Hippo Signaling Regulates High-NaCl-Induced Increase in RORγt+ Pro-Inflammatory Lymphocytes
Abstract
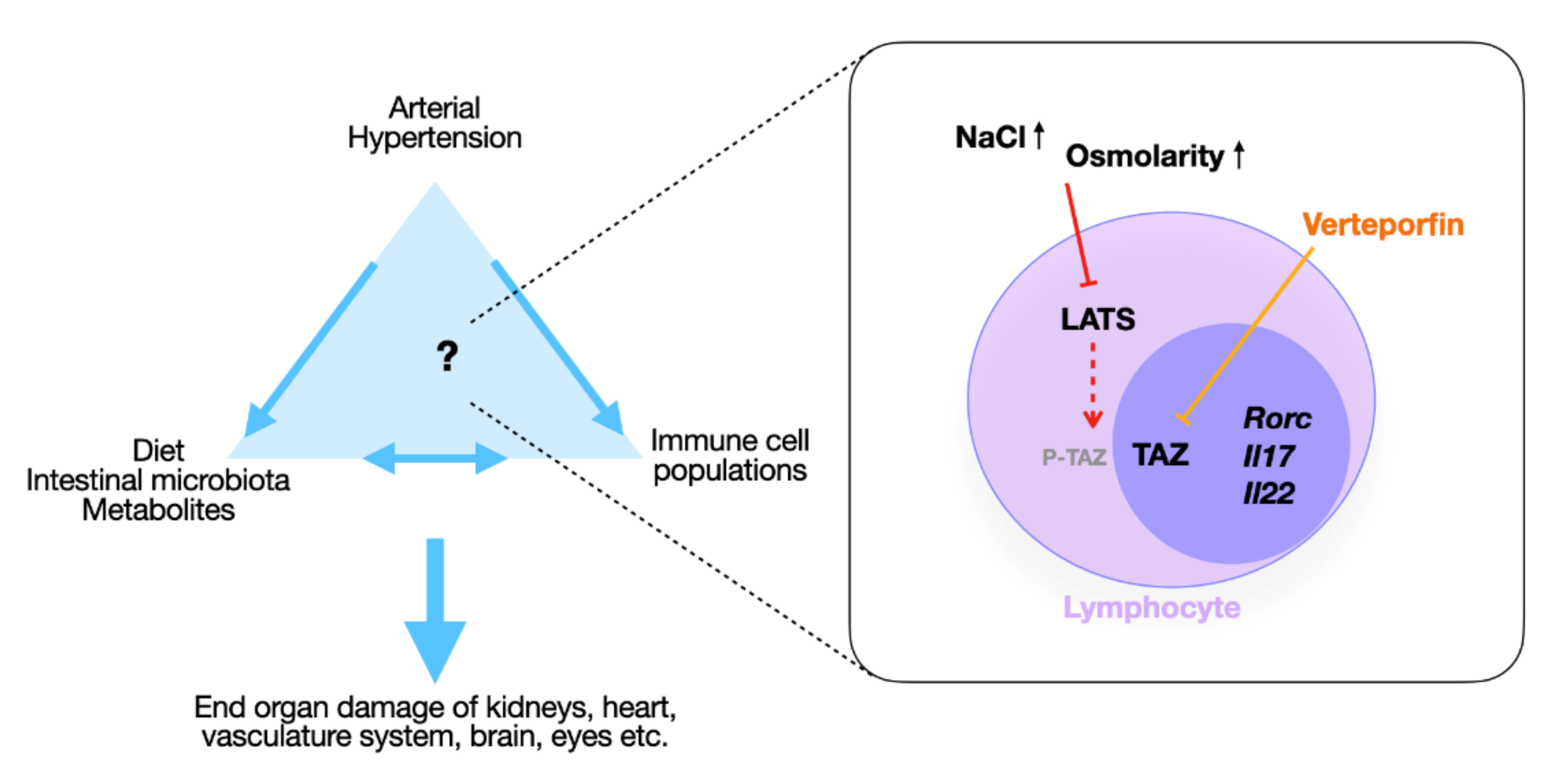
1. Introduction
2. Results
2.1. Ang II and High NaCl Increased RORγt-Positive T Lymphocytes In Vivo
2.2. NaCl but Not Ang II Increases RORγt-Expressing Splenic Lymphocytes
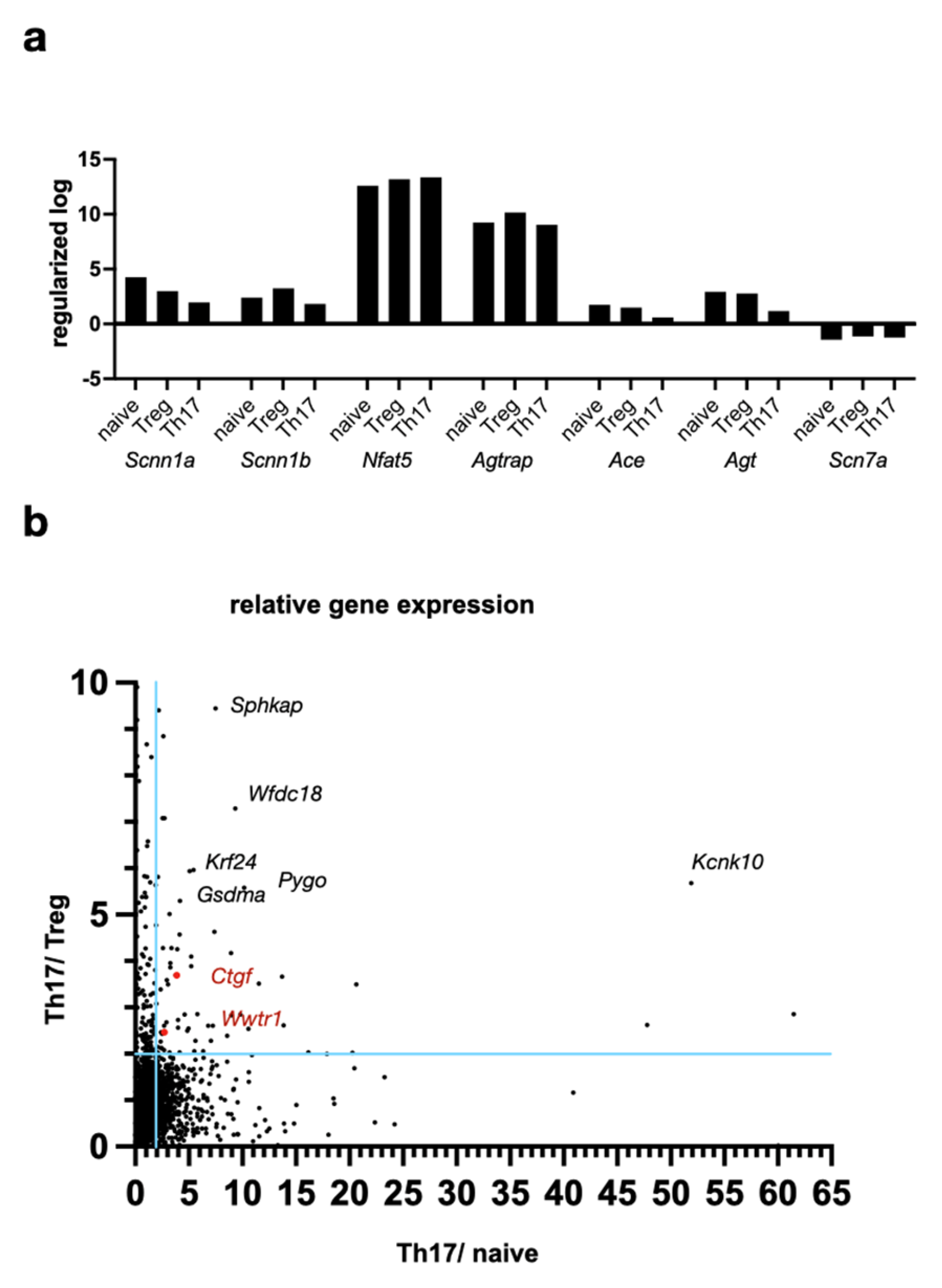
2.3. Hippo Pathway Transcription Factor TAZ Is Overexpressed in Th17 Compared to Treg Lymphocytes
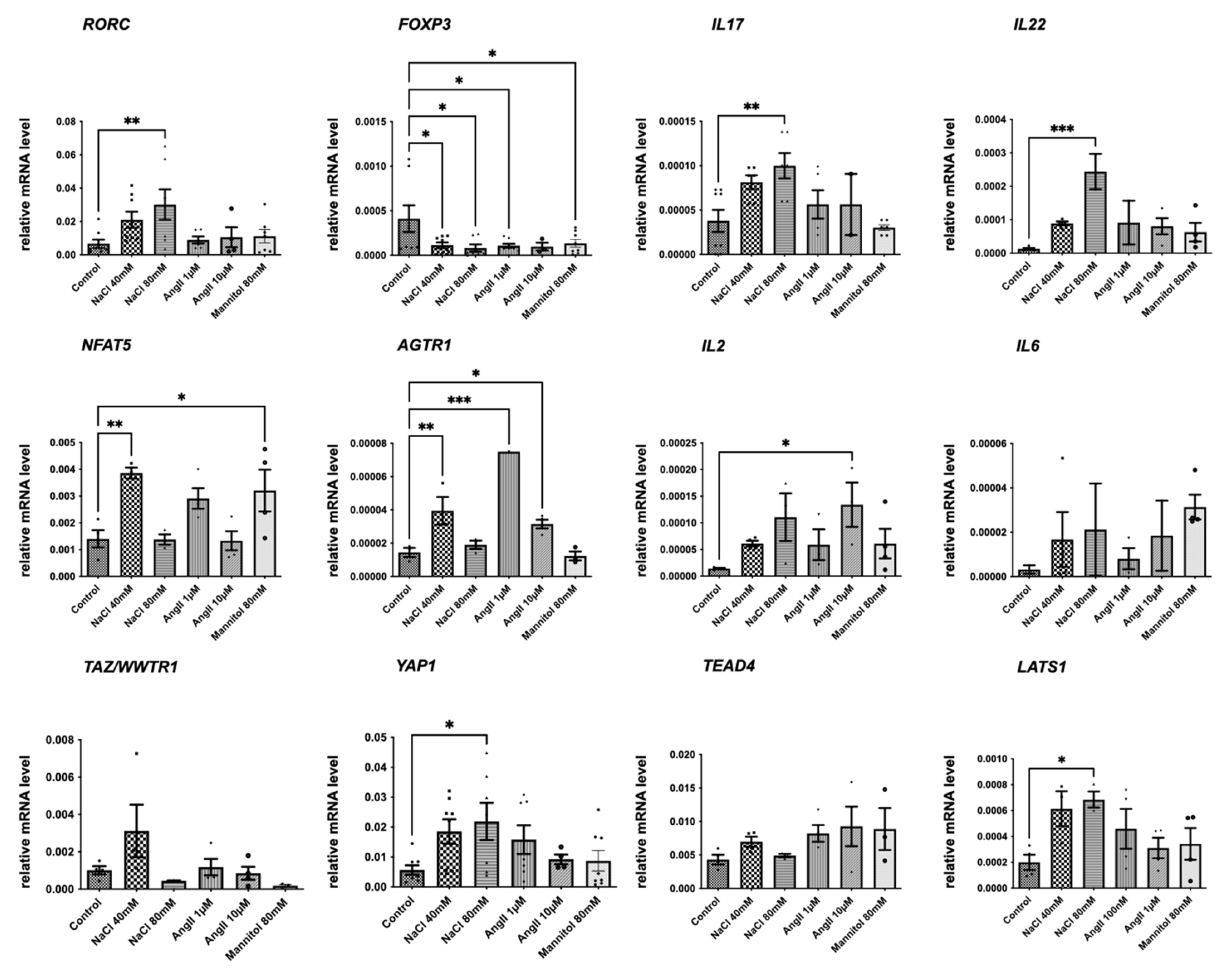
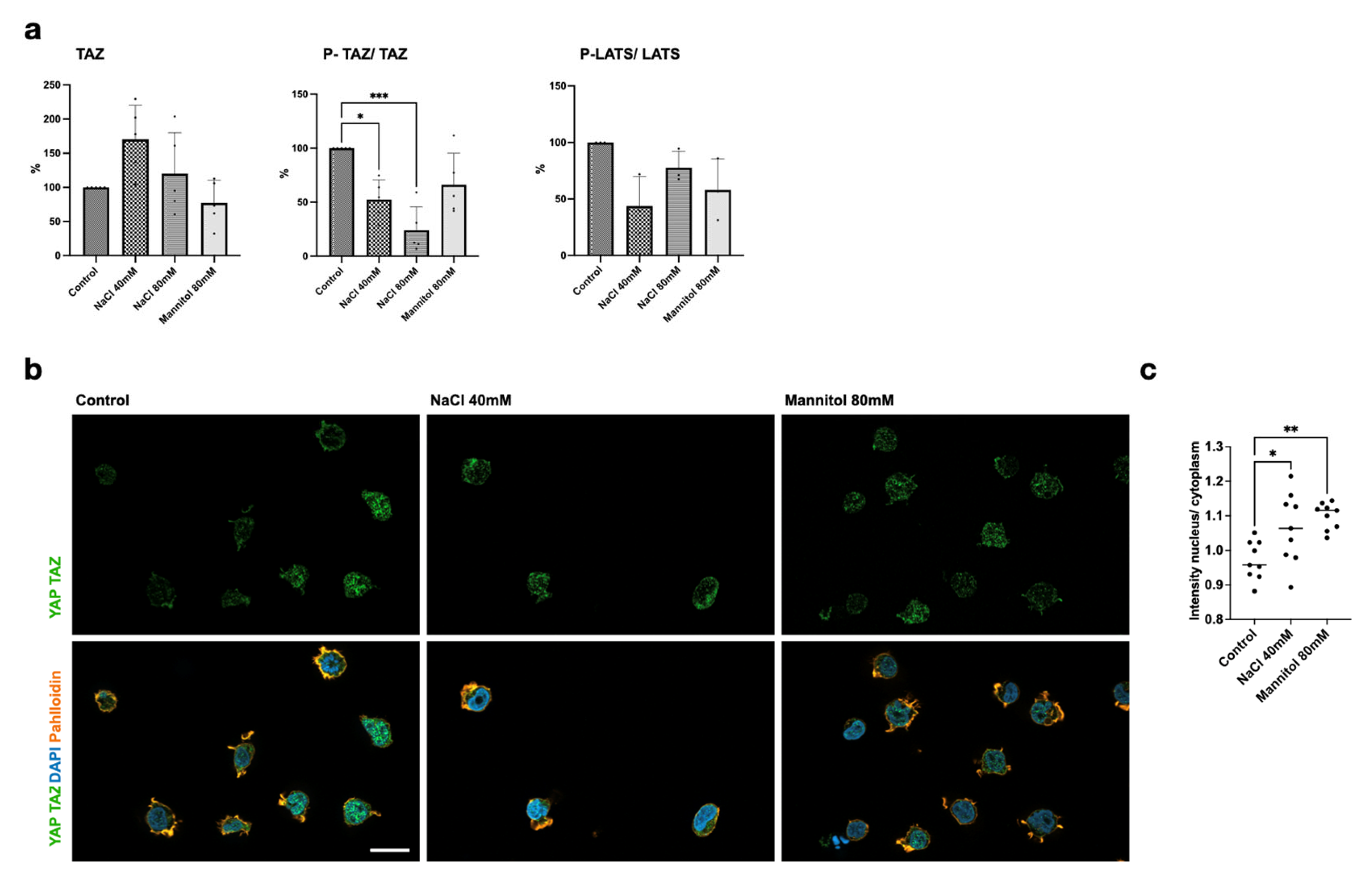
2.4. NaCl Induces Hippo Pathway Activation in Lymphocytes
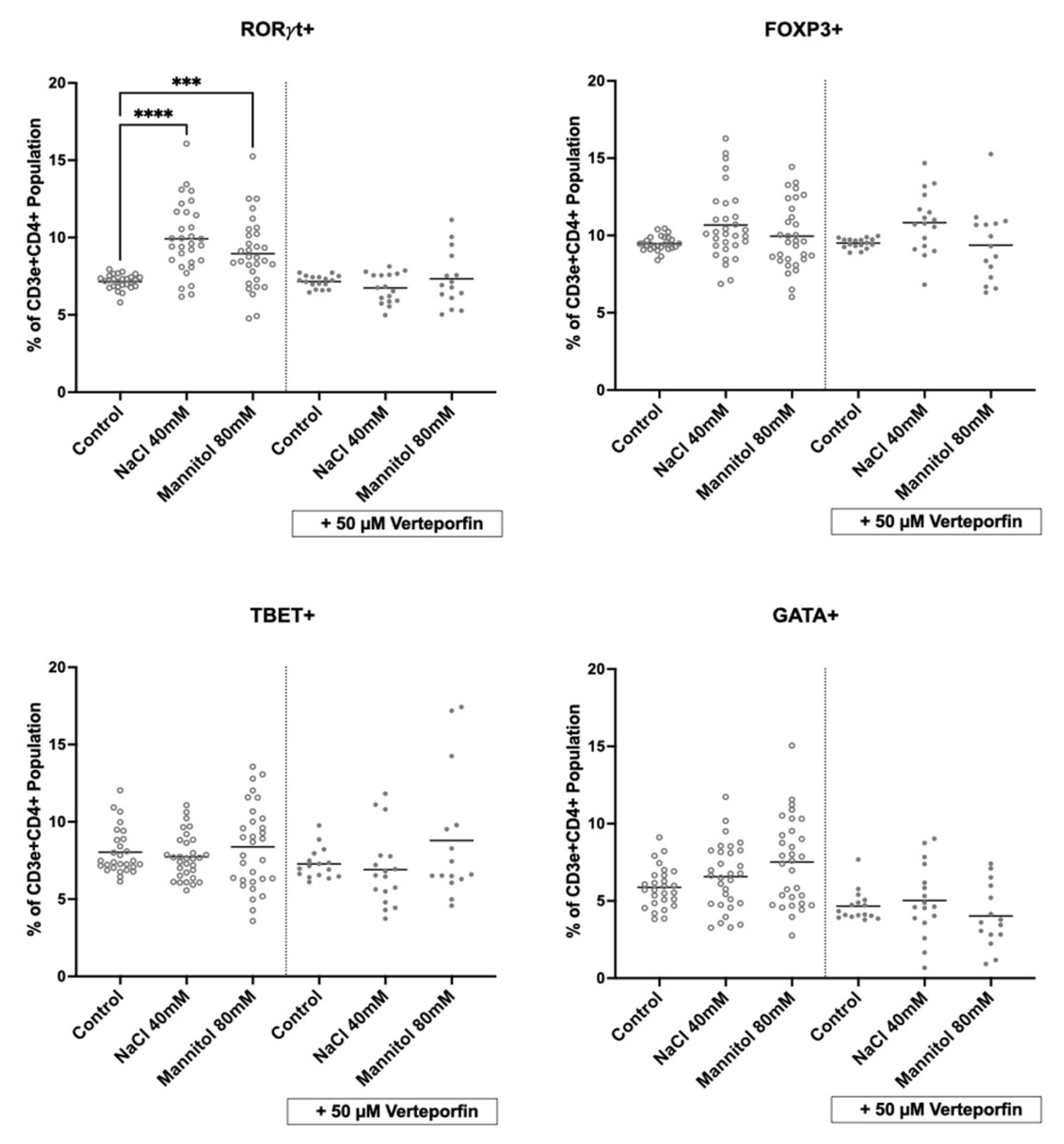
2.5. Inhibition of Hippo Signaling Abrogates NaCl’s Effect on Lymphocytes
3. Discussion
4. Methods
4.1. Animal Model of Hypertension and Immune Phenotyping
4.2. Cultivation of Murine Splenocytes
4.3. Cell Culture
4.4. Flow Cytometry
4.5. Analyses of Murine Splenocyte Expression Arrays
4.6. Western Blot
4.7. RNA Isolation, cDNA Synthesis, and qPCR
4.8. Immunofluorescent Staining
4.9. Graphic Representation and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arterial hypertension | AH |
| Angiotensin II receptor type 1 | AGTR1 |
| Angiotensin II | Ang II |
| Conventional T-helper cells | Tconv |
| Cytotoxic T cells | Tc |
| Fluorescence-activated cell sorting | FACS |
| GATA-binding protein 3 | Gata3 |
| Innate lymphoid cells | ILC |
| Large tumor suppressor kinase 1 | LATS1 |
| Sodium chloride | NaCl |
| Macrophage-stimulating 1 | MST1 |
| Nuclear factor of activated T cells 5 | NFAT5 |
| nuclear factor ’kappa-light-chain-enhancer’ of activated B cells | NF-kB |
| Phosphate-buffered saline | PBS |
| Phospho-S89 TAZ | P-TAZ |
| Renin–angiotensin–aldosterone system | RAAS |
| Retinoid-related orphan receptor gamma | RORγt |
| γδ T cells | γδT |
| Transcriptional coactivator with PDZ-binding motif | TAZ |
| TEA domain transcription factor | TEAD |
| T-box transcription factor TBX21 | T-bet |
| T-helper cells | Th |
| Regulatory T cell | Treg |
| Yes-associated protein | YAP |
References
- World Health Organization. Global Report on Hypertension: The Race Against a Silent Killer; World Health Organization: Geneva, Switzerland, 2023; pp. 1–276. [Google Scholar]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 Promotes Angiotensin II–Induced Hypertension and Vascular Dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Mancia Chairperson, G.; Kreutz Co-Chair, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar]
- Geng, J.; Yu, S.; Zhao, H.; Sun, X.; Li, X.; Wang, P.; Xiong, X.; Hong, L.; Xie, C.; Gao, J.; et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat. Immunol. 2017, 18, 800–812. [Google Scholar] [CrossRef]
- Kurz, A.R.M.; Pruenster, M.; Rohwedder, I.; Ramadass, M.; Schäfer, K.; Harrison, U.; Gouveia, G.; Nussbaum, C.; Immler, R.; Wiessner, J.R.; et al. MST1-dependent vesicle trafficking regulates neutrophil transmigration through the vascular basement membrane. J. Clin. Investig. 2016, 126, 4125–4139. [Google Scholar] [CrossRef]
- Stubbington, M.J.; Mahata, B.; Svensson, V.; Deonarine, A.; Nissen, J.K.; Betz, A.G.; Teichmann, S.A. An atlas of mouse CD4+ T cell transcriptomes. Biol. Direct 2015, 10, 14. [Google Scholar] [CrossRef]
- Wei, C.; Li, X. Verteporfin inhibits cell proliferation and induces apoptosis in different subtypes of breast cancer cell lines without light activation. BMC Cancer 2020, 20, 1042. [Google Scholar] [CrossRef]
- Bailey, M.A.; Dhaun, N. Salt Sensitivity: Causes, Consequences, and Recent Advances. Hypertension 2024, 81, 476–489. [Google Scholar] [CrossRef]
- Maifeld, A.; Wild, J.; Karlsen, T.V.; Rakova, N.; Wistorf, E.; Linz, P.; Jung, R.; Birukov, A.; Gimenez-Rivera, V.-A.; Wilck, N.; et al. Skin Sodium Accumulates in Psoriasis and Reflects Disease Severity. J. Investig. Dermatol. 2022, 142, 166–178.e8. [Google Scholar] [CrossRef]
- Faraco, G.; Brea, D.; Garcia-Bonilla, L.; Wang, G.; Racchumi, G.; Chang, H.; Buendia, I.; Santisteban, M.M.; Segarra, S.G.; Koizumi, K.; et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 2018, 2, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Gast, L.V.; Baier, L.-M.; Meixner, C.R.M.; Chaudry, O.; Engelke, K.; Uder, M.; Nagel, A.M.; Heiss, R. MRI of Potassium and Sodium Enables Comprehensive Analysis of Ion Perturbations in Skeletal Muscle Tissue After Eccentric Exercise. Investig. Radiol. 2023, 58, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Norlander, A.E.; Saleh, M.A.; Pandey, A.K.; Itani, H.A.; Wu, J.; Xiao, L.; Kang, J.; Dale, B.L.; Goleva, S.B.; Laroumanie, F.; et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight. 2017, 2, e92801. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.T.; Andrade, J.; Armbruster, M.; Shi, C.; Castro, M.; Costa, A.S.H.; Sugino, T.; Eelen, G.; Zimmermann, B.; Wilhelm, K.; et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat. Metab. 2022, 6, 672–682. [Google Scholar] [CrossRef]
- Karmaus, P.W.F.; Chen, X.; Lim, S.A.; Herrada, A.A.; Nguyen, T.-L.M.; Xu, B.; Dhungana, Y.; Rankin, S.; Chen, W.; Rosencrance, C.; et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature 2019, 7737, 101–105. [Google Scholar] [CrossRef]
- Crowley, S.D.; Frey, C.W.; Gould, S.K.; Griffiths, R.; Ruiz, P.; Burchette, J.L.; Howell, D.N.; Makhanova, N.; Yan, M.; Kim, H.-S.; et al. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am. J. Physiol.-Ren. Physiol. 2008, 295, F515–F524. [Google Scholar] [CrossRef]
- Serrador, J.M.; Nieto, M.; Sánchez-Madrid, F. Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol. 1999, 9, 228–233. [Google Scholar] [CrossRef]
- Pfeifer, M.; Brammeld, J.S.; Price, S.; Pilling, J.; Bhavsar, D.; Farcas, A.; Bateson, J.; Sundarrajan, A.; Miragaia, R.J.; Guan, N.; et al. Genome-wide CRISPR screens identify the YAP/TEAD axis as a driver of persister cells in EGFR mutant lung cancer. Commun. Biol. 2024, 7, 497. [Google Scholar] [CrossRef]
- He, L.; Pratt, H.; Gao, M.; Wei, F.; Weng, Z.; Struhl, K. YAP and TAZ are transcriptional co-activators of AP-1 proteins and STAT3 during breast cellular transformation. eLife 2021, 10, e67312. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, L.; Li, H.; Zhang, X.; Li, S.; Wang, K.; Yang, M.; Ma, X.; Zhang, D.; Xiang, S.; et al. Hippo-signaling-controlled MHC class I antigen processing and presentation pathway potentiates antitumor immunity. Cell Rep. 2024, 43, 114003. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Yang, Y.; Zhao, S.; You, J.; Wang, J.; Cai, J.; Wang, H.; Wang, J.; Zhang, W.; et al. Scarless wound healing programmed by core-shell microneedles. Nat. Commun. 2023, 14, 3431. [Google Scholar] [CrossRef] [PubMed]
- Markó, L.; Park, J.-K.; Henke, N.; Rong, S.; Balogh, A.; Klamer, S.; Bartolomaeus, H.; Wilck, N.; Ruland, J.; Forslund, S.K.; et al. B-cell lymphoma/leukaemia 10 and angiotensin II-induced kidney injury. Cardiovasc. Res. 2020, 5, 1059–1070. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeeb, B.L.; Weber-Stiehl, S.; Escudero-Hernández, C.; Müller, D.N.; Maifeld, A.; Sommer, F.; Schmitt, R.; Sievers, L.K. Hippo Signaling Regulates High-NaCl-Induced Increase in RORγt+ Pro-Inflammatory Lymphocytes. Int. J. Mol. Sci. 2025, 26, 2143. https://doi.org/10.3390/ijms26052143
Zeeb BL, Weber-Stiehl S, Escudero-Hernández C, Müller DN, Maifeld A, Sommer F, Schmitt R, Sievers LK. Hippo Signaling Regulates High-NaCl-Induced Increase in RORγt+ Pro-Inflammatory Lymphocytes. International Journal of Molecular Sciences. 2025; 26(5):2143. https://doi.org/10.3390/ijms26052143
Chicago/Turabian StyleZeeb, Bastian Lukas, Saskia Weber-Stiehl, Celia Escudero-Hernández, Dominik N. Müller, Andras Maifeld, Felix Sommer, Roland Schmitt, and Laura Katharina Sievers. 2025. "Hippo Signaling Regulates High-NaCl-Induced Increase in RORγt+ Pro-Inflammatory Lymphocytes" International Journal of Molecular Sciences 26, no. 5: 2143. https://doi.org/10.3390/ijms26052143
APA StyleZeeb, B. L., Weber-Stiehl, S., Escudero-Hernández, C., Müller, D. N., Maifeld, A., Sommer, F., Schmitt, R., & Sievers, L. K. (2025). Hippo Signaling Regulates High-NaCl-Induced Increase in RORγt+ Pro-Inflammatory Lymphocytes. International Journal of Molecular Sciences, 26(5), 2143. https://doi.org/10.3390/ijms26052143




