Graphene-Based Far-Infrared Therapy Promotes Adipose Tissue Thermogenesis and UCP1 Activation to Combat Obesity in Mice
Abstract
1. Introduction
2. Results
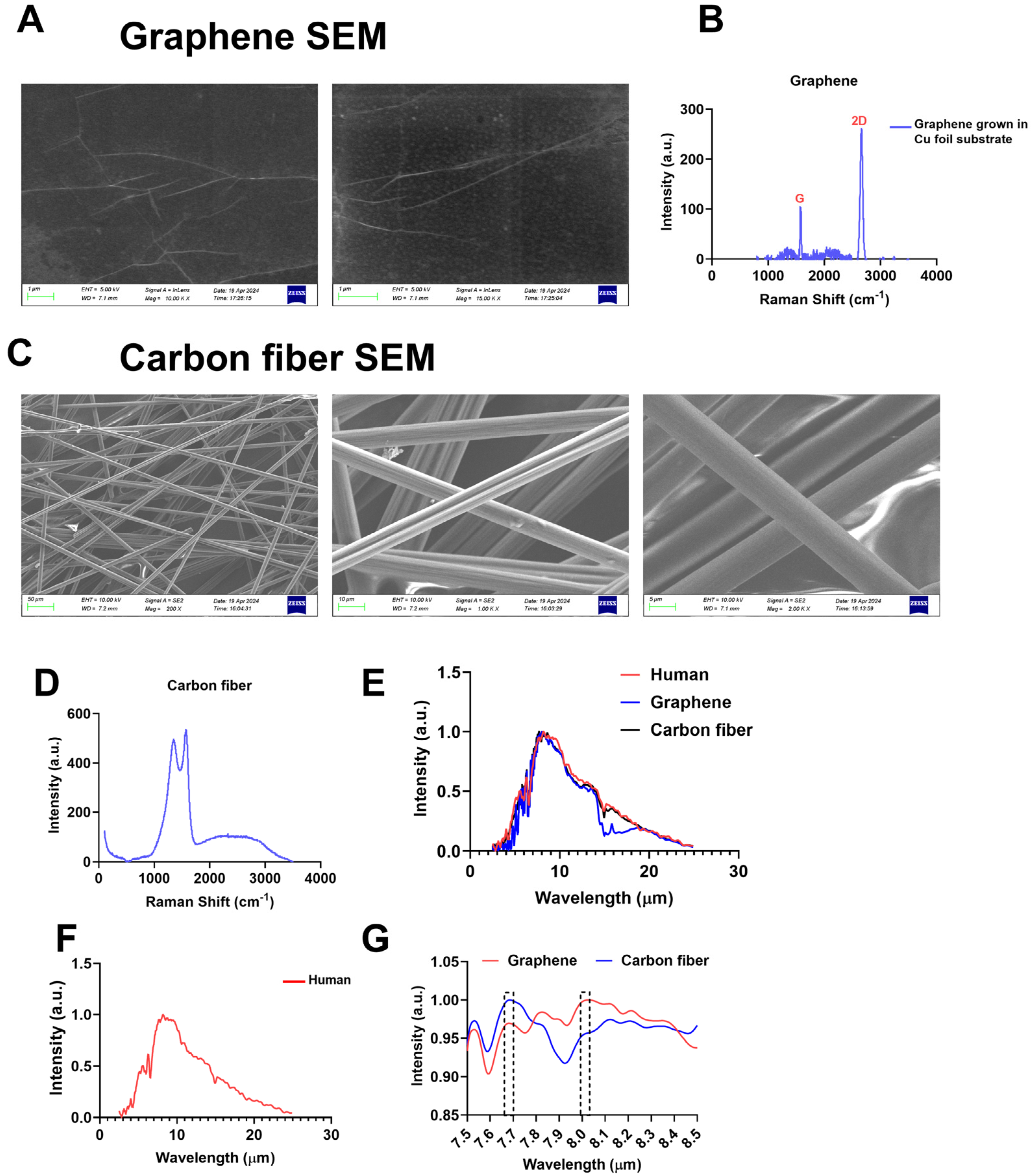
2.1. Materialistics of Graphene and Carbon Fibers
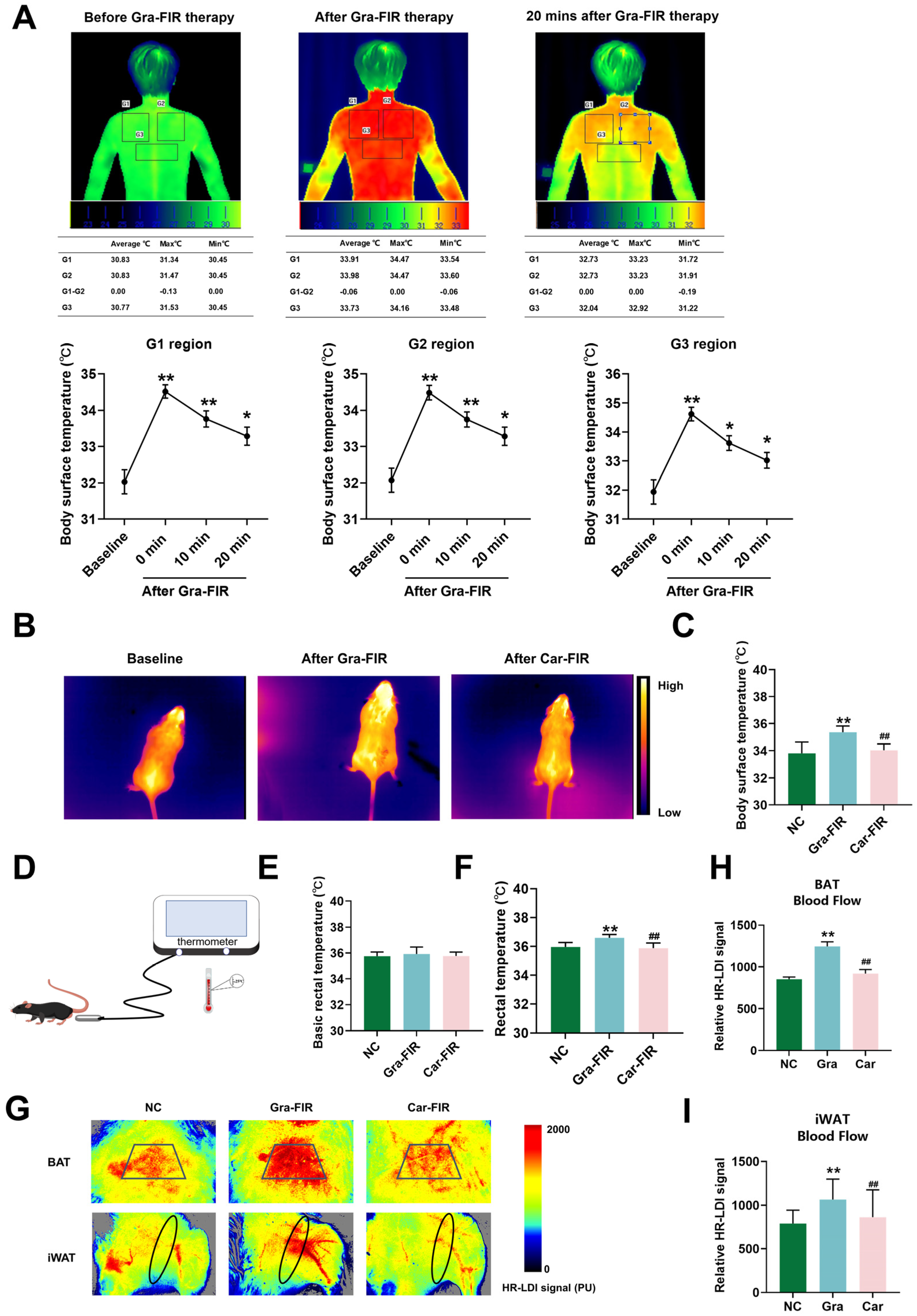
2.2. Graphene-FIR Therapy Can Increase Body Temperature and Blood Flow
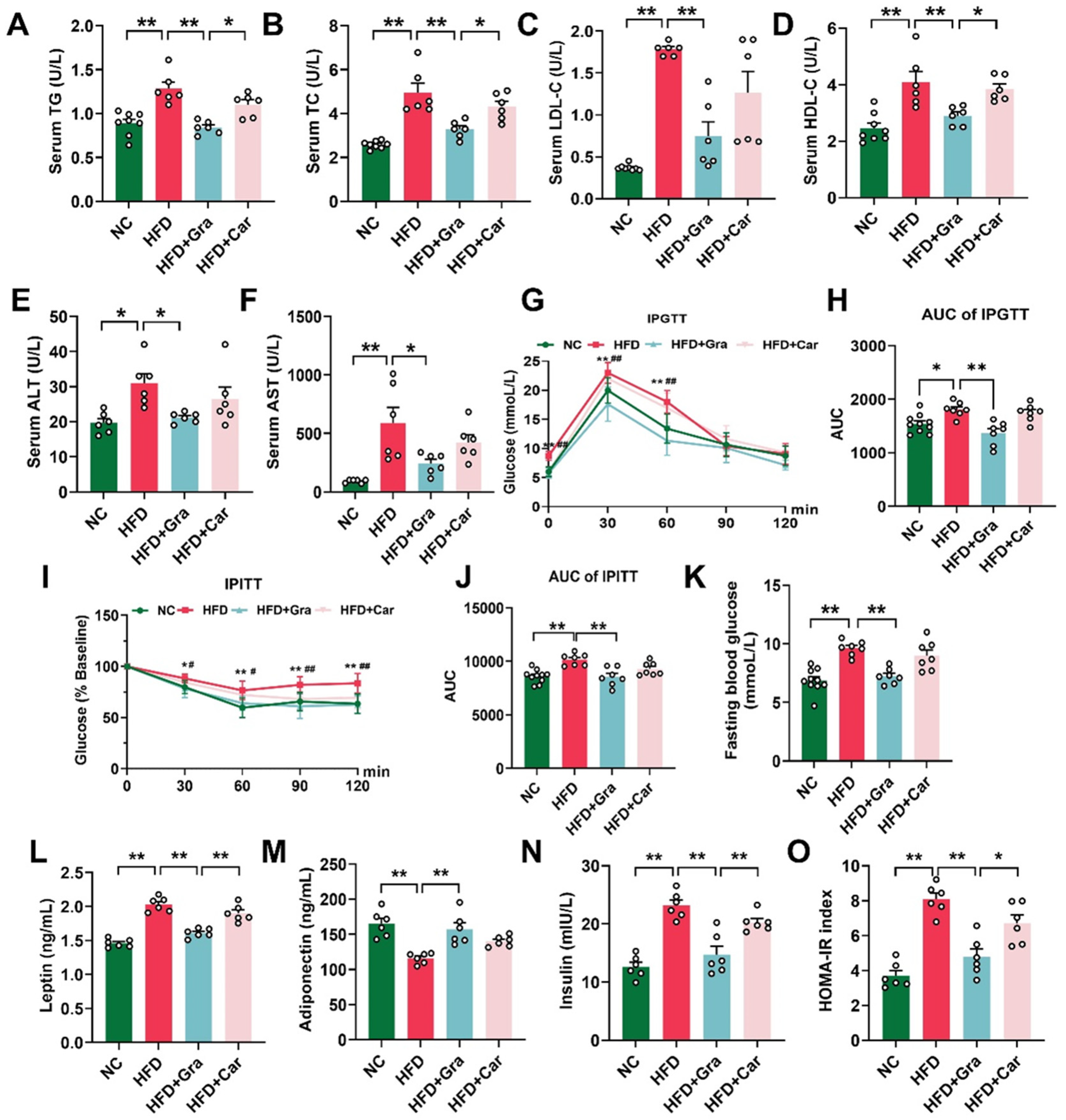
2.3. Graphene-FIR Therapy Can Improve Dyslipidemia and Insulin Resistance in HFD Mice
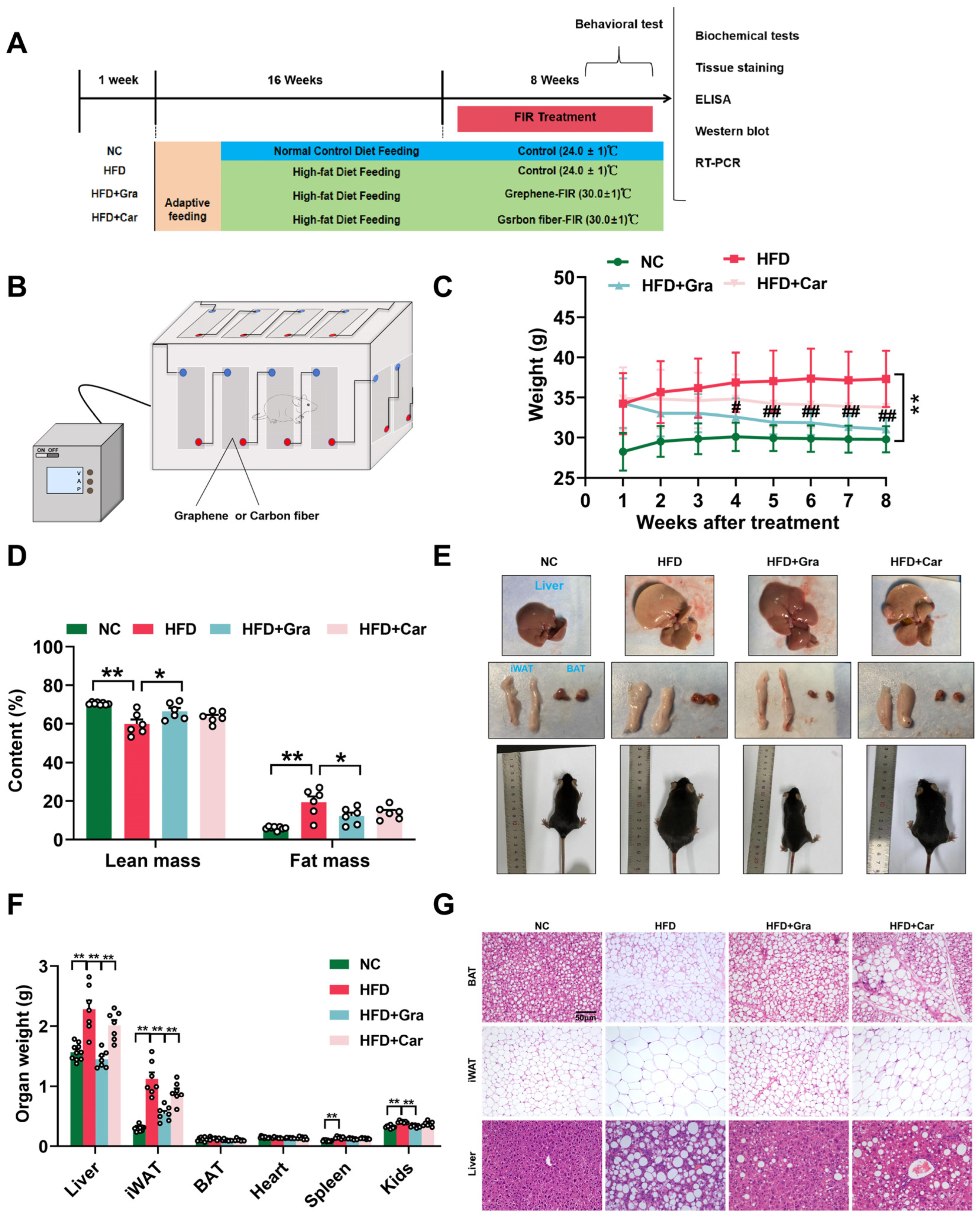
2.4. Graphene-FIR Therapy Has No Impact on Energy Intake but Increases EE in HFD Mice
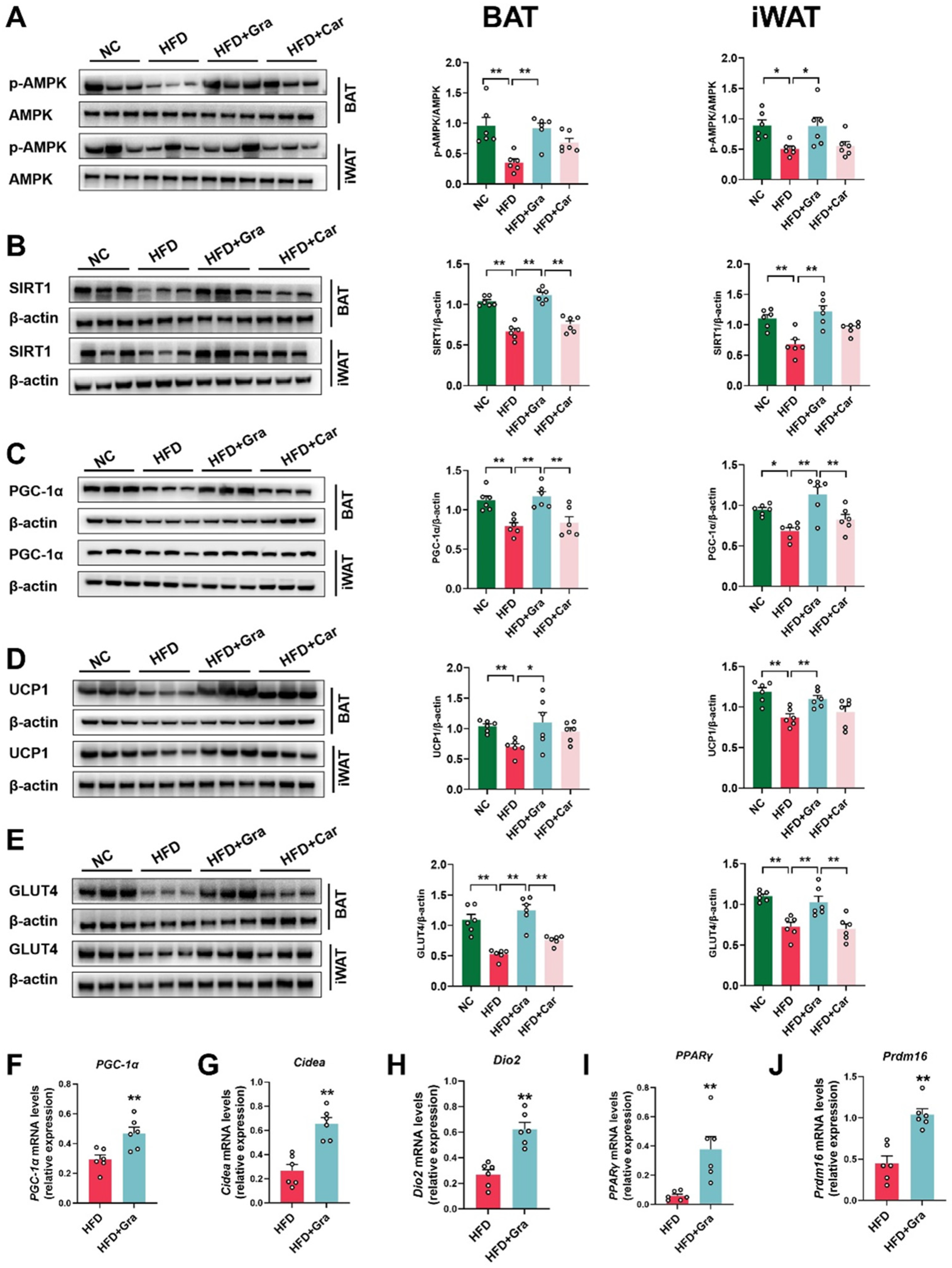
2.5. Graphene-FIR Therapy Can Activate Adipose Tissue Thermogenic Program and Increase AMPK/SIRT1/UCP1 Protein Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mice Experiments
4.3. Hyperthermia Therapy in Human Subjects
4.4. Glucose Tolerance Test (GTT) and Insulin Resistance Test (ITT)
4.5. Cold-Induced Thermogenesis
4.6. Body Composition Analysis
4.7. Determination of EE by Indirect Calorimetry
4.8. Biochemical Analyses
4.9. Tissue Staining
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. RNA Extraction and Quantitative Real-Time PCR
4.12. Western Blot
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alarming-Projection-by-2035-More-Than-Half-of-The-Global-Population-Will-Be-Obese [M/OL]. Miami, World Obesity Federation. 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 15 March 2023).
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue-A Translational Perspective. Endocr. Rev. 2023, 44, 143–192. [Google Scholar] [PubMed]
- Yan, C.; Zeng, T.; Lee, K.; Nobis, M.; Loh, K.; Gou, L.; Xia, Z.; Gao, Z.; Bensellam, M.; Hughes, W.; et al. Peripheral-specific Y1 receptor antagonism increases thermogenesis and protects against diet-induced obesity. Nat. Commun. 2021, 12, 2622. [Google Scholar] [CrossRef]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.-E.; Mantzoros, C.S. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metabolism 2019, 92, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.B.; Nie, Y.M.; Feng, G.P.; Deng, M.J.; Hu, Y.M.; Zhang, H.J.; Zhao, Y.Y.; Feng, Y.W.; Yu, T.T.; et al. Self-Organization Formation of Multicellular Spheroids Mediated by Mechanically Tunable Hydrogel Platform: Toward Revealing the Synergy of Chemo- and Noninvasive Photothermal Therapy against Colon Microtumor. Macromol. Biosci. 2022, 22, e2100498. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Ping, X.; Zhang, Y.; Zhang, T.; Wang, L.; Jin, L.; Zhao, W.; Guo, M.; Shen, F.; et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell 2022, 185, 949–966.e19. [Google Scholar] [CrossRef]
- Chevalier, C.; Kieser, S.; Çolakoğlu, M.; Hadadi, N.; Brun, J.; Rigo, D.; Suárez-Zamorano, N.; Spiljar, M.; Fabbiano, S.; Busse, B.; et al. Warmth Prevents Bone Loss Through the Gut Microbiota. Cell Metab. 2020, 32, 575–590.e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, M.C.; Hsu, Y.H.; Chou, T.C. Far-infrared radiation alleviates cisplatin-induced vascular damage and impaired circulation via activation of HIF-1α. Cancer Sci. 2022, 113, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Miao, X.-Y.; Zhang, J.-S.; Wei, D.-D.; Dong, H.-J.; Xue, R.; Li, J.-C.; Zhang, Y.; Feng, X.-X.; Li, J.; et al. Far-infrared therapy promotes exercise capacity and glucose metabolism in mice by modulating microbiota homeostasis and activating AMPK. Sci. Rep. 2024, 14, 16314. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Li, S.; Cheng, X.; Tan, X.-C.; Huang, Y.-L.; Dong, H.-J.; Xue, R.; Zhang, Y.; Li, J.-C.; Feng, X.-X.; et al. Far-Infrared Therapy Based on Graphene Ameliorates High-Fat Diet-Induced Anxiety-Like Behavior in Obese Mice via Alleviating Intestinal Barrier Damage and Neuroinflammation. Neurochem. Res. 2024, 49, 1735–1750. [Google Scholar] [CrossRef]
- Göransson, O.; Kopietz, F.; Rider, M.H. Metabolic control by AMPK in white adipose tissue. Trends Endocrinol. Metab. 2023, 34, 704–717. [Google Scholar] [CrossRef]
- Zhang, C.-S.; Li, M.; Wang, Y.; Li, X.; Zong, Y.; Long, S.; Zhang, M.; Feng, J.-W.; Wei, X.; Liu, Y.-H.; et al. The aldolase inhibitor aldometanib mimics glucose starvation to activate lysosomal AMPK. Nat. Metab. 2022, 4, 1369–1401. [Google Scholar] [CrossRef]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.; Xu, Z.; Liu, H.; Gu, L.Q.; Ye, J.M.; Huang, Z.S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef]
- Zhang, Q.; He, C.X.; Wang, L.Y.; Qian, D.; Tang, D.D.; Jiang, S.N.; Chen, W.W.; Wu, C.J.; Peng, W. Hydroxy-α-sanshool from the fruits of Zanthoxylum bungeanum Maxim. promotes browning of white fat by activating TRPV1 to induce PPAR-γ deacetylation. Phytomedicine 2023, 121, 155113. [Google Scholar] [CrossRef]
- Zhang, E.; Jin, L.; Wang, Y.; Tu, J.; Zheng, R.; Ding, L.; Fang, Z.; Fan, M.; Al-Abdullah, I.; Natarajan, R.; et al. Intestinal AMPK modulation of microbiota mediates crosstalk with brown fat to control thermogenesis. Nat. Commun. 2022, 13, 1135. [Google Scholar] [CrossRef]
- Li, R.; Xue, Z.; Li, S.; Zhou, J.; Liu, J.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Mulberry leaf polysaccharides ameliorate obesity through activation of brown adipose tissue and modulation of the gut microbiota in high-fat diet fed mice. Food Funct. 2022, 13, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Lu, H.; You, Y.; Zhou, X.; He, Q.; Wang, M.; Chen, L.; Zhou, L.; Sun, X.; Liu, Y.; Jiang, P.; et al. Citrus reticulatae pericarpium Extract Decreases the Susceptibility to HFD-Induced Glycolipid Metabolism Disorder in Mice Exposed to Azithromycin in Early Life. Front. Immunol. 2021, 12, 774433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, X.; Lin, S.; Xu, H.; Liang, X.; Wang, Y.; Xu, J.; Wang, K.; Guo, X.; Wang, J.; et al. Limosilactobacillus reuteri and caffeoylquinic acid synergistically promote adipose browning and ameliorate obesity-associated disorders. Microbiome 2022, 10, 226. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- van Baak, M.A.; Mariman, E.C.M. Mechanisms of weight regain after weight loss—The role of adipose tissue. Nat. Rev. Endocrinol. 2019, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Ji, L.; Zhao, Y.; He, L.; Zhao, J.; Gao, T.; Liu, F.; Qi, B.; Kang, F.; Wang, G.; Zhao, Y.; et al. AKAP1 Deficiency Attenuates Diet-Induced Obesity and Insulin Resistance by Promoting Fatty Acid Oxidation and Thermogenesis in Brown Adipocytes. Adv. Sci. 2021, 8, 2002794. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, H.; Ren, L.; Li, Q.; Li, N. Animal models for the atherosclerosis research: A review. Protein Cell 2011, 2, 189–201. [Google Scholar]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 2021, 23, 218. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Rajitha, B.; Aliya, S.; Kotipatruni, R.P.; Madanraj, A.S.; Hammond, A.; Park, D.; Chigurupati, S.; Alam, A.; Pattnaik, S. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor. Rev. 2016, 31, 37–48. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.J.; Enerbäck, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 2018, 14, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, M.T.; Myung, C.S. Garcinia Cambogia Improves High-Fat Diet-Induced Glucose Imbalance by Enhancing Calcium/CaMKII/AMPK/GLUT4-Mediated Glucose Uptake in Skeletal Muscle. Mol. Nutr. Food Res. 2022, 66, e2100669. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Zhang, X.; Zhao, B.; Burley, G.; Yang, Z.; Luo, Y.; Li, A.; Zhang, R.; Liu, Z.; et al. The combined effect of metformin and mirabegron on diet-induced obesity. MedComm 2023, 4, e207. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, S.; Cheng, X.; Tan, X.; Shi, Y.; Su, G.; Huang, Y.; Zhang, Y.; Xue, R.; Li, J.; et al. Graphene-Based Far-Infrared Therapy Promotes Adipose Tissue Thermogenesis and UCP1 Activation to Combat Obesity in Mice. Int. J. Mol. Sci. 2025, 26, 2225. https://doi.org/10.3390/ijms26052225
Zhang J, Li S, Cheng X, Tan X, Shi Y, Su G, Huang Y, Zhang Y, Xue R, Li J, et al. Graphene-Based Far-Infrared Therapy Promotes Adipose Tissue Thermogenesis and UCP1 Activation to Combat Obesity in Mice. International Journal of Molecular Sciences. 2025; 26(5):2225. https://doi.org/10.3390/ijms26052225
Chicago/Turabian StyleZhang, Jinshui, Shuo Li, Xin Cheng, Xiaocui Tan, Yingxian Shi, Guixin Su, Yulong Huang, Yang Zhang, Rui Xue, Jingcao Li, and et al. 2025. "Graphene-Based Far-Infrared Therapy Promotes Adipose Tissue Thermogenesis and UCP1 Activation to Combat Obesity in Mice" International Journal of Molecular Sciences 26, no. 5: 2225. https://doi.org/10.3390/ijms26052225
APA StyleZhang, J., Li, S., Cheng, X., Tan, X., Shi, Y., Su, G., Huang, Y., Zhang, Y., Xue, R., Li, J., Fan, Q., Dong, H., Deng, Y., & Zhang, Y. (2025). Graphene-Based Far-Infrared Therapy Promotes Adipose Tissue Thermogenesis and UCP1 Activation to Combat Obesity in Mice. International Journal of Molecular Sciences, 26(5), 2225. https://doi.org/10.3390/ijms26052225




