In Vitro and In Silico Cytotoxic Activity of Isocordoin from Adesmia balsamica Against Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. In Silico Approach
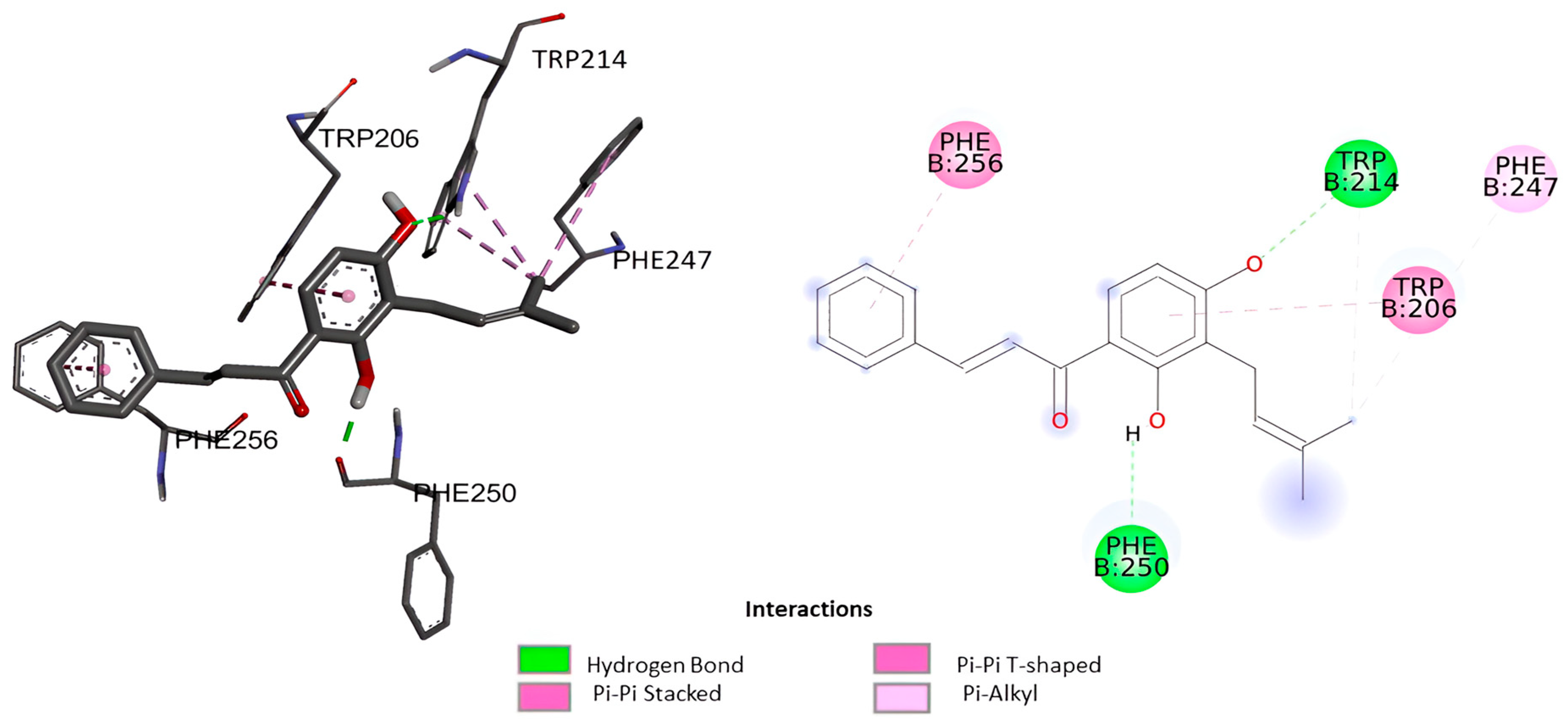
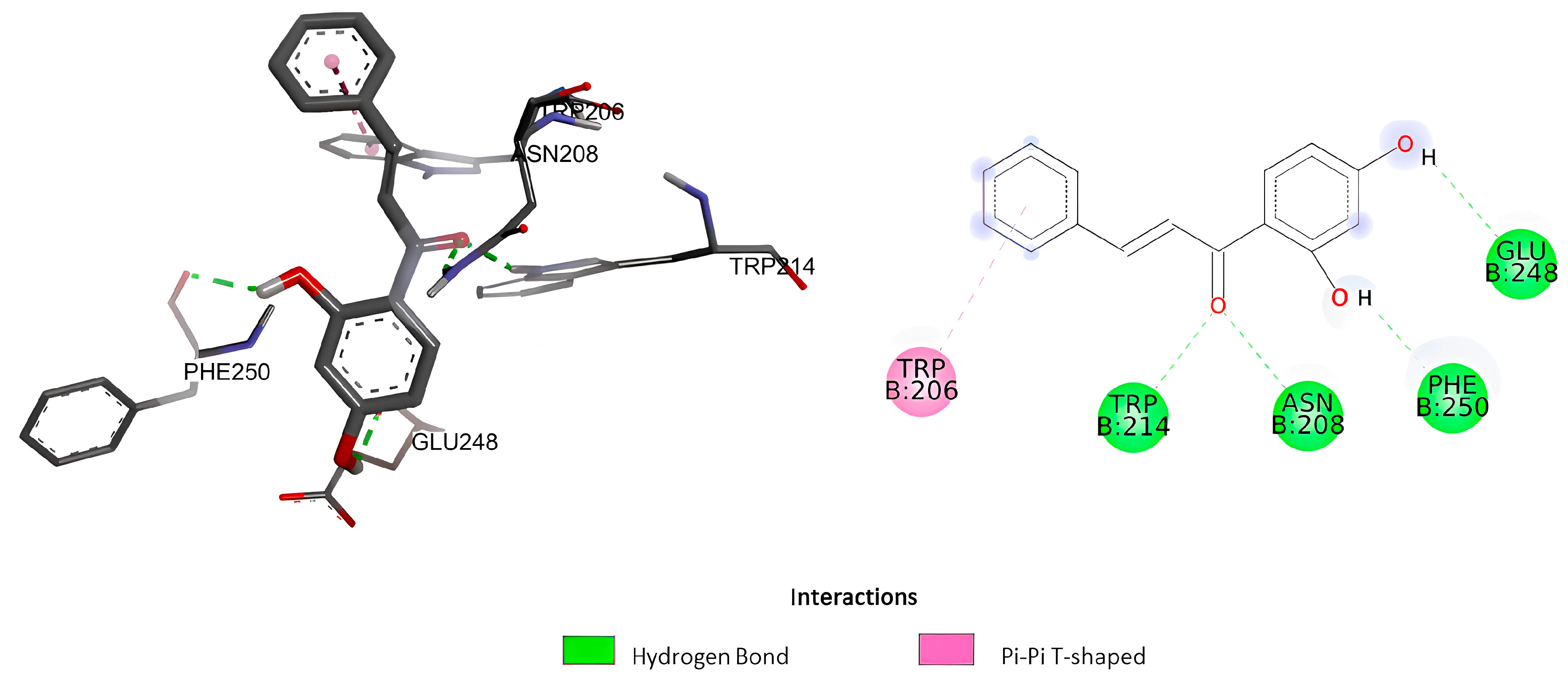
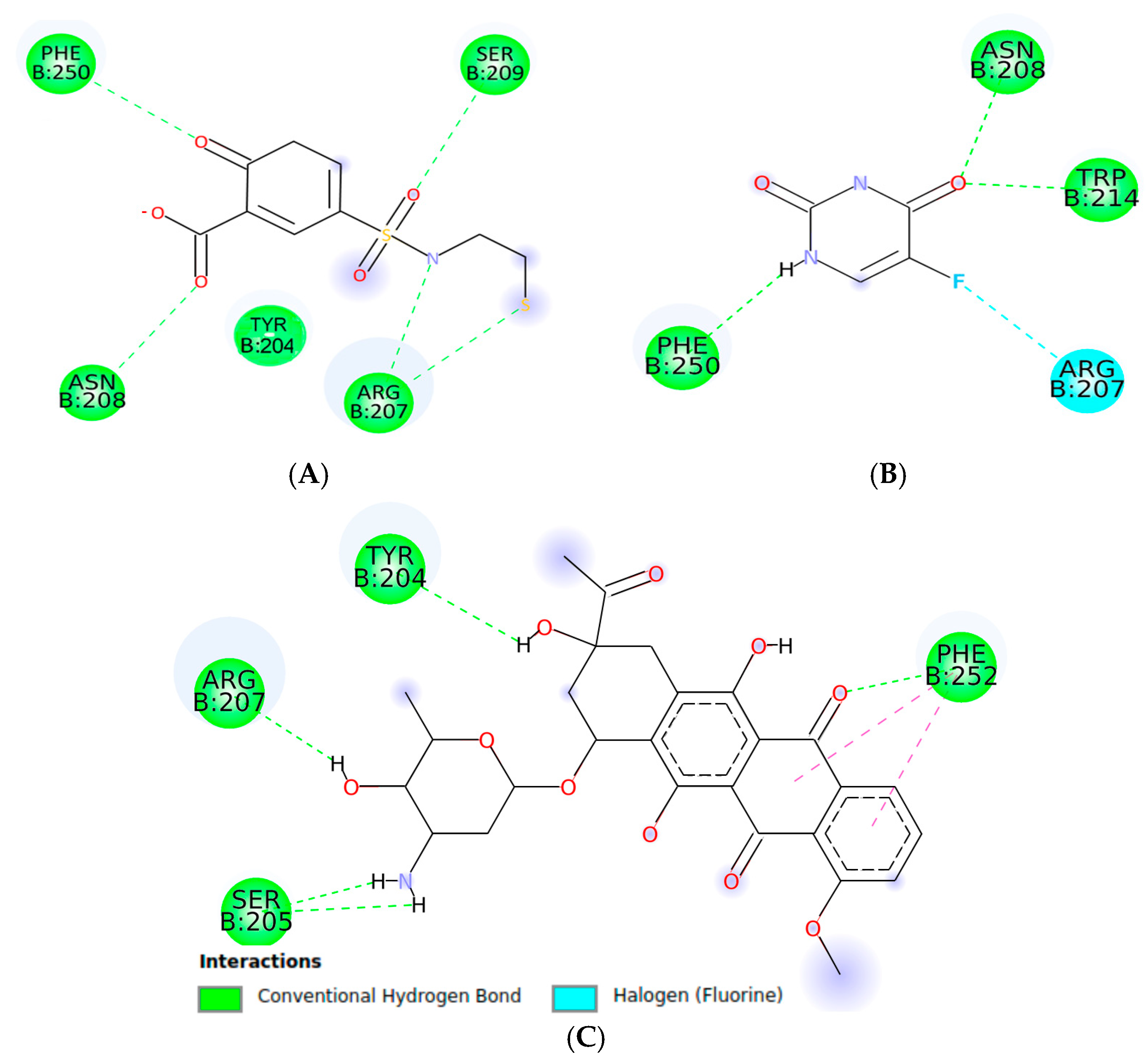
2.1.1. Molecular Docking
2.1.2. Prediction of Physicochemical Properties, Pharmacokinetics, and Drug-likeness Profile
3. Materials and Methods
3.1. Plant Material
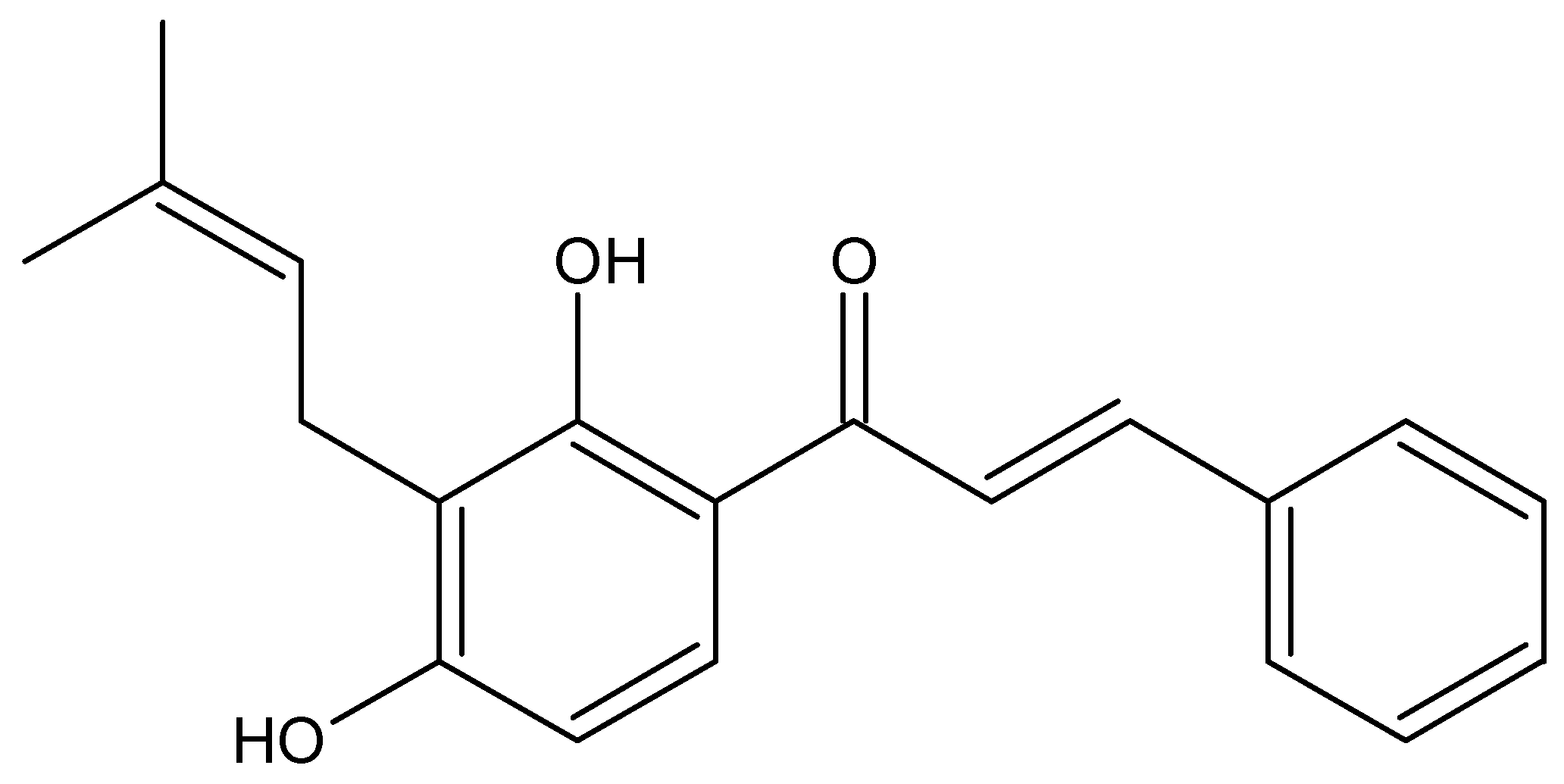
3.2. Extraction and Isolation of Isocordoin
3.3. Cell Lines and Culture Conditions
3.4. Cytotoxicity Assay
3.5. Selectivity Index
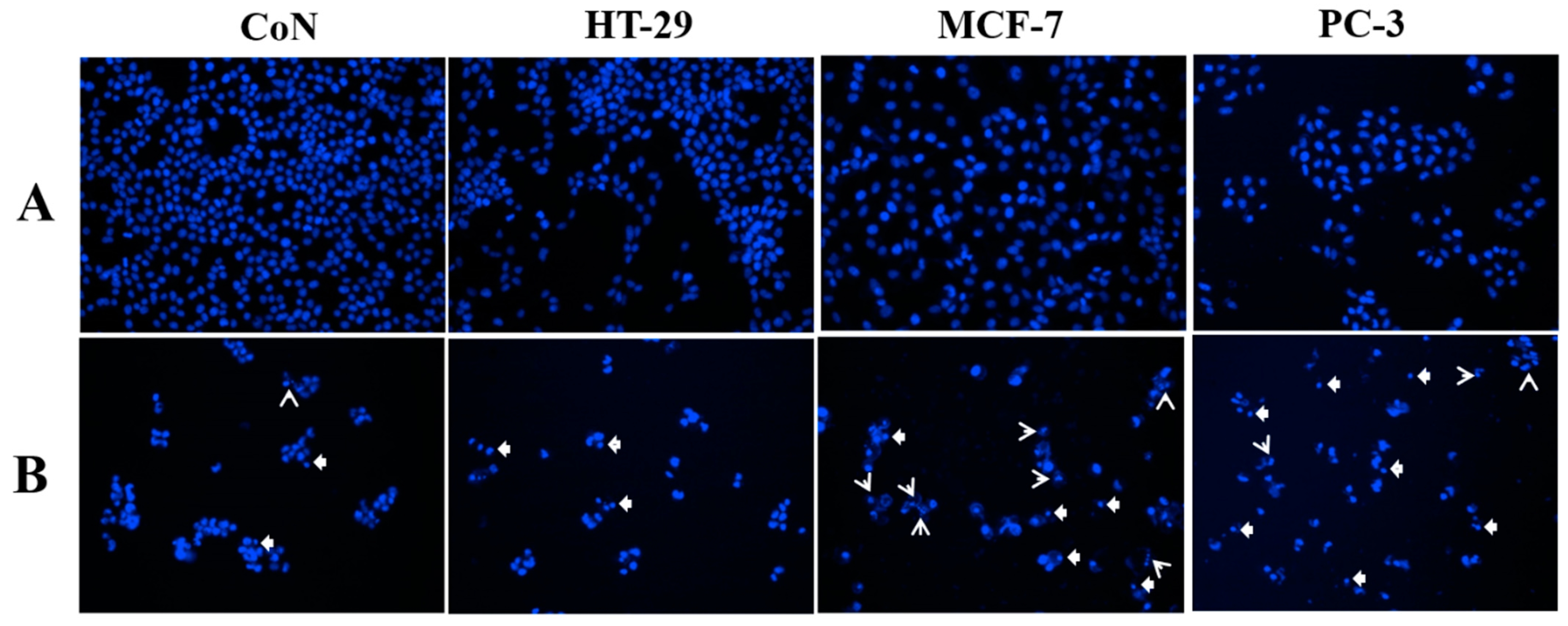
3.6. Cellular and Nuclear Morphology
3.7. Reactive Oxygen Species (ROS) Generation
3.8. Mitochondrial Membrane Permeability
3.9. Caspase Activation
3.10. In Silico Assays
3.10.1. Materials
3.10.2. Construction of Ligands
3.10.3. Molecular Docking
3.10.4. Prediction of Physicochemical Properties, Pharmacokinetics, and Drug-likeness Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Orzetti, S.; Tommasi, F.; Bertola, A.; Bortolin, G.; Caccin, E.; Cecco, S.; Ferrarin, E.; Giacomin, E.; Baldo, P. Genetic therapy and molecular targeted therapy in oncology: Safety, pharmacovigilance, and perspectives for research and clinical practice. Int. J. Mol. Sci. 2022, 23, 3012. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Gui, Y.; Li, Q.; An, J.; Wang, D. The signaling pathways and targets of natural products from traditional Chinese medicine treating gastric cancer provide new candidate therapeutic strategies. Biochim. Biophys. Acta 2023, 1878, 188998. [Google Scholar] [CrossRef]
- Ziech, D.; Anestopoulos, I.; Hanafi, R.; Voulgaridou, G.P.; Franco, R.; Georgakilas, A.; Pappa, A.; Panayiotidis, M.I. Pleiotrophic effects of natural products in ROS-induced carcinogenesis: The role of plant-derived natural products in oral cancer hemoprevention. Cancer Lett. 2012, 327, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rashid, K.; AlAmodi, A.; Agha, M.; Akhtar, S.; Hakeem, I.; Raza, S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhong, M.; Liu, Y.; Xiong, Y.; Gao, Z.; Ma, J.; Zhuang, G.; Hong, X. Application of natural products for inducing ferroptosis in tumor cells. Appl. Biochem. Biotechnol. 2022, V69, 190–197. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433. [Google Scholar] [CrossRef]
- Bhalla, V.X.; Kayak, U.R.; Dev, S. Some new flavonoids from Psolarea corylifolia. Tetrahedron Lett. 1968, 20, 2401–2406. [Google Scholar] [CrossRef]
- Fang, S.C.; Hsu, C.L.; Yu, Y.S.; Yen, G.C. Cytotoxic effects of new geranyl chalcone derivatives isolated from the leaves of Artocarpus communis in SW 872 human liposarcoma cells. J. Agric. Food Chem. 2008, 56, 8859–8868. [Google Scholar] [CrossRef]
- Kuete, V.; Sandj, L.P. Isobavachalcone: An overview. Chin. J. Integr. Med. 2012, 18, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Blaney, W.M.; Delle, F.; Monache, D.; Bettolo, G. Insect antifeedant activity associated with compounds isolated from species of Lonchocarpus and Tephrosia. J. Chem. Ecol. 1990, 16, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Vela, T.; Yam-Puc, A.; Chan-Bacab, M.J.; Moo-Puc, R.E.; Cáceres-Farfán, M. Antiprotozoal and cytotoxic studies on some isocordoin derivatives. Planta Med. 2009, 75, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Villarreal, A.; Hernández-Abreu, O.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Escalante-Erosa, F.; Peña-Rodríguez, L.M.; Villalobos-Molina, R.; Estrada-Soto, S. Antihypertensive and vasorelaxant effects of dihydrospinochalcone-A isolated from Lonchocarpus xuul Lundell by NO production: Computational and ex vivo approaches. Phytomedicine 2013, 20, 1241–1246. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Escobar, B.; Montenegro, I.; Villena, J.; Werner, E.; Godoy, P.; Olguín, Y.; Madrid, A. Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin. Molecules 2017, 22, 968. [Google Scholar] [CrossRef]
- Da Silva, A.F.; De Campos, F.; Santin, J.R.; Yam-Puc, A.; Escalante-Erosa, F.; Sosa, K.G.; Klein, L.C.; Peña Rodriguez, L.M.; Cechinel, V.; Quintã, N. Anti-inflammatory and anti-hypersensitive effects of the chalcone isocordoin and its semisynthetic derivatives in mice. Behav. Pharmacol. 2020, 31, 716–727. [Google Scholar] [CrossRef]
- Caamal-Fuentes, S.R.; Peraza-Sánchez, L.W.; Torres-Tapia, R.; Moo-Puc, A. Isolation and identification of cytotoxic compounds from Aeschynomene fascicularis, a Mayan medicinal plant. Molecules 2015, 20, 13563–13574. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Avola, R.; Graziano, A.; Montenegro, I.; Said, B.; Madrid, A. Isocordoin analogues promote apoptosis in human melanoma cells via Hsp70. Phytother. Res. 2019, 33, 3242–3250. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Duveau, D.Y.; Arce, P.M.; Schoenfeld, R.A.; Raghav, N.; Cortopassi, G.A.; Hecht, S.M. Synthesis and characterization of mitoQ and idebenone analogues as mediators of oxygen consumption in mitochondria. Bioorganic Med. Chem. Lett. 2010, 18, 6429–6441. [Google Scholar] [CrossRef]
- Urra, F.A.; Martínez-Cifuentes, M.; Pavani, M.; Lapier, M.; Jaña-Prado, F.; Parra, E.; Maya, J.D.; Pessoa-Mahana, H.; Ferreira, J.; Araya-Maturana, R. An ortho-carbonyl substituted hydroquinone derivative is an anticancer agent that acts by inhibiting mitochondrial bioenergetics and by inducing G2/M-phase arrest in mammary adenocarcinoma TA3. Toxicol. Appl. Pharmacol. 2013, 267, 218–227. [Google Scholar] [CrossRef]
- Gruber, E.; Hoelscher, G.L.; Bethea, S.; Hanley, E.N. Mitochondrial Membrane Potential and Nuclear and Gene Expression Changes During Human Disc Cell Apoptosis. Spine 2015, 40, 876–882. [Google Scholar] [CrossRef]
- Ulte, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Henderson, J. Ion transport by energy-conserving biological membranes. Annu. Rev. Microbiol. 1971, 25, 393–428. [Google Scholar] [CrossRef]
- Ávila, H.P.; Smânia, E.F.; Monache, F.D.; Smânia, A.J. Structure–activity relationship of antibacterial chalcones. Bioorganic Med. Chem. Lett. 2008, 16, 9790–9794. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Zhang, G.; Chen, H.; Luo, Z.; Deng, X.; Kang, B. Role of caspase family in intervertebral disc degeneration and its therapeutic prospects. Biomolecules 2022, 12, 1074. [Google Scholar] [CrossRef]
- Araya, L.E.; Soni, I.V.; Hardy, J.A.; Julien, O. Deorphanizing caspase-3 and caspase-9 substrates in and out of apoptosis with deep substrate profiling. ACS Chem. Biol. 2021, 16, 2280–2296. [Google Scholar] [CrossRef]
- Aly, A.A.; Sayed, S.M.; Abdelhafez, E.S.M.N.; Abdelhafez, W.Y.; Abdelzaher, M.A.; Raslan, A.E.; Thabet, K.A.; El-Reedy, A.A.; Brown, A.B. New quinoline-2-one/pyrazole derivatives; design, synthesis, molecular docking, anti-apoptotic evaluation, and caspase-3 inhibition assay. Bioorganic Chem. 2020, 94, 103348. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Krishna, S.R.; Nagaraju, D.; Reddy, K.G.; Kishore, B.; Reddy, Y. Environmentally benign synthesis, molecular properties prediction and anti-inflammatory activity of novel isoxazolo[5,4-d]isoxazol-3-yl-aryl-methanones via vinylogous Henry nitroaldol adducts as synthons. Bioorganic Med. Chem. Lett. 2015, 25, 1630–1634. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Moorthy, N.S.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activities. Recent Pat. Anticancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef]
- Villena, J.; Montenegro, I.; Said, B.; Werner, E.; Flores, S.; Madrid, A. Ultrasound assisted synthesis and cytotoxicity evaluation of known 2′,4′-dihydroxychalcone derivatives against cancer cell lines. Food Chem. Toxicol. 2021, 148, 111969. [Google Scholar] [CrossRef]
- Alabsi, A.M.; Lim, K.L.; Paterson, I.C.; Ali-Saeed, R.; Muharram, B.A. Cell Cycle Arrest and Apoptosis Induction via Modulation of Mitochondrial Integrity by Bcl-2 Family Members and Caspase Dependence in Dracaena cinnabari-Treated H400 Human Oral Squamous Cell Carcinoma. Biomed Res. Int. 2016, 2016, 4904016. [Google Scholar] [CrossRef]
- Nichani, K.; Li, J.; Suzuki, M.; Houston, J.P. Evaluation of Caspase-3 Activity During Apoptosis with Fluorescence Lifetime-Based Cytometry Measurements and Phasor Analyses. Cytometry A 2020, 97, 1265–1275. [Google Scholar] [CrossRef]
- Erlanson, D.A.; Lam, J.W.; Wiesmann, C.; Luong, T.N.; Simmons, R.L.; DeLano, W.L.; Choong, I.C.; Burdett, M.T.; Flanagan, W.M.; Lee, D.; et al. In situ assembly of enzyme inhibitors using extended tethering. Nat. Biotechnol. 2003, 21, 308–314. [Google Scholar] [CrossRef]
- Morris, G.; Goodsell, D.; Halliday, R.; Huey, R.; Hart, W.; Belew, R.; Olson, A. Automated docking using a Lamarckian Genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Febrina, E.; Asnawi, A.; Abdulah, R.; Lestari, K.; Supratman, U. Identification of flavonoids from Acalypha indica L.(Euphorbiaceae) as caspase-3 activators using molecular docking and molecular dynamics. Int. J. Appl. Pharm. 2022, 14, 162–166. [Google Scholar] [CrossRef]
| Sample | Cell lines IC50 (µM) a (SI b) | |||
|---|---|---|---|---|
| HT-29 | MCF-7 | PC-3 | CoN CCD841 | |
| Isocordoin | 27.2 ± 0.3 * (2.9) | 21.1 ± 0.2 * (3.7) | 15.2 ± 0.6 * (5.2) | 78.7 ± 0.1 |
| Daunorubicin | 15.1 ± 0.5 (<1) | 0.33 ± 0.2 (42) | 0.41 ± 0.4 (33.9) | 13.9 ± 0.3 |
| 5-FU | 2.9 ± 0.7 (19.3) | 22.3 ± 0.2 (2.5) | 16.4 ± 0.6 (3.4) | 56.1 ± 0.5 |
| Sample | Concentration (µM) | HT-29 | MCF-7 |
|---|---|---|---|
| Isocordoin | 25 | 27.8 ± 1.9 * | 5.0 ± 1.1 |
| 50 | 45.5 ± 3.7 * | 8.3 ± 1.2 | |
| Control a | --- | 15.7 ± 0.9 | 6.5 ± 0.9 |
| Sample | Concentration (µM) | HT-29 | MCF-7 |
|---|---|---|---|
| Isocordoin | 25 | 40.8 ± 5.1 * | 37.7 ± 2.2 * |
| 50 | 37.0 ±2.9 * | 54.9 ± 6.1 * | |
| Control | --- | 7.6 ± 0.9 | 13.2 ± 1.6 |
| Sample | Concentration (µM) | HT-29 | MCF-7 |
|---|---|---|---|
| Isocordoin | 25 | 22.8 ± 1.4 * | 8.3 ± 1.0 |
| 50 | 27.7 ± 1.6 * | 25.6 ± 2.6 * | |
| Control a | --- | 13.6 ± 1.1 | 8.8 ± 0.9 |
| Ligand | Docking Score (ΔG) (Kcal/mol) | Estimated Inhibition Constant (Ki) (µM) | Amino Acid Involved |
|---|---|---|---|
| Isocordoin | −6.13 | 32.01 | Phe256, Trp214, Phe247, Trp 206, Phe250 |
| 2,4’-dihydroxychalcone | −5.58 | 81.57 | Phe250, Glu248, Asn208, Trp214, Trp206 |
| NLBA * | −5.52 | 66.53 | Arg207, Tyr204, Asn208, Phe250, Ser209 |
| Daunorubicin | −7.09 | 6.33 | Tyr204, Arg207, Ser205, Phe252 |
| 5-FU | −3.68 | 2000 | Asn208, Trp214, Arg207, Phe250 |
| Compound | Lipophilicity log P | Heavy Atoms | Aromatic Heavy Atoms | Rot. Bond | H-Bond Acc. | H-Bond Don. | MR a | TPSA b (A2) | %ABS c |
|---|---|---|---|---|---|---|---|---|---|
| Isocordoin | 4.24 | 23 | 12 | 5 | 3 | 2 | 94.01 | 57.53 | 89.15 |
| 2,4’-dihydroxychalcone | 2.75 | 18 | 12 | 3 | 3 | 2 | 70.29 | 57.53 | 89.15 |
| Compound | MW a | Log S b | LV c | Bioavailability Score | GI Absorption d | BBB Permeant e | P-gp Substrate f |
|---|---|---|---|---|---|---|---|
| Isocordoin | 308.37 | −5.25 | 0 | 0.55 | High | Yes | No |
| 2,4’-dihydroxychalcone | 240.25 | −3.85 | 0 | 0.55 | High | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Muñoz, E.; Ferreira, C.; Russo, A.; Villena, J.; Montenegro, I.; Birchmeier, D.; Madrid, A. In Vitro and In Silico Cytotoxic Activity of Isocordoin from Adesmia balsamica Against Cancer Cells. Int. J. Mol. Sci. 2025, 26, 2238. https://doi.org/10.3390/ijms26052238
Silva V, Muñoz E, Ferreira C, Russo A, Villena J, Montenegro I, Birchmeier D, Madrid A. In Vitro and In Silico Cytotoxic Activity of Isocordoin from Adesmia balsamica Against Cancer Cells. International Journal of Molecular Sciences. 2025; 26(5):2238. https://doi.org/10.3390/ijms26052238
Chicago/Turabian StyleSilva, Valentina, Evelyn Muñoz, Catalina Ferreira, Alessandra Russo, Joan Villena, Iván Montenegro, Daniela Birchmeier, and Alejandro Madrid. 2025. "In Vitro and In Silico Cytotoxic Activity of Isocordoin from Adesmia balsamica Against Cancer Cells" International Journal of Molecular Sciences 26, no. 5: 2238. https://doi.org/10.3390/ijms26052238
APA StyleSilva, V., Muñoz, E., Ferreira, C., Russo, A., Villena, J., Montenegro, I., Birchmeier, D., & Madrid, A. (2025). In Vitro and In Silico Cytotoxic Activity of Isocordoin from Adesmia balsamica Against Cancer Cells. International Journal of Molecular Sciences, 26(5), 2238. https://doi.org/10.3390/ijms26052238






