; Zhao, R.S.; Chen, Z.J.; Gao, F.; Zhao, H.; Zhao, S.G. Cellular atlases of ovarian microenvironment alterations by diet and genetically-induced obesity. Sci. China-Life Sci. 2024, 67, 51–66. [Google Scholar] [CrossRef]
- Niu, W.B.; Spradling, A.C. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc. Natl. Acad. Sci. USA 2020, 117, 20015–20026. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.-M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Mol. Cell 2018, 72, 1021–1034.e4. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Li, C.-Y.; Meng, T.-G.; Wang, Y.; Liu, W.-B.; Li, A.; Cai, Y.-J.; Hou, Y.; Schatten, H.; Wang, Z.-B.; et al. Single-cell RNA sequencing reveals regulation of fetal ovary development in the monkey (Macaca fascicularis). Cell Discov. 2020, 6, 97. [Google Scholar] [CrossRef]
- Wang, J.J.; Ge, W.; Zhai, Q.Y.; Liu, J.C.; Sun, X.W.; Liu, W.X.; Li, L.; Lei, C.Z.; Dyce, P.W.; De Felici, M.; et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol. 2020, 18, e3001025. [Google Scholar] [CrossRef]
- Pei, J.; Xiong, L.; Guo, S.; Wang, X.; Bao, P.; Wu, X.; Yan, P.; Guo, X. A single-cell transcriptomic atlas characterizes cell types and their molecular features in yak ovarian cortex. FASEB J. 2023, 37, e22718. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Y.; Tian, Y.; Wu, S.; Gao, F.; Yuan, X. Deciphering Cellular Heterogeneity and Communication Patterns in Porcine Antral Follicles by Single-Cell RNA Sequencing. Animals 2023, 13, 3019. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.; Yang, Y.; Chen, K.; Xu, H. Transcriptome Profiling of the Ovarian Cells at the Single-Cell Resolution in Adult Asian Seabass. Front. Cell. Dev. Biol. 2021, 9, 647892. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.Y.; Tan, F.J.; Wang, H.Y.; Chen, J.Y.; Zhang, X.H.; Zhao, X.N.; Liu, K.Q.; Wang, Q.; Liu, S.S.; et al. Single-Cell Atlas of the Chinese Tongue Sole (Cynoglossus semilaevis) Ovary Reveals Transcriptional Programs of Oogenesis in Fish. Front. Cell. Dev. Biol. 2022, 10, 828124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Kossack, M.E.; McFaul, M.E.; Christensen, L.N.; Siebert, S.; Wyatt, S.R.; Kamei, C.N.; Horst, S.; Arroyo, N.; Drummond, I.A.; et al. Single-cell transcriptome reveals insights into the development and function of the zebrafish ovary. eLife 2022, 11, e76014. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, Y.; Ai, J.; Li, K. Application of Single-Cell RNA Sequencing in Ovarian Development. Biomolecules 2023, 13, 47. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Xu, M.; Hu, N.; Miao, J.; Zhao, Y.; Wang, L. Single-nucleus RNA Sequencing reveals the mechanism of cigarette smoke exposure on diminished ovarian reserve in mice. Ecotox. Environ. Safe 2022, 245, 114093. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, Y.R.; Gao, X.T.; Song, Y.; Huang, Y.N.; Jiang, Q.Y. Unveiling the Ovarian Cell Characteristics and Molecular Mechanism of Prolificacy in Goats via Single-Nucleus Transcriptomics Data Analysis. Curr. Issues Mol. Biol. 2024, 46, 2301–2319. [Google Scholar] [CrossRef]
- Yao, Z.L.; Wang, X.; Hu, C.L.; Chen, F.X.; Chen, H.J.; Jiang, S.J.; Zhao, Y.; Ji, X.S. A single-nucleus transcriptomic atlas characterizes cell types and their molecular features in the ovary of adult Nile tilapia. J. Fish Biol. 2024, 105, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Adiconis, X.; Simmons, S.K.; Kowalczyk, M.S.; Hession, C.C.; Marjanovic, N.D.; Hughes, T.K.; Wadsworth, M.H.; Burks, T.; Nguyen, L.T.; et al. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat. Biotechnol. 2020, 38, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.B.; Codeluppi, S.; Yung, Y.C.; Gao, D.; Chun, J.; Kharchenko, P.V.; Linnarsson, S.; Zhang, K. A comparative strategy for single-nucleus and single-cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Sci. Rep. 2017, 7, 6031. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, C.Y.; Zhang, T.; Wang, Z.J. The Reproductive Biology of Sinibrama taeniatus from the Minjiang River (in Meishan City). Chin. J. Zool. 2015, 50, 563–570. [Google Scholar] [CrossRef]
- Zhao, Z.; Peng, L.; Zhao, Q.; Wang, Z.J. Genome-Wide Identification, Expression and Response to Estrogen of Vitellogenin Gene Family in Sichuan Bream (Sinibrama taeniatus). Int. J. Mol. Sci. 2024, 25, 6739. [Google Scholar] [CrossRef]
- Yoon, C.; Kawakami, K.; Hopkins, N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 1997, 124, 3157–3165. [Google Scholar] [CrossRef]
- Bertho, S.; Clapp, M.; Banisch, T.U.; Bandemer, J.; Raz, E.; Marlow, F.L. Zebrafish dazl regulates cystogenesis and germline stem cell specification during the primordial germ cell to germline stem cell transition. Development 2021, 148, dev187773. [Google Scholar] [CrossRef]
- Liu, L.; Ge, W. Growth Differentiation Factor 9 and Its Spatiotemporal Expression and Regulation in the Zebrafish Ovary1. Biol. Reprod. 2007, 76, 294–302. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Dranow, D.B.; Hu, K.; Bird, A.M.; Lawry, S.T.; Adams, M.T.; Sanchez, A.; Amatruda, J.F.; Draper, B.W. Bmp15 Is an Oocyte-Produced Signal Required for Maintenance of the Adult Female Sexual Phenotype in Zebrafish. PLoS Genet. 2016, 12, e1006323. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Oates, A.C.; Crowhurst, M.O.; Ward, A.C.; Layton, J.E. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001, 98, 3087–3096. [Google Scholar] [CrossRef]
- Wu, H.; Liu, K.; Zhang, J. Excess fibronectin 1 participates in pathogenesis of pre-eclampsia by promoting apoptosis and autophagy in vascular endothelial cells. Mol. Hum. Reprod. 2021, 27, gaab030. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.J.; de Leij, L.M.F.H.; Harmsen, M.C. Epithelial Cell Adhesion Molecule: More than a Carcinoma Marker and Adhesion Molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef]
- Okamoto, K.; Okazawa, H.; Okuda, A.; Sakai, M.; Muramatsu, M.; Hamada, H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell 1990, 60, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Schöler, H.R.; Ruppert, S.; Suzuki, N.; Chowdhury, K.; Gruss, P. New type of POU domain in germ line-specific protein Oct-4. Nature 1990, 344, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Kostyrko, K.; Bosshard, S.; Urban, Z.; Mermod, N. A role for homologous recombination proteins in cell cycle regulation. Cell Cycle 2015, 14, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Völkening, L.; Vatselia, A.; Asgedom, G.; Bastians, H.; Lavin, M.; Schindler, D.; Schambach, A.; Bousset, K.; Dörk, T. RAD50 regulates mitotic progression independent of DNA repair functions. FASEB J. 2020, 34, 2812–2820. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef]
- La, H.; Yoo, H.; Lee, E.J.; Thang, N.X.; Choi, H.J.; Oh, J.; Park, J.H.; Hong, K.H. Insights from the Applications of Single-Cell Transcriptomic Analysis in Germ Cell Development and Reproductive Medicine. Int. J. Mol. Sci. 2021, 22, 823. [Google Scholar] [CrossRef]
- Suzuki, S.; Diaz, V.D.; Hermann, B.P. What has single-cell RNA-seq taught us about mammalian spermatogenesis? Biol. Reprod. 2019, 101, 617–634. [Google Scholar] [CrossRef]
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Yoshihara, M.; Douagi, I.; Damdimopoulos, A.; Panula, S.; Petropoulos, S.; Lu, H.J.; Pettersson, K.; Palm, K.; Katayama, S.; et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 2020, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Diff. 2008, 50, S195–S219. [Google Scholar] [CrossRef]
- Viana, I.K.S.; Gonçalves, L.A.B.; Ferreira, M.A.P.; Mendes, Y.A.; Rocha, R.M. Oocyte growth, follicular complex formation and extracellular-matrix remodeling in ovarian maturation of the imperial zebra pleco fish Hypancistrus zebra. Sci. Rep. 2018, 8, 13760. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yoshikuni, M.; Yamashita, M.; Tokumoto, T.; Katsu, Y. Regulation of Oocyte Growth and Maturation in Fish. Curr. Top. Dev. Biol. 1995, 30, 103–145. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.-F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Chapter Three-Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Hard, J.J.; Utter, F. Genetic variation and fitness in salmonids. Conserv. Genet. 2002, 3, 321–333. [Google Scholar] [CrossRef]
- Sousa-Santos, C.; Robalo, J.I.; Pereira, A.M.; Branco, P.; Santos, J.M.; Ferreira, M.T.; Sousa, M.; Doadrio, I. Broad-scale sampling of primary freshwater fish populations reveals the role of intrinsic traits, inter-basin connectivity, drainage area and latitude on shaping contemporary patterns of genetic diversity. PeerJ 2016, 4, e1694. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 2017, 20, 858–873.e4. [Google Scholar] [CrossRef]
- Ishiguro, K.; Matsuura, K.; Tani, N.; Takeda, N.; Usuki, S.; Yamane, M.; Sugimoto, M.; Fujimura, S.; Hosokawa, M.; Chuma, S.; et al. MEIOSIN Directs the Switch from Mitosis to Meiosis in Mammalian Germ Cells. Dev. Cell 2020, 52, 429–445. [Google Scholar] [CrossRef]
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600. [Google Scholar] [CrossRef]
- Koubova, J.; Menke, D.B.; Zhou, Q.; Capel, B.; Griswold, M.D.; Page, D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 2474–2479. [Google Scholar] [CrossRef]
- Rodríguez-Marí, A.; Cañestro, C.; BreMiller, R.A.; Catchen, J.M.; Yan, Y.L.; Postlethwait, J.H. Retinoic Acid Metabolic Genes, Meiosis, and Gonadal Sex Differentiation in Zebrafish. PLoS ONE 2013, 8, e73951. [Google Scholar] [CrossRef]
- Zhong, C.Y.; Liu, M.; Tao, Y.; Wu, X.; Yang, Y.; Wang, T.; Meng, Z.; Xu, H.; Liu, X. Pou5f1 and Nanog Are Reliable Germ Cell-Specific Genes in Gonad of a Protogynous Hermaphroditic Fish, Orange-Spotted Grouper (Epinephelus coioides). Genes 2022, 13, 79. [Google Scholar] [CrossRef]
- Wallace, R.A.; Selman, K. Ultrastructural aspects of oogenesis and oocyte growth in fish and amphibians. J. Electron Microsc. Tech. Electron. Microsc. Tech. 1990, 16, 175–201. [Google Scholar] [CrossRef]
- Tang, W.W.C.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Jie, H.Y.; Huang, K.J.; Cai, B.; Zhou, X.; Liang, M.Y.; Zhou, C.Q.; Mai, Q.Y. The dynamic expression of SOX17 in germ cells from human female foetus and adult ovaries after specification. Front. Endocrinol. 2023, 14, 1124143. [Google Scholar] [CrossRef]
- De Jong, J.; Stoop, H.; Gillis, A.; van Gurp, R.; van de Geijn, G.-J.; Boer, M.D.; Hersmus, R.; Saunders, P.; Anderson, R.A.; Oosterhuis, J.W.; et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J. Pathol. J. Pathol. 2008, 215, 21–30. [Google Scholar] [CrossRef]
- Peng, F.; Zhou, Y.; Wang, J.; Guo, B.; Wei, Y.; Deng, H.; Wu, Z.; Zhang, C.; Shi, K.; Li, Y.; et al. The transcription factor Sp1 modulates RNA polymerase III gene transcription by controlling BRF1 and GTF3C2 expression in human cells. J. Biol. Chem. 2020, 295, 4617–4630. [Google Scholar] [CrossRef]
- Cai, H.; Liu, B.; Wang, H.; Sun, G.; Feng, L.; Chen, Z.; Zhou, J.; Zhang, J.; Zhang, T.; He, M.; et al. SP1 governs primordial folliculogenesis by regulating pregranulosa cell development in mice. J. Mol. Cell Biol. 2020, 12, 230–244. [Google Scholar] [CrossRef]
- Sloan, R.S.; Swanson, C.I.; Gavilano, L.; Smith, K.N.; Malek, P.Y.; Snow-Smith, M.; Duronio, R.J.; Key, S. Characterization of null and hypomorphic alleles of the Drosophila l(2)dtl/cdt2 gene Larval lethality and male fertility. Fly 2012, 6, 173–183. [Google Scholar] [CrossRef][Green Version]
- Sun, L.; Huang, Y.; Wei, Q.; Tong, X.; Cai, R.; Nalepa, G.; Ye, X. Cyclin E-CDK2 Protein Phosphorylates Plant Homeodomain Finger Protein 8 (PHF8) and Regulates Its Function in the Cell Cycle. J. Biol. Chem. 2015, 290, 4075–4085. [Google Scholar] [CrossRef]
- Irvin, J.D.; Pugh, B.F. Genome-wide Transcriptional Dependence on TAF1 Functional Domains. J. Biol. Chem. 2006, 281, 6404–6412. [Google Scholar] [CrossRef]
- Jambaldorj, J.; Makino, S.; Munkhbat, B.; Tamiya, G. Sustained expression of a neuron-specific isoform of the Taf1 gene in development stages and aging in mice. Biochem. Biophys. Res. Commun. 2012, 425, 273–277. [Google Scholar] [CrossRef]
- Peer, N.R.; Law, S.M.; Murdoch, B.; Goulding, E.H.; Eddy, E.M.; Kim, K. Germ Cell–Specific Retinoic Acid Receptor α Functions in Germ Cell Organization, Meiotic Integrity, and Spermatogonia. Endocrinology 2018, 159, 3403–3420. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kim, D.H.; Kwak, S.-N.; Jeong, S.-W.; Kwon, O.-J. X-box binding protein 1 enhances adipogenic differentiation of 3T3-L1 cells through the downregulation of Wnt10b expression. FEBS Lett. 2013, 587, 1644–1649. [Google Scholar] [CrossRef]
- Moore, B.D.; Jin, R.U.; Lo, H.; Jung, M.; Wang, H.; Battle, M.A.; Wollheim, C.B.; Urano, F.; Mills, J.C. Transcriptional Regulation of X-Box-binding Protein One (XBP1) by Hepatocyte Nuclear Factor 4α (HNF4A) Is Vital to Beta-cell Function. J. Biol. Chem. 2016, 291, 6146–6157. [Google Scholar] [CrossRef]
- Glimcher, L.H.; Lee, A.H. From Sugar to Fat How the Transcription Factor XBP1 Regulates Hepatic Lipogenesis. Integr. Physiol. 2009, 1173, E2–E9. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, H.; Xiao, X.; Wang, L.; Jia, L.; Shi, Y.; Zhang, M.; Fang, C.; Zeng, Y.; Wu, T.; et al. Global crotonylome identifies EP300-regulated ANXA2 crotonylation in cumulus cells as a regulator of oocyte maturation. Int. J. Biol. Macromol. 2024, 259, 129149. [Google Scholar] [CrossRef]
- Pursell, Z.F.; Kunkel, T.A. DNA polymerase epsilon: A polymerase of unusual size (and complexity). Prog. Nucleic Acid Res. Mol. Biol. 2008, 82, 101–145. [Google Scholar] [CrossRef]
- Holt, J.E.; Jones, K.T. Control of homologous chromosome division in the mammalian oocyte. Mol. Hum. Reprod. 2009, 15, 139–147. [Google Scholar] [CrossRef]
- Bellelli, R.; Belan, O.; Pye, V.E.; Clement, C.; Maslen, S.L.; Skehel, J.M.; Cherepanov, P.; Almouzni, G.; Boulton, S.J. POLE3-POLE4 Is a Histone H3-H4 Chaperone that Maintains Chromatin Integrity during DNA Replication. Mol. Cell 2018, 72, 112–126.e5. [Google Scholar] [CrossRef]
- Hill, B.R.; Ozgencil, M.; Buckley-Benbow, L.; Skingsley, S.L.P.; Tomlinson, D.; Eizmendi, C.O.; Agnarelli, A.; Bellelli, R. Loss of POLE3-POLE4 unleashes replicative gap accumulation upon treatment with PARP inhibitors. Cell Rep. 2024, 43, 114205. [Google Scholar] [CrossRef]
- Turan, V.; Oktay, K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update 2019, 26, 43–57. [Google Scholar] [CrossRef]
- Xu, X.; Duan, S.; Hua, X.; Li, Z.; He, R.; Zhang, Z. Stable inheritance of H3.3-containing nucleosomes during mitotic cell divisions. Nat. Commun. 2022, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Sumpter, J.P. Oocyte growth and development in teleosts. Rev. Fish Biol. Fish. 1996, 6, 287–318. [Google Scholar] [CrossRef]
- Pawshe, C.H.; Appa Rao, K.B.C.; Totey, S.M. Effect of insulin-like growth factor I and its interaction with gonadotropins on in vitro maturation and embryonic development, cell proliferation, and biosynthetic activity of cumulus-oocyte complexes and granulosa cells in buffalo. Mol. Reprod. Dev. 1998, 49, 277–285. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; García-García, R.M.; Cajas, Y.N.; Fierro, N.; Lorenzo, P.L.; Arias-Álvarez, M. Improvement of oocyte competence and in vitro oocyte maturation with EGF and IGF-I in Guinea pig model. Theriogenology 2024, 214, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, B.B.; Gabillard, J.-C.; Bobe, J. Insulin-Like Growth Factor-Binding Protein (IGFBP)-1, -2, -3, -4, -5, and -6 and IGFBP-Related Protein 1 during Rainbow Trout Postvitellogenesis and Oocyte Maturation: Molecular Characterization, Expression Profiles, and Hormonal Regulation. Endocrinology 2006, 147, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Xu, S.; Chang, Y.; Ye, Y.; Guo, A.; Kang, Y.; Guo, H.; Xu, H.; Chen, L.; et al. Loss of Gsdf leads to a dysregulation of Igf2bp3-mediated oocyte development in medaka. Gen. Comp. Endocrinol. 2019, 277, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Choi, K.H.; Jun, J.C.; Choi, M.S.; Lee, K.Y. Ultrastructural Studies on Oocyte Development and Vitellogenesis During Oogenesis in Female Boleophthalmus pectinirostris. Anim. Cells Syst. 2009, 13, 49–57. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; Fu, Y.; Liu, Z.; Zhao, M.; Xu, L.; Zhan, T.; Huang, T.; Luo, M.; Chen, W.; et al. Comparative Transcriptome Analysis Identifies MAPK Signaling Pathway Associated with Regulating Ovarian Lipid Metabolism during Vitellogenesis in the Mud Crab, Scylla paramamosain. Fishes 2023, 8, 145. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Wang, X.-P. Dpp-mediated TGF-β signaling regulates vitellogenesis through 20-hydroxyecdysone signaling in the cabbage beetle, Colaphellus bowringi. Pestic. Biochem. Physiol. 2023, 197, 105706. [Google Scholar] [CrossRef]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef]
- Clelland, E.; Peng, C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009, 312, 42–52. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Zhang, Y.; Li, Y.-Y.; Liu, X.-M.; Wang, X.-X.; Zhang, C.-L.; Hao, C.-F.; Deng, S.-L. Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front. Biosci.-Landmark 2019, 24, 983–993. [Google Scholar] [CrossRef]
- Lv, X.M.; He, C.B.; Huang, C.; Wang, H.B.; Hua, G.H.; Wang, Z.F.; Zhou, J.; Chen, X.C.; Ma, B.W.; Timm, B.K.; et al. Timely expression and activation of YAP1 in granulosa cells is essential for ovarian follicle development. FASEB J. 2019, 33, 10049–10064. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Sugiura, K.; Wigglesworth, K.; O’Brien, M.J.; Affourtit, J.P.; Pangas, S.A.; Matzuk, M.M.; Eppig, J.J. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008, 135, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, A.D.; Gilbert, I.; Scantland, S.; Fournier, E.; Ashkar, F.; Bastien, A.; Saadi, H.A.S.; Gagné, D.; Sirard, M.-A.; Khandjian, É.W.; et al. Cumulus Cell Transcripts Transit to the Bovine Oocyte in Preparation for Maturation1. Biol. Reprod. 2016, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. Altered Energy Metabolism, Mitochondrial Dysfunction, and Redox Imbalance Influencing Reproductive Performance in Granulosa Cells and Oocyte During Aging. Reprod. Sci. 2024, 31, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Burns, K.H.; Viveiros, M.M.; Eppig, J.J. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science 2002, 296, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xizhe, W.; Adam, D.H.; Amir, G.; Mingzhuo, P.; Seungsoo, K.; Daniela, C.; Jan, H.; Judith, C.; Rogerio, L.; et al. The regulatory landscapes of human ovarian ageing. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kulus, J.; Kulus, M.; Kranc, W.; Jopek, K.; Zdun, M.; Józkowiak, M.; Jaśkowski, J.M.; Piotrowska-Kempisty, H.; Bukowska, D.; Antosik, P.; et al. Transcriptomic Profile of New Gene Markers Encoding Proteins Responsible for Structure of Porcine Ovarian Granulosa Cells. Biology 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Ożegowska, K.; Brązert, M.; Ciesiółka, S.; Nawrocki, M.J.; Kranc, W.; Celichowski, P.; Jankowski, M.; Bryja, A.; Jeseta, M.; Antosik, P.; et al. Genes Involved in the Processes of Cell Proliferation, Migration, Adhesion, and Tissue Development as New Potential Markers of Porcine Granulosa Cellular Processes In Vitro: A Microarray Approach. DNA Cell Biol. 2019, 38, 549–560. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, Y.S.; Xie, J.; Wang, T.X.; Zhang, L.H.; Zhang, W.M. Growth differentiation factor 9 (Gdf9) was localized in the female as well as male germ cells in a protogynous hermaphroditic teleost fish, ricefield eel Monopterus albus. Gen. Comp. Endocrinol. 2012, 178, 355–362. [Google Scholar] [CrossRef]
- Halm, S.; Ibañez, A.J.; Tyler, C.R.; Prat, F. Molecular characterisation of growth differentiation factor 9 (gdf9) and bone morphogenetic protein 15 (bmp15) and their patterns of gene expression during the ovarian reproductive cycle in the European sea bass. Mol. Cell. Endocrinol. 2008, 291, 95–103. [Google Scholar] [CrossRef]
- Kawagishi, R.; Tahara, M.; Morishige, K.; Sakata, M.; Tasaka, K.; Ikeda, W.; Morimoto, K.; Takai, Y.; Murata, Y. Expression of nectin-2 in mouse granulosa cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 71–76. [Google Scholar] [CrossRef]
- Kobayashi, I.; Kobayashi-Sun, J.; Kim, A.D.; Pouget, C.; Fujita, N.; Suda, T.; Traver, D. Jam1a–Jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature 2014, 512, 319–323. [Google Scholar] [CrossRef]
- Stephens, C.S.; Johnson, P.A. Occludin expression and regulation in small follicles of the layer and broiler breeder hen. Gen. Comp. Endocrinol. 2017, 248, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.K.; Schmierer, B.; Shkumatava, A.; Kuchler, K. Activin A and follicle-stimulating hormone control tight junctions in avian granulosa cells by regulating occludin expression. Biol. Reprod. 2004, 70, 1493–1499. [Google Scholar] [CrossRef] [PubMed]



| Sample | nCount RNA | nFeature RNA | Percent Double Cell | Num Orig | Num Filterd |
|---|---|---|---|---|---|
| II-1 | 13,963 | 200–4132 | 8.41% | 11,073 | 9448 |
| II-2 | 11,337 | 200–3595 | 9.64% | 11,514 | 9660 |
| II-3 | 16,998 | 200–4512 | 5.90% | 7524 | 6593 |
| III-1 | 31,489 | 200–6869 | 10.80% | 12,665 | 10,701 |
| III-2 | 31,489 | 200–6869 | 10.80% | 12,665 | 10,701 |
| III-3 | 17,891 | 200–5316 | 8.27% | 10,242 | 8786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Huang, S.; Feng, Q.; Peng, L.; Zhao, Q.; Wang, Z. Characterizing the Ovarian Cytogenetic Dynamics of Sichuan Bream (Sinibrama taeniatus) During Vitellogenesis at a Single-Cell Resolution. Int. J. Mol. Sci. 2025, 26, 2265. https://doi.org/10.3390/ijms26052265
Zhao Z, Huang S, Feng Q, Peng L, Zhao Q, Wang Z. Characterizing the Ovarian Cytogenetic Dynamics of Sichuan Bream (Sinibrama taeniatus) During Vitellogenesis at a Single-Cell Resolution. International Journal of Molecular Sciences. 2025; 26(5):2265. https://doi.org/10.3390/ijms26052265
Chicago/Turabian StyleZhao, Zhe, Shixia Huang, Qilin Feng, Li Peng, Qiang Zhao, and Zhijian Wang. 2025. "Characterizing the Ovarian Cytogenetic Dynamics of Sichuan Bream (Sinibrama taeniatus) During Vitellogenesis at a Single-Cell Resolution" International Journal of Molecular Sciences 26, no. 5: 2265. https://doi.org/10.3390/ijms26052265
APA StyleZhao, Z., Huang, S., Feng, Q., Peng, L., Zhao, Q., & Wang, Z. (2025). Characterizing the Ovarian Cytogenetic Dynamics of Sichuan Bream (Sinibrama taeniatus) During Vitellogenesis at a Single-Cell Resolution. International Journal of Molecular Sciences, 26(5), 2265. https://doi.org/10.3390/ijms26052265






