Insights on the Role of Sialic Acids in Acute Lymphoblastic Leukemia in Children
Abstract
1. Introduction
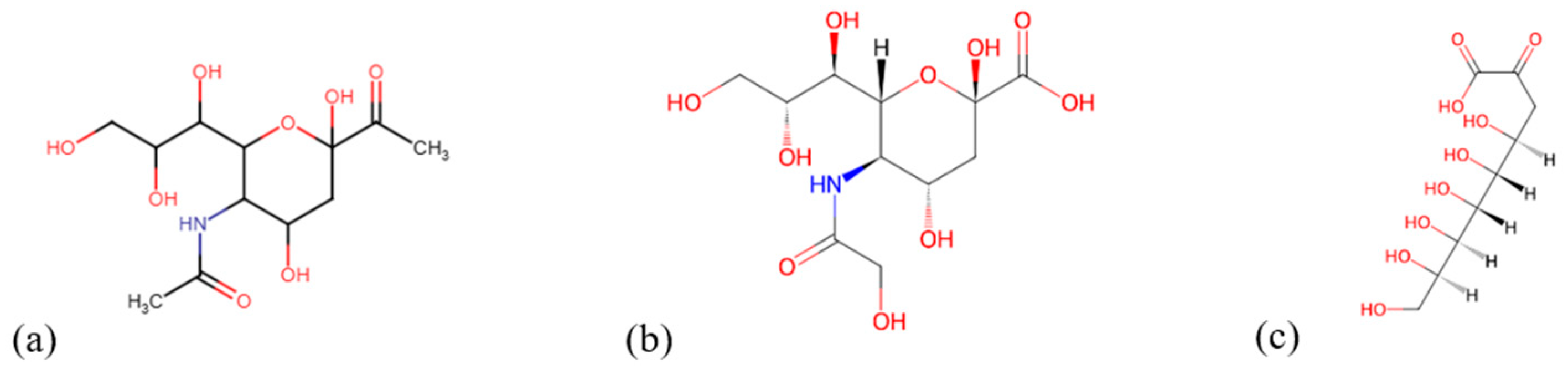
2. Sialic Acid
2.1. Chemical Structure
2.2. Functional Features
3. General Functions of Sialic Acid
3.1. Roles of Sialic Acid in the Body
3.1.1. Sialic Acids Synthesis in Cancer
3.1.2. Different Roles of Sialic Acids
3.2. Hypersialylation and Cancer
4. Acute Lymphoblastic Leukemia (ALL)
4.1. Brief Background on ALL
4.2. Molecular Mechanisms Leading to ALL
4.3. Clinical Trials and Treatments
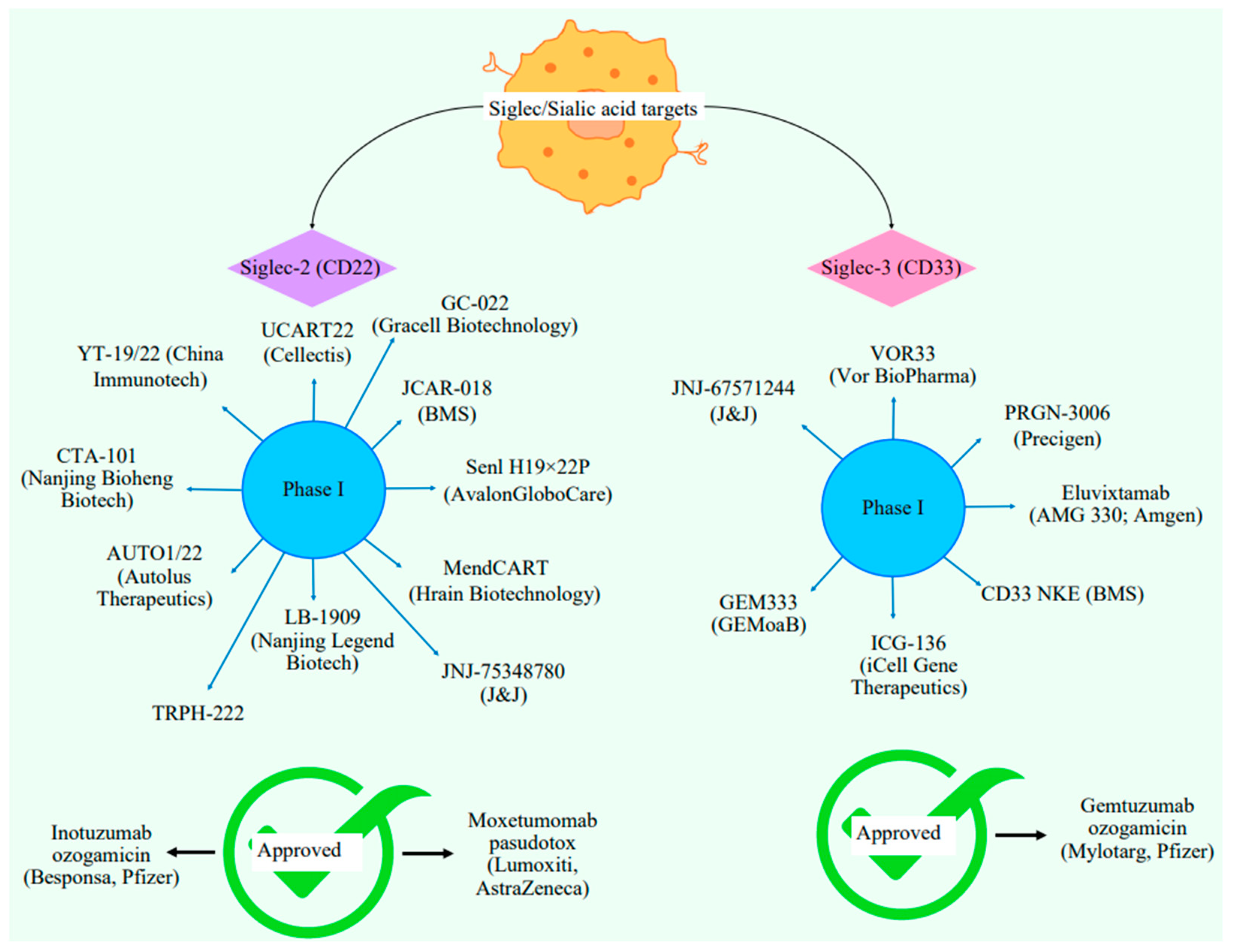
5. Siglecs Against ALL
5.1. Types of Siglecs
5.2. Siglec Checkpoint and Interplay Resulting in Immunotolerance
Medicinal Therapeutic Reports Against Host Pathogens
6. Drug Resistance in Cancer
7. Current Clinical and Approved Treatments
Immune Therapies Safe for Children
8. Conclusions and Further Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhide, G.P.; Colley, K.J. Sialylation of N-glycans: Mechanism, cellular compartmentalization & function. Histochem. Cell Biol. 2017, 147, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Varki, A. Chemical diversity in the sialic acids & related r-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Glycoproteins: Research booming on long-ignored ubiquitous compounds. Mol. Cell. Biochem. 1982, 42, 167–187. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K. Introduction to Glycobiology; Oxford University Press: Oxford, MI, USA, 2011. [Google Scholar]
- Varki, A.; Angata, T. Siglecs-the major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef]
- Lehmann, F.; Tiralongo, E.; Tiralongo, J. Sialic acid-specific lectins: Occurrence, specificity and function. Cell. Mol. Life Sci. 2006, 63, 1331–1354. [Google Scholar] [CrossRef]
- Roberts, K.G. Genetics & prognosis of ALL in children vs adults. Am. Soc. Hematol. Educ. Program 2018, 2018, 137–145. [Google Scholar] [CrossRef]
- Dinner, S.; Liedtke, M. Antibody-based therapies in patients with acute lymphoblastic leukemia. Am. Soc. Hematol. Educ. Program 2018, 2018, 9–15. [Google Scholar] [CrossRef]
- Weigel, P.H.; Yik, J.H. Glycans as endocytosis signals: The cases of the asialoglycoprotein and hyaluronan/chondroitin sulfate receptors. Biochim. Biophys. Acta Gen. 2002, 1572, 341–363. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Jastrząb, P.; Narejko, K.; Car, H.; Wielgat, P. Cell membrane sialome: Sialic acids as therapeutic targets and regulators of drug resistance in human cancer management. Cancer 2023, 15, 5103. [Google Scholar] [CrossRef] [PubMed]
- DeRenzo, C.; Krenciute, G.; Gottschalk, S. The landscape of CAR T cells beyond acute lymphoblastic leukemia for pediatric solid tumors. Hematol. Am. Soc. Hematol. Educ. Program 2018, 38, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Puckett, Y.; Chan, O. Acute Lymphocytic Leukemia; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cohen, M.; Varki, A. The sialome—Far more than the sum of its parts. J. Integr. Biol. 2010, 14, 455–464. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic acid and biology of life: An introduction. Sialic Acids Sialoglycoconjugates Biol. Life Health Dis. 2020, 2020, 1–60. [Google Scholar] [CrossRef]
- Gray, M.A.; Stanczak, M.A.; Mantuano, N.R.; Xiao, H.; Pijnenborg, J.F.A.; Malaker, S.A.; Miller, C.A.; Weidenbacher, P.A.; Tanzo, J.T.; Ahn, G.; et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 2020, 16, 1376–8134. [Google Scholar] [CrossRef]
- Ankenbauer, K.E.; Rao, T.C.; Mattheyses, A.L.; Bellis, S.L. Sialylation of EGFR by ST6GAL1 induces receptor activation and modulates trafficking dynamics. J. Biol. Chem. 2023, 299, 105217. [Google Scholar] [CrossRef]
- Fliniaux, I.; Marchand, G.; Molinaro, C.; Decloquement, M.; Martoriati, A.; Marin, M.; Bodart, J.F.; Harduin-Lepers, A.; Cailliau, K. Diversity of sialic acids and sialoglycoproteins in gametes and at fertilization. Front. Cell. Dev. Biol. 2022, 10, 982931. [Google Scholar] [CrossRef]
- Yu, Y.; Peng, W. Recent progress in targeting the sialylated glycan-SIGLEC axis in cancer immunotherapy. Cancer Biol. Med. 2020, 20, 369–384. [Google Scholar] [CrossRef]
- Visser, E.A.; Moons, S.J.; Timmermans, S.B.P.E.; de Jong, H.; Boltje, T.J.; Bull, C.J. Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021, 297, 100906. [Google Scholar] [CrossRef]
- Varki, A.; Schnaar, R.L.; Schauer, R. Sialic acids & other nonulosonic acids. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; Volume 2015–2017. [Google Scholar]
- Mahajan, V.S.; Pillai, S. Sialic acids & autoimmune disease. Immunol. Rev. 2016, 269, 145–161. [Google Scholar] [CrossRef]
- Gowthaman, U.; Eswarakumar, V.P. Molecular mimicry: Good artists copy, great artists steal. Virulence 2013, 4, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Bergfeld, A.K.; Pearce, O.M.; Diaz, S.L.; Pham, T.; Varki, A. Metabolism of vertebrate amino sugars with N-glycolyl groups: Elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J. Biol. Chem. 2012, 287, 28865–28881. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, & implications for hominid evolution. Am. J. Phys. Anthropol. 2001, 33, 54–69. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Büll, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Miyagi, T.; Takahashi, K.; Hata, K.; Shiozaki, K.; Yamaguchi, K. Sialidase significance for cancer progression. Glycoconj. J. 2012, 29, 567–577. [Google Scholar] [CrossRef]
- Dorsett, K.A.; Marciel, M.P.; Hwang, J.; Ankenbauer, K.E.; Bhalerao, N.; Bellis, S.L. Regulation of ST6GAL1 sialyltransferase expression in cancer cells. J. Glycobiol. 2021, 31, 530–539. [Google Scholar] [CrossRef]
- Davies, L.R.; Varki, A. Why is N-glycolylneuraminic acid rare in the vertebrate brain? Top. Curr. Chem. 2015, 366, 31–54. [Google Scholar] [CrossRef]
- Freud, A.G.; Caligiuri, M.A. Human natural killer cell development. Immunol. Rev. 2006, 214, 56–72. [Google Scholar] [CrossRef]
- Sato, C.; Hane, M.; Kitajima, K. Relationship between ST8SIA2, polysialic acid & its binding moleculates, & psychiatric disorders. Biochim. Biophys. Acta. 2016, 1860, 1739–1752. [Google Scholar] [CrossRef]
- Dedola, S.; Ahmadipour, S.; de Andrade, P.; Baker, A.N.; Boshra, A.N.; Chessa, S.; Gibson, M.I.; Hernando, P.J.; Ivanova, I.M.; Lloyd, J.E.; et al. Sialic acids in infection and their potential use in detection and protection against pathogens. RSC Chem. Biol. 2024, 5, 167. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.E. The enzymes of sialic acid biosynthesis. Bioorg. Chem. 2005, 33, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Feng, J.; Han, X.; Ying, Y.; Lou, W.; Liu, L.; Zhang, L. The potential of siglecs and sialic acids as biomarkers and therapeutic targets in tumor immunotherapy. Cancers 2024, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Macauley, M.S. Hypersialylation in cancer: Modulation of inflammation and therapeutic opportunities. Cancer 2018, 10, 207. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Irons, E.E.; Gc, S.; Lau, J.T. Sialic acid in the regulation of blood cell production, differentiation and turnover. Immunology 2024, 172, 517–532. [Google Scholar] [CrossRef]
- Bowles, W.H.; Gloster, T.M. Sialidase and sialyltransferase inhibitors: Targeting pathogenicity and disease. Front. Mol. Biosci. 2021, 8, 705133. [Google Scholar] [CrossRef]
- Suzuki, O. Regulation of cell adhesion to galectins by glycosylation: A new concept in lymphoma cell adhesion. Adv. Cancer Biol.-Metastasis 2021, 3, 100016. [Google Scholar] [CrossRef]
- Irons, E.E.; Cortes Gomez, E.; Andersen, V.L.; Lau, J.T. Bacterial colonization and TH17 immunity are shaped by intestinal sialylation in neonatal mice. J. Glycobiol. 2022, 32, 414–428. [Google Scholar] [CrossRef]
- Lee-Sundlov, M.M.; Ashline, D.J.; Hanneman, A.J.; Grozovsky, R.; Reinhold, V.N.; Hoffmeister, K.M.; Lau, J.T. Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. J. Glycobiol. 2017, 27, 188–198. [Google Scholar] [CrossRef]
- Yu, R.K.; Tsai, Y.T.; Ariga, T. Functional roles of gangliosides in neurodevelopment: An overview of recent advances. Neurochem. Res. 2012, 37, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides & polysialic acid in nervous system development, stability, disease, & regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Gökbuge, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an important regulator of antibody function. Front. Immunol. 2022, 13, 818736. [Google Scholar] [CrossRef]
- Pearce, O.M.; Läubli, H. Sialic acids in cancer biology and immunity. J. Glycobiol. 2016, 26, 111–128. [Google Scholar] [CrossRef]
- Natoni, A.; Macauley, M.S.; O’Dwyer, M.E. Targeting selectins and their ligands in cancer. Front. Oncol. 2016, 6, 93. [Google Scholar] [CrossRef]
- Li, R.E.; van Vliet, S.J.; Van Kooyk, Y. Using the glycan toolbox for pathogenic interventions and glycan immunotherapy. Curr. Opin. Biotechnol. 2018, 51, 24–31. [Google Scholar] [CrossRef]
- Zhang, Z.; Wuhrer, M.; Holst, S. Serum sialylation changes in cancer. Glycoconj. J. 2018, 35, 139–160. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, G.; Guan, F. Biological functions & analytical strategies of sialic acids in tumor. Cells 2020, 9, 273. [Google Scholar] [CrossRef]
- Chang, J.S.; Wiemels, J.L.; Chokkalingam, A.P.; Metayer, C.; Barcellos, L.F.; Hansen, H.M.; Aldrich, M.C.; Guha, N.; Urayama, K.Y.; Scélo, G.; et al. Genetic polymorphisms in adaptive immunity genes & childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2152–2163. [Google Scholar] [CrossRef]
- Tandon, S. Acute leukemia treatment in low- and middle-income countries: Is it time for tailored therapy? Cancer Res. Stat. Treat. 2020, 3, 642–643. [Google Scholar] [CrossRef]
- Aziz, J.; Zahid, M.; Mahmood, R.; Maqbool, S. Modified BFM protocol for childhood acute lymphoblastic leukemia: A retrospective analysis. Med. Pediatr. Oncol. 1997, 28, 48–53. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Accute Lymphocytic Leukemia (ALL). Available online: https://www.cancer.org/cancer/types/acute-lymphocytic-leukemia/about/key-statistics.html (accessed on 16 May 2023).
- Foà, R.; Chiaretti, S. Philadelphia chromosome-positive acute lymphoblastic leukemia. N. Engl. J. Med. 2022, 386, 2399–2411. [Google Scholar] [CrossRef]
- He, J.; Munir, F.; Catueno, S.; Connors, J.S.; Gibson, A.; Robusto, L.; McCall, D.; Nunez, C.; Roth, M.; Tewari, P.; et al. Biological markers of high-Risk childhood acute lymphoblastic leukemia. Cancers 2024, 16, 858. [Google Scholar] [CrossRef]
- Huang, F.L.; Liao, E.C.; Li, C.L.; Yen, C.Y.; Yu, S.J. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: Molecular pathways and disease treatments. Oncol. Lett. 2020, 20, 448–454. [Google Scholar] [CrossRef]
- Zhou, Y.; You, M.J.; Young, K.H.; Lin, P.; Lu, G.; Medeiros, L.J.; Bueso-Ramos, C.E. Advances in the molecular pathobiology of B-lymphoblastic leukemia. Hum. Pathol. 2012, 43, 1347–1362. [Google Scholar] [CrossRef]
- Cobaleda, C.; Godley, L.A.; Nichols, K.E.; Wlodarski, M.W.; Sanchez-Garcia, I. Insights into the Molecular Mechanisms of Genetic Predisposition to Hematopoietic Malignancies: The Importance of Gene–Environment Interactions. Cancer Discov. 2024, 14, 396–405. [Google Scholar] [CrossRef]
- Mahmood, N.; Shahid, S.; Bakhshi, T.; Riaz, S.; Ghufran, H.; Yaqoob, M. Identification of significant risks in pediatric acute lymphoblastic (ALL) through machine learning (ML approach). Med. Biol. Eng. Comput. 2020, 58, 2631–2640. [Google Scholar] [CrossRef]
- Jensen, C.D.; Block, G.; Buffler, P.; Ma, X.; Selvin, S.; Month, S. Maternal dietary risk factors in childhood acute lymphoblastic leukemia (United States). Cancer Causes Control 2004, 15, 559–570. [Google Scholar] [CrossRef]
- Hoelzer, D.; Bassan, R.; Dombret, H.; Fielding, A.; Ribera, J.M.; Buske, C. ESMO Guidelines Committee. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment & follow-up. Ann. Oncol. 2016, 27, 69–82. [Google Scholar] [CrossRef]
- Heo, Y.-A.; Syed, Y.Y.; Keam, S.J. Pegaspargase: A review in acute lymbhoblastic leukaemia. Drugs 2019, 79, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yang, Y.; Weng, L.; Wu, Q.; Zhang, J.; Zao, P.; Fang, L.; Shi, Y.; Wang, P. Emerging phagocytosis checkpoints in cancer immuno therapy. Signal Transduct. Target. Ther. 2023, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Webster, R. The immune checkpoint inhibitors: Where are we now? Nat. Rev. Drug Discov. 2014, 13, 883–884. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, H.; Fan, Y.; Liu, Q.; Song, J.; Yao, S.; Cao, F. Immune and clinical features of CD96 expression in glioma by in silico analysis. Front. Bioeng. Biotechnol. 2020, 8, 592. [Google Scholar] [CrossRef]
- Liotta, F.; Angeli, R.; Cosmi, L.; Fili, L.; Manuelli, C.; Frosali, F.; Mazzinghu, B.; Maggi, L.; Pasini, A.; Lisi, V.; et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 2009, 26, 279–289. [Google Scholar] [CrossRef]
- Lin, H.; Kryczek, I.; Li, S.; Green, M.D.; Ali, A.; Hammasha, R.; Wei, S.; Vatan, L.; Szeliga, W.; Grove, S.; et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell 2021, 39, 480–493.e6. [Google Scholar] [CrossRef]
- Kegyes, D.; Jitaru, C.; Ghiaur, G.; Ciurea, S.; Hoelzer, D.; Tomuleasa, C.; Gale, R.P. Switching from salvage chemotherapy to immunotherapy in adult B-cell acute lymphoblastic leukemia. Blood Rev. 2023, 59, 101042. [Google Scholar] [CrossRef]
- Iacobucci, I.; Mullighan, C.G. Genetic basis of acute lymphoblastic leukemia. J. Clin. Oncol. 2017, 35, 975–983. [Google Scholar] [CrossRef]
- Bloom, M.; Maciaszek, J.L.; Clark, M.E.; Pui, C.-H.; Nichols, K.E. Recent advances in genetic predisposition to pediatric acute lymphoblastic leukemia. Expert Rev. Hematol. 2020, 13, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, P.; Kaufmann, T. Sialic acids & their influence on human NK cell function. Cells 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Clark, E.A.; Filbin, M.; Gordon, S.; Jones, Y.; Kehrl, J.H.; Kelm, S.; Le Douarin, N.; Powell, L.; Roder, J.; et al. Siglecs: A family of sialic-acid binding lectins. J. Glycobiol. 1998, 8, 5–6. [Google Scholar] [CrossRef]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef]
- Movsisyan, L.D.; Macauley, M.S. Structural advances of siglecs: Insight into synthetic glycan ligands for immunomodulation. Org. Biomol. Chem. 2020, 18, 5784–5797. [Google Scholar] [CrossRef]
- Dharmadhikari, G.; Stolz, K.; Hauke, M.; Morgan, N.G.; Varki, A.; De Koning, E.; Kelm, S.; Maedler, K. Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci. Rep. 2017, 7, 45319. [Google Scholar] [CrossRef]
- Filipsky, F.; Läubli, H. Regulation of sialic acid metabolism in cancer. Carbohydr. Res. 2024, 539, 109123. [Google Scholar] [CrossRef]
- Oliveira, T.; Zhang, M.; Joo, E.J.; Abdel-Azim, H.; Chen, C.W.; Yang, L.; Chou, C.H.; Qin, X.; Chen, J.; Alagesan, K.; et al. Glycoproteome remodeling in MLL-rearranged B-cell precursor acute lymphoblastic leukemia. Theranostics 2021, 11, 9519–9537. [Google Scholar] [CrossRef]
- Lanza, F.; Maffini, E.; Rondoni, M.; Massari, E.; Faini, A.C.; Malavasi, F. CD22 Expression in B-Cell Acute Lymphoblastic Leukemia: Biological Significance and Implications for Inotuzumab Therapy in Adults. Cancers 2020, 12, 303. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Arena, A.; Fierabracci, A. Sialic acid-siglec axis in human immune regulation, involvement in autoimmunity and cancer and potential therapeutic treatments. Int. J. Mol. Sci. 2021, 22, 5774. [Google Scholar] [CrossRef]
- Nicoll, G.; Avril, T.; Lock, K.; Furukawa, K.; Bovin, N.; Crocker, P.R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and-independent mechanisms. Eur. J. Immunol. 2003, 33, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Démoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell–dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef]
- Läubli, H.; Pearce, O.M.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef]
- Lanza, F. Issue Highlight—July 2018. Cytom. Part B Clin. Cytom. 2018, 94, 557–560. [Google Scholar] [CrossRef][Green Version]
- Adachi, T.; Harumiya, S.; Takematsu, H.; Kozutsumi, Y.; Wabl, M.; Fujimoto, M.; Tedder, T.F. CD22 serves as a receptor for soluble IgM. Eur. J. Immunol. 2012, 42, 241–247. [Google Scholar] [CrossRef]
- Lunn, M.P.; Nobile-Orazio, E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst. Rev. 2016, 10, CD002827. [Google Scholar] [CrossRef]
- Campagnolo, M.; Zambello, R.; Nobile-Orazio, E.; Benedetti, L.; Marfia, G.A.; Riva, N.; Castellani, F.; Bianco, M.; Salvalaggio, A.; Garnero, M.; et al. IgM MGUS and Waldenstrom-associated anti-MAG neuropathies display similar response to rituximab therapy. J. Neurol. Neurosurg. Psychiatry 2017, 88, 1094–1097. [Google Scholar] [CrossRef]
- Briani, C.; Visentin, A.; Salvalaggio, A.; Cacciavillani, M.; Trentin, L. Obinutuzumab, a new anti-CD20 antibody, and chlorambucil are active and effective in anti-myelin-associated glycoprotein antibody polyneuropathy. Eur. J. Neurol. 2018, 26, 371–375. [Google Scholar] [CrossRef]
- Kimbara, S.; Kondo, S. Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma. World J. Gastroenterol. 2016, 22, 7440–7452. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor microenvironment: Lactic acid promotes tumor development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Wei, A.; Fan, B.; Zhao, Y.; Zhang, H.; Wang, L.; Yu, X.; Yuan, Q.; Yang, D.; Wang, S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin signaling pathway. Oncotarget 2016, 7, 65374. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mandal, C.; Chatterjee, U.; Mandal, C. Association of cytosolic sialidase Neu2 with plasma membrane enhances Fas-mediated apoptosis by impairing PI3K-Akt/mTOR-mediated pathway in pancreatic cancer cells. Cell Death Dis. 2018, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yao, S.; Hu, Y.; Feng, Y.; Li, M.; Bian, Z.; Zhang, J.; Qin, Y.; Qi, X.; Zhou, L.; et al. The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin. Cancer Res. 2017, 23, 7375–7387. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Zhang, H.; Zhang, P.; Zheng, Y.S.; Zhang, X.J.; Han, B.W.; Luo, X.Q.; Xu, L.; Zhou, H.; Qu, L.H.; et al. Down-regulated miR-331–5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J. Cell. Mol. Med. 2011, 15, 2164–2175. [Google Scholar] [CrossRef]
- Kawagashira, Y.; Koike, H.; Tomita, M.; Morozumi, S.; Iijima, M.; Nakamura, T.; Katsuno, M.; Tanaka, F.; Sobue, G. Morphological progression of myelin abnormalities in IgM-monoclonal gammopathy of undetermined significance anti-myelin-associated glycoprotein neuropathy. J. Neuropathol. Exp. Neurol. 2010, 69, 1143–1157. [Google Scholar] [CrossRef]
- Blixt, O.; Collins, B.E.; van den Nieuwenhof, I.M.; Crocker, P.R.; Paulson, J.C. Sialoside specificity of the siglec family assessed using novel multivalent probes. Am. J. Biochem. Mol. Biol. 2003, 278, 31007–31019. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef]
- Lenza, M.P.; Atxabal, U.; Oyenarte, I.; Jimenez-Barbero, J.; Eleonore, J. Current status on therapeutic molecules targeting Siglec receptors. Cells 2020, 9, 2691. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Tallman, M.S.; Robak, T.; Coutre, S.; Wilson, W.H.; Stetler-Stevenson, M.; Fitzgerald, D.J.; Lechleider, R.; Pastan, I. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J. Clin. Oncol. 2012, 30, 1822–1828. [Google Scholar] [CrossRef]
- Einsele, H.; Borghaei, H.; Orlowski, R.Z.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020, 126, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Slaney, C.Y.; Wang, P.; Darcy, P.K.; Kershaw, M.H. CARs versus BiTEs: A comparison between T cell-redirection strategies for cancer treatment. Cancer Discov. 2018, 8, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Queudeville, M.; Ebinger, M. Blinatumomab in pediatric acute lymphoblastic leukemia-from salvage to first line therapy (A systematic review). J. Clin. Med. 2021, 10, 2544. [Google Scholar] [CrossRef] [PubMed]
- Von Stackelberg, A.; Locatelli, F.; Zugmaier, G.; Handgretinger, R.; Trippett, T.M.; Rizzari, C.; Bader, P.; O’Brien, M.M.; Brethon, B.; Bhojwani, D.; et al. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J. Clin. Oncol. 2016, 34, 4381–4389. [Google Scholar] [CrossRef]
- Läubli, H.; Nalle, S.C.; Maslyar, D. Targeting the siglec–sialic acid immune axis in cancer: Current and future approaches. Cancer Immunol. Res. 2022, 10, 1423–1432. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Patel, S.; Muffly, L.; Hossain, N.M.; Oak, J.; Baird, J.H.; Frank, M.J.; Shiraz, P.; Sahaf, B.; Craig, J.; et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat. Med. 2021, 27, 1419–1431. [Google Scholar] [CrossRef]
- Levine, J.E.; Grupp, S.A.; Pulsipher, M.A.; DIetz, A.C.; Rives, S.; Myers, G.D.; August, K.J.; Verneris, M.R.; Buechner, J.; Laetsch, T.W.; et al. Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukemia. J. Immunother. Cancer 2021, 9, e002287. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, K.R.; Baek, K.-H. Insights on the Role of Sialic Acids in Acute Lymphoblastic Leukemia in Children. Int. J. Mol. Sci. 2025, 26, 2233. https://doi.org/10.3390/ijms26052233
Radu KR, Baek K-H. Insights on the Role of Sialic Acids in Acute Lymphoblastic Leukemia in Children. International Journal of Molecular Sciences. 2025; 26(5):2233. https://doi.org/10.3390/ijms26052233
Chicago/Turabian StyleRadu, Kimberley Rinai, and Kwang-Hyun Baek. 2025. "Insights on the Role of Sialic Acids in Acute Lymphoblastic Leukemia in Children" International Journal of Molecular Sciences 26, no. 5: 2233. https://doi.org/10.3390/ijms26052233
APA StyleRadu, K. R., & Baek, K.-H. (2025). Insights on the Role of Sialic Acids in Acute Lymphoblastic Leukemia in Children. International Journal of Molecular Sciences, 26(5), 2233. https://doi.org/10.3390/ijms26052233






