Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection
Abstract
1. Introduction
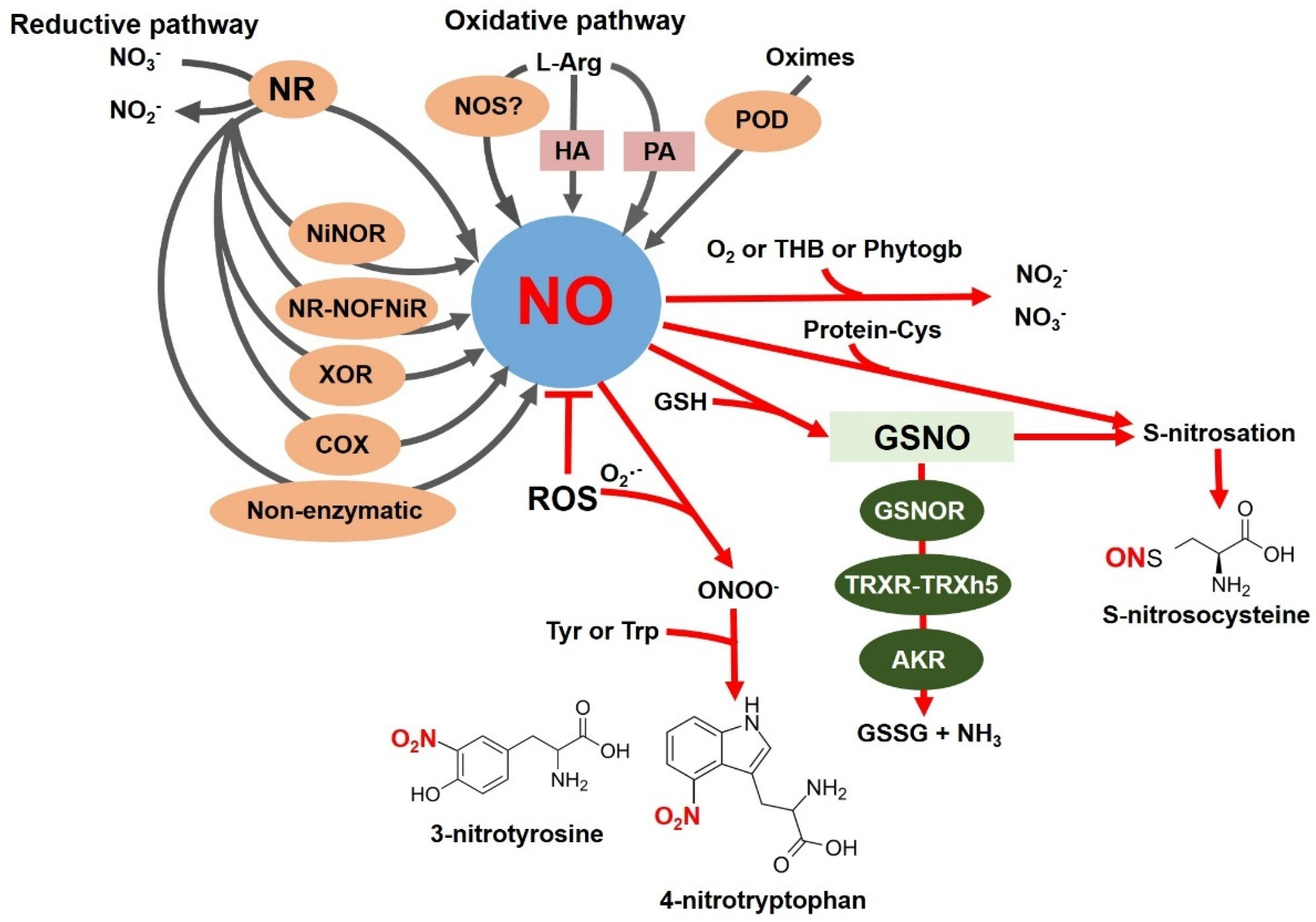
2. NO Synthesis and Degradation in Plants
3. NO-Mediated Post-Translational Protein Modifications
4. Lipid-Mediated NO Signaling
5. Plant Immunity Concepts
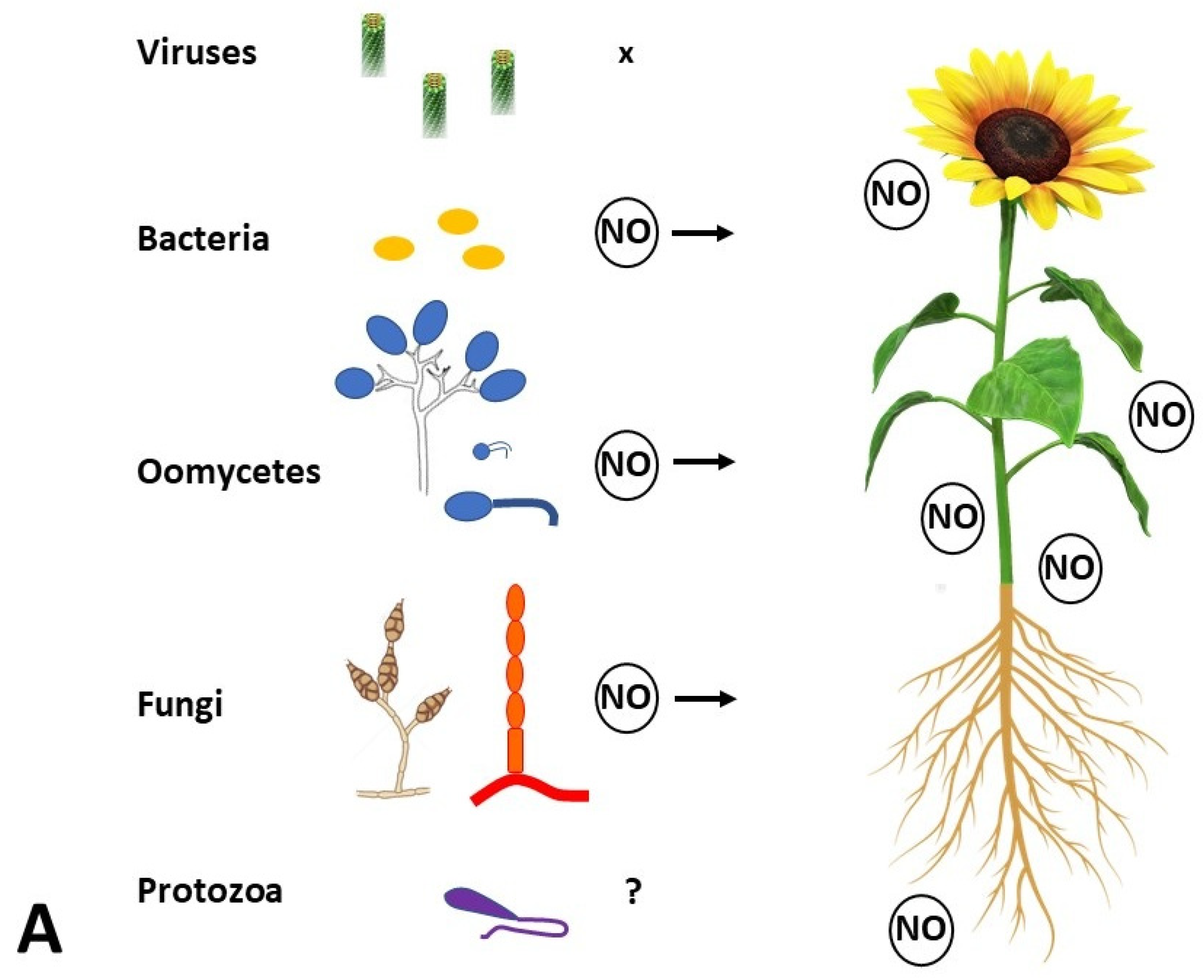
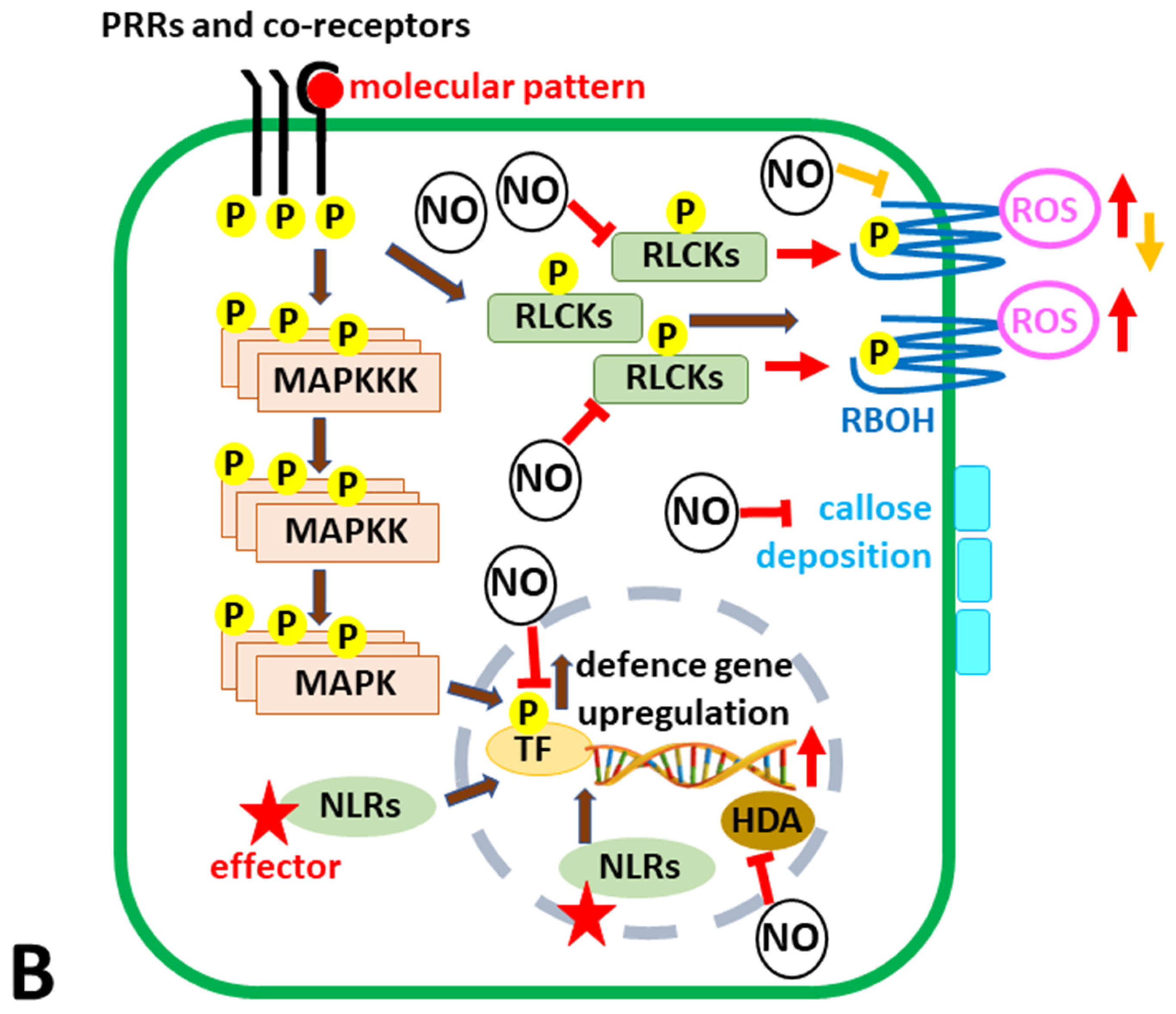
6. A Piece of NO in the Plant Immunity Puzzle
7. Exogenous Application of Nitric Oxide Donors
8. NO-Based NPs in Crop Protection in Agriculture
9. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| AKR | aldo-keto reductase |
| APX | ascorbate peroxidase |
| BIK1 | botrytis-induced kinase 1 |
| COX | cytochrome c oxidase |
| DAMP | damage-associated molecular pattern |
| ETI | effector-triggered immunity |
| GSH | reduced glutathione |
| GSNO | S-nitrosoglutathione |
| GSNOR | S-nitrosoglutathione reductase |
| GSSG | oxidized glutathione |
| HAMP | herbivore-associated molecular pattern |
| HDA | histone deacetylase |
| HR | hypersensitive reaction |
| JA | jasmonic acid |
| MAMP | microbe-associated molecular pattern |
| MDAR | monodehydroascorbate reductase |
| NLRs | nucleotide-binding leucine-rich repeat receptors |
| NO2-Ln | nitro-linolenic acid |
| NiNOR | nitrite: NO reductase |
| NOS | nitric oxide synthase |
| NO2-FA | nitrated fatty acid |
| NR | nitrate reductase |
| ONOO− | peroxynitrite |
| PAMP | pathogen-associated molecular pattern |
| PR1 | pathogenesis-related 1 protein |
| PRR | pattern recognition receptor |
| PTI | pattern-triggered immunity |
| PTM | post-translational modification |
| RBOHD | respiratory burst oxidase homolog D |
| RLCK | receptor-like cytoplasmic kinase |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SNO | S-nitrosothiol |
| SOD | superoxide dismutase |
| TRX | thioredoxin |
| TRXR | thioredoxin reductase |
| XOR | xanthine oxidoreductase |
References
- Freh, N.; Gao, J.; Petersen, M.; Panstruga, R. Plant autoimmunity-fresh insights into an old phenomenon. Plant Physiol. 2022, 188, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Suarez, S.; Doctorovich, F.; Sobieszczuk-Nowicka, E.; Bruce King, S.; Milczarek, G.; Rębiś, T.; Gajewska, J.; Jagodzik, P.; et al. Discovery of endogenous nitroxyl as a new redox player in Arabidopsis thaliana. Nat. Plants 2023, 9, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Barroso, J.B.; Boscari, A.; Corpas, F.J.; Gupta, K.J.; Hancock, J.T.; Lindermayr, C.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. Interorgan, intraorgan and interplant communication mediated by nitric oxide and related species. New Phytol. 2024, 244, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Wang, X.; Zhao, C.; Shen, H.; Yang, L. Nitric Oxide Regulates Seed Germination by Integrating Multiple Signalling Pathways. Int. J. Mol. Sci. 2023, 24, 9052. [Google Scholar] [CrossRef]
- Kumari, R.; Kapoor, P.; Mir, B.A.; Singh, M.; Parrey, Z.A.; Rakhra, G.; Parihar, P.; Khan, M.N.; Rakhra, G. Unlocking the versatility of nitric oxide in plants and insights into its molecular interplays under biotic and abiotic stress. Nitric Oxide 2024, 150, 1–17. [Google Scholar] [CrossRef]
- Corpas, F.J. NO and H2S Contribute to Crop Resilience against Atmospheric Stressors. Int. J. Mol. Sci. 2024, 25, 3509. [Google Scholar] [CrossRef]
- Freschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef]
- Graska, J.; Fidler, J.; Gietler, M.; Prabucka, B.; Nykiel, M.; Labudda, M. Nitric Oxide in Plant Functioning: Metabolism, Signaling, and Responses to Infestation with Ecdysozoa Parasites. Biology 2023, 12, 927. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Yun, B.-W. Nitric Oxide Acts as a Key Signaling Molecule in Plant Development under Stressful Conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef]
- Hussain, A.; Faheem, B.; Jang, H.-S.; Lee, D.-S.; Mun, B.-G.; Rolly, N.K.; Yun, B.-W. Melatonin–Nitric Oxide Crosstalk in Plants and the Prospects of NOMela as a Nitric Oxide Donor. Int. J. Mol. Sci. 2024, 25, 8535. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Silveira, N.M.; Ribeiro, R.V.; Pieretti, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M.; Hancock, J.T.; Petřivalský, M.; Gupta, K.J.; et al. Nitric oxide-releasing nanomaterials: From basic research to potential biotechnological applications in agriculture. New Phytol. 2022, 234, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Thomas, D.D. Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide. Redox Biol. 2015, 5, 225–233. [Google Scholar] [CrossRef]
- Mur, L.A.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelstrup, K.H.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef]
- Allagulova, C.R.; Lubyanova, A.R.; Avalbaev, A.M. Multiple Ways of Nitric Oxide Production in Plants and Its Functional Activity under Abiotic Stress Conditions. Int. J. Mol. Sci. 2023, 24, 11637. [Google Scholar] [CrossRef]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002, 53, 103–110. [Google Scholar] [CrossRef]
- Planchet, E.; Kaiser, W.M. Nitric oxide production in plants. Plant Signal. Behav. 2006, 2, 46–51. [Google Scholar] [CrossRef]
- Kolbert, Z.; Ortega, L.; Erdei, L. Involvement of nitrate reductase (NR) in osmotic stress-induced NO generation of Arabidopsis thaliana L. roots. J. Plant Physiol. 2010, 167, 77–80. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Lamattina, L. Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J. Exp. Bot. 2012, 63, 4875–4885. [Google Scholar] [CrossRef]
- Mohn, M.A.; Thaqi, B.; Fischer-Schrader, K. Isoform-specific NO synthesis by Arabidopsis thaliana nitrate reductase. Plants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Reda, M.; Kabała, K.; Stanisławski, J.; Szczepski, K.; Janicka, M. Regulation of NO-generating system activity in cucumber root response to cold. Int. J. Mol. Sci. 2025, 26, 1599. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, C.; Stremlau, S. Formation and possible roles of nitric oxide in plant roots. J. Exp. Bot. 2006, 57, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, Á.; Ocaña-Calahorro, F.; Mariscal, V.; Carreras, A.; Barroso, J.B.; Galván, A.; Fernández, E. A dual system formed by the ARC and NR molybdoenzymes mediates nitrite-dependent NO production in Chlamydomonas. Plant Cell Environ. 2016, 39, 2097–2107. [Google Scholar] [CrossRef]
- Maiber, L.; Koprivova, A.; Bender, D.; Kopriva, S.; Fischer-Schrader, K. Characterization of the amidoxime reducing components ARC1 and ARC2 from Arabidopsis thaliana. FEBS J. 2022, 289, 5656–5669. [Google Scholar] [CrossRef]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef]
- Barroso, J.B.; Corpas, F.J.; Carreras, A.; Sandalio, L.M.; Valderrama, R.; Palma, J.; Lupiáñez, J.A.; del Rıo, L.A. Localization of nitric-oxide synthase in plant peroxisomes. J. Biol. Chem. 1999, 274, 36729–36733. [Google Scholar] [CrossRef]
- Kumari, A.; Kaladhar, V.C.; Yadav, N.; Singh, P.; Reddy, K.; Gupta, K.J. Nitric oxide regulates mitochondrial biogenesis in plants. Plant Cell Environ. 2023, 46, 2492–2506. [Google Scholar] [CrossRef]
- Stöhr, C.; Ullrich, W.R. Generation and possible roles of NO in plant roots and their apoplastic space. J. Exp. Bot. 2002, 53, 2293–2303. [Google Scholar] [CrossRef]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef]
- Cooney, R.V.; Harwood, P.J.; Custer, L.J.; Franke, A.A. Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ. Health Perspect. 1994, 102, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Jeandroz, S.; Wipf, D.; Stuehr, D.J.; Lamattina, L.; Melkonian, M.; Tian, Z.; Zhu, Y.; Carpenter, E.J.; Wong, G.K.-S.; Wendehenne, D. Occurrence, structure, and evolution of nitric oxide synthase–like proteins in the plant kingdom. Sci. Signal. 2016, 9, re2. [Google Scholar] [CrossRef] [PubMed]
- Foresi, N.; Correa-Aragunde, N.; Parisi, G.; Calo, G.; Salerno, G.; Lamattina, L. Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 2010, 22, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; Del Castello, F.; Lamattina, L. A singular nitric oxide synthase with a globin domain found in Synechococcus PCC 7335 mobilizes N from arginine to nitrate. Sci. Rep. 2018, 8, 12505. [Google Scholar] [CrossRef]
- Rümer, S.; Gupta, K.J.; Kaiser, W.M. Plant cells oxidize hydroxylamines to NO. J. Exp. Bot. 2009, 60, 2065–2072. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef]
- Groß, F.; Rudolf, E.E.; Thiele, B.; Durner, J.; Astier, J. Copper amine oxidase 8 regulates arginine-dependent nitric oxide production in Arabidopsis thaliana. J. Exp. Bot. 2017, 68, 2149–2162. [Google Scholar] [CrossRef]
- López-Gómez, P.; Buezo, J.; Urra, M.; Cornejo, A.; Esteban, R.; de Los Reyes, J.F.; Urarte, E.; Rodríguez-Dobreva, E.; Chamizo-Ampudia, A.; Eguaras, A.; et al. A new oxidative pathway of nitric oxide production from oximes in plants. Mol. Plant 2024, 17, 178–198. [Google Scholar] [CrossRef]
- Liao, W.; Igamberdiev, A.U.; Palma, J.M. Advances in Nitric Oxide Signalling and Metabolism in Plants. Int. J. Mol. Sci. 2023, 24, 6397. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wu, X.; Fang, H.; Huang, D.; Pan, X.; Liao, W. Role of protein S-nitrosylation in plant growth and development. Plant Cell Rep. 2024, 43, 204. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Protein nitration: A connecting bridge between nitric oxide (NO) and plant stress. Plant Stress 2021, 2, 100026. [Google Scholar] [CrossRef]
- León, J. Protein tyrosine nitration in plant nitric oxide signaling. Front. Plant Sci. 2022, 13, 859374. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, S.; Ferrer-Sueta, G.; Peluffo, G.; Valez, V.; Zhang, H.; Kalyanaraman, B.; Radi, R. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids 2007, 32, 501–515. [Google Scholar] [CrossRef]
- Ischiropoulos, H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003, 305, 776–783. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; López-Jaramillo, J.; Padilla, M.N.; Carreras, A.; Corpas, F.J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Mata-Pérez, C.; Valderrama, R.; Padilla, M.N.; López-Jaramillo, J.; Luque, F.; Corpas, F.J.; Barroso, J.B. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J. Exp. Bot. 2015, 66, 5983–5996. [Google Scholar] [CrossRef]
- Holzmeister, C.; Gaupels, F.; Geerlof, A.; Sarioglu, H.; Sattler, M.; Durner, J.; Lindermayr, C. Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J. Exp. Bot. 2015, 66, 989–999. [Google Scholar] [CrossRef]
- Boutin, C.; Clément, C.; Rivoal, J. Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction. Int. J. Mol. Sci. 2024, 25, 9845. [Google Scholar] [CrossRef]
- Lindermayr, C.; Saalbach, G.; Durner, J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2005, 137, 921–930. [Google Scholar] [CrossRef]
- Kovacs, I.; Ageeva, A.; König, E.-E.; Lindermayr, C. Chapter Two—S-Nitrosylation of Nuclear Proteins: New Pathways in Regulation of Gene Expression. Adv. Bot. Res. 2016, 77, 15–39. [Google Scholar] [CrossRef]
- Ageeva-Kieferle, A.; Rudolf, E.E.; Lindermayr, C. Redox-Dependent Chromatin Remodeling: A New Function of Nitric Oxide as Architect of Chromatin Structure in Plants. Front. Plant Sci. 2019, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Borrowman, S.; Kapuganti, J.G.; Loake, G.J. Expanding roles for S-nitrosylation in the regulation of plant immunity. Free Radic. Biol. Med. 2023, 194, 357–368. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Soybean (Glycine max L.) Lipoxygenase 1 (LOX 1) is modulated by nitric oxide and hydrogen sulfide: An in vitro approach. Int. J. Mol. Sci. 2023, 24, 8001. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Gow, A.J.; Stamler, J.S.; Glazebrook, J. Ancient origins of nitric oxide signaling in biological systems. Proc. Natl. Acad. Sci. USA 1999, 96, 14206–14207. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Liu, L.; Hausladen, A.; Zeng, M.; Que, L.; Heitman, J.; Stamler, J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001, 410, 490–494. [Google Scholar] [CrossRef]
- Jahnová, J.; Luhová, L.; Petřivalský, M. S-nitrosoglutathione reductase—The master regulator of protein S-nitrosation in plant NO signaling. Plants 2019, 8, 48. [Google Scholar] [CrossRef]
- Kneeshaw, S.; Gelineau, S.; Tada, Y.; Loake, G.J.; Spoel, S.H. Selective protein denitrosylation activity of thioredoxin-h5 modulates plant immunity. Mol. Cell 2014, 56, 153–162. [Google Scholar] [CrossRef]
- Jedelská, T.; Luhová, L.; Petřivalský, M. Thioredoxins: Emerging players in the regulation of protein S-nitrosation in plants. Plants 2020, 9, 1426. [Google Scholar] [CrossRef]
- Treffon, P.; Vierling, E. Focus on nitric oxide homeostasis: Direct and indirect enzymatic regulation of protein denitrosation reactions in plants. Antioxidants 2022, 11, 1411. [Google Scholar] [CrossRef]
- Treffon, P.; Rossi, J.; Gabellini, G.; Trost, P.; Zaffagnini, M.; Vierling, E. Quantitative proteome profiling of a S-nitrosoglutathione reductase (GSNOR) null mutant reveals a new class of enzymes involved in nitric oxide homeostasis in plants. Front. Plant Sci. 2021, 12, 787435. [Google Scholar] [CrossRef] [PubMed]
- Stomberski, C.T.; Anand, P.; Venetos, N.M.; Hausladen, A.; Zhou, H.L.; Premont, R.T.; Stamler, J.S. AKR1A1 is a novel mammalian S-nitroso-glutathione reductase. J. Biol. Chem. 2019, 294, 18285–18293. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Pothiraj, R.; Suthanthiram, B.; Somasundaram, S.M.; Subbaraya, U. Phylogenomic classification and synteny network analyses deciphered the evolutionary landscape of aldo–keto reductase (AKR) gene superfamily in the plant kingdom. Gene 2022, 816, 146169. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Zhang, J.; Hou, Y.; Zhang, T.; Kang, J.; Wang, Z.; Yang, Q.; Long, R. Analysis of aldo–keto reductase gene family and their responses to salt, drought, and abscisic acid stresses in Medicago truncatula. Int. J. Mol. Sci. 2020, 21, 754. [Google Scholar] [CrossRef]
- Niranjan, V.; Uttarkar, A.; Dadi, S.; Dawane, A.; Vargheese, A.; Kumar, J.H.G.; Makarla, U.; Ramu, V.S. Stress-induced detoxification enzymes in rice have broad substrate affinity. ACS Omega 2021, 6, 3399–3410. [Google Scholar] [CrossRef]
- Javidi, M.R.; Maali-Amiri, R.; Poormazaheri, H.; Niaraki, M.S.; Kariman, K. Cold stress-induced changes in metabolism of carbonyl compounds and membrane fatty acid composition in chickpea. Plant Physiol. Biochem. 2022, 192, 10–19. [Google Scholar] [CrossRef]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Gupta, V. Transcriptome analysis for heat stress related genes in wheat genotype WH-730. Cereal Res. Commun. 2024, 1–13. [Google Scholar] [CrossRef]
- Guan, X.; Yu, L.; Wang, A. Genome-Wide Identification and Characterization of Aldo-Keto Reductase (AKR) Gene Family in Response to Abiotic Stresses in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 24, 1272. [Google Scholar] [CrossRef]
- Yadav, S.; Preethi, V.; Dadi, S.; Seth, C.S.; Chandrashekar, B.K.; Vemanna, R.S. Small chemical molecules regulating the phytohormone signalling alter the plant’s physiological processes to improve stress adaptation, growth and productivity. Physiol. Mol. Biol. Plants 2024, 30, 1593–1610. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Miyata, S.; Takahashi, M. Pleiotropic actions of aldehyde reductase (AKR1A). Metabolites 2021, 11, 343. [Google Scholar] [CrossRef]
- Treffon, P.; Vierling, E. Disrupted nitric oxide homeostasis impacts fertility through multiple processes including protein quality control. Plant Physiol. 2025, 197, kiae609. [Google Scholar] [CrossRef]
- Hancock, J.T.; Veal, D. Nitric oxide, other reactive signalling compounds, redox, and reductive stress. J. Exp. Bot. 2021, 72, 819–829. [Google Scholar] [CrossRef]
- Gupta, K.J.; Hancock, J.T.; Petřivalský, M.; Kolbert, Z.; Lindermayr, C.; Durner, J.; Barroso, J.B.; Palma, J.M.; Brouquisse, R.; Wendehenne, D.; et al. Recommendations on terminology and experimental best practice associated with plant nitric oxide research. New Phytol. 2020, 225, 1828–1834. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Noctor, G.; Cohen, M.; Trémulot, L.; Châtel-Innocenti, G.; Van Breusegem, F.; Mhamdi, A. Glutathione: A key modulator of plant defence and metabolism through multiple mechanisms. J. Exp. Bot. 2024, 75, erae194. [Google Scholar] [CrossRef]
- Di Fino, L.; Arruebarrena Di Palma, A.; Perk, E.A.; García-Mata, C.; Schopfer, F.J.; Laxalt, A.M. Nitro-fatty acids: Electrophilic signaling molecules in plant physiology. Planta 2021, 254, 120. [Google Scholar] [CrossRef]
- Jouhet, J.; Alves, E.; Boutté, Y.; Darnet, S.; Domergue, F.; Durand, T.; Fischer, P.; Fouillen, L.; Grube, M.; Joubès, J.; et al. Plant and algal lipidomes: Analysis, composition, and their societal significance. Prog. Lipid Res. 2024, 96, 101290. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Luque, F.; Melguizo, M.; Jiménez-Ruiz, J.; Fierro-Risco, J.; Peñas-Sanjuán, A.; Valderrama, R.; et al. Nitro-Fatty Acids in Plant Signaling: Nitro-Linolenic Acid Induces the Molecular Chaperone Network in Arabidopsis. Plant Physiol. 2016, 170, 686–701. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol. 2017, 11, 554–561. [Google Scholar] [CrossRef]
- Vollár, M.; Feigl, G.; Oláh, D.; Horváth, A.; Molnár, Á.; Kúsz, N.; Ördög, A.; Csupor, D.; Kolbert, Z. Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica napus L. Plants 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Mata-Pérez, C.; Padilla, M.N.; Chaki, M.; Valderrama, R.; Aranda-Caño, L.; Barroso, J.B. Role of electrophilic nitrated fatty acids during development and response to abiotic stress processes in plants. J. Exp. Bot. 2021, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Caño, L.; Valderrama, R.; Chaki, M.; Begara-Morales, J.C.; Melguizo, M.; Barroso, J.B. Nitrated fatty-acids distribution in storage biomolecules during Arabidopsis thaliana development. Antioxidants 2022, 11, 1869. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.; Dixon, R.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz, M.; Floryszak-Wieczorek, J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007, 172, 876–887. [Google Scholar] [CrossRef]
- Scheler, C.; Durner, J.; Astier, J. Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 2013, 16, 534–539. [Google Scholar] [CrossRef]
- Sytykiewicz, H.; Czerniewicz, P.; Ruszczyńska, M.; Kmieć, K. The Interplay of Nitric Oxide and Nitrosative Modifications in Maize: Implications for Aphid Herbivory and Drought Stress. Int. J. Mol. Sci. 2024, 25, 11280. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of plants to overcome abiotic and biotic stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Zhang, L.; Hua, C.; Janocha, D.; Fliegmann, J.; Nürnberger, T. Plant cell surface immune receptors—Novel insights into function and evolution. Curr. Opin. Plant Biol. 2023, 74, 102384. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Moratto, E.; Savage, Z.; Hamilton, E.; Adachi, H.; Wu, C.H.; Leary, A.Y.; Tumtas, Y.; Rothery, S.M.; Maqbool, A.; et al. Dynamic localization of a helper NLR at the plant-pathogen interface underpins pathogen recognition. Proc. Natl. Acad. Sci. USA 2021, 118, e2104997118. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Haxim, Y.; Islam, W.; Kahar, G.; Liu, X.; Zhang, D. Role of pathogen’s effectors in understanding host-pathogen interaction. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2022, 1869, 119347. [Google Scholar] [CrossRef] [PubMed]
- Locci, F.; Parker, J.E. Plant NLR immunity activation and execution: A biochemical perspective. Open Biol. 2024, 14, 230387. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.J.; Xin, X.-F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Yu, N.-N.; Park, G. Nitric Oxide in Fungi: Production and Function. J. Fungi 2024, 10, 155. [Google Scholar] [CrossRef]
- Harris, J.M.; Balint-Kurti, P.; Bede, J.C.; Day, B.; Gold, S.; Goss, E.M.; Grenville-Briggs, L.J.; Jones, K.M.; Wang, A.; Wang, Y.; et al. What are the Top 10 Unanswered Questions in Molecular Plant-Microbe Interactions? Mol. Plant-Microbe Interact. 2020, 33, 1354–1365. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Wang, J.; Geldner, N.; Zhou, F. Spatiotemporal control of root immune responses during microbial colonization. Curr. Opin. Plant Biol. 2023, 74, 102369. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Zhang, Y. Plant immunity: Danger perception and signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-L.; Kim, S.-T.; Castel, B.; Charoennit, N.; Chae, E. Genetics of autoimmunity in plants: An evolutionary genetics perspective. New Phytol. 2021, 229, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Thoms, D.; Liang, Y.; Haney, C.H. Maintaining symbiotic homeostasis: How do plants engage with beneficial microorganisms while at the same time restricting pathogens? Mol. Plant-Microbe Interact. 2021, 34, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Chen, C.; Sun, Y.; Wang, D.; Nawaz, T.; El-Kahtany, K.; Fahad, S. Mechanisms of nitric oxide involvement in plant-microbe interaction and its enhancement of stress resistance. Plant Stress 2023, 10, 100191. [Google Scholar] [CrossRef]
- Piterková, J.; Petřivalský, M.; Luhová, L.; Mieslerová, B.; Sedlářová, M.; Lebeda, A. Local and systemic production of nitric oxide in tomato responses to powdery mildew infection. Mol. Plant Pathol. 2009, 10, 501–513. [Google Scholar] [CrossRef]
- Jedelská, T.; Sedlářová, M.; Lochman, J.; Činčalová, L.; Luhová, L.; Petřivalský, M. Protein S-nitrosation differentially modulates tomato responses to infection by hemi-biotrophic oomycetes of Phytophthora spp. Hortic. Res. 2021, 8, 34. [Google Scholar] [CrossRef]
- Khan, E.A.; Aftab, S.; Hasanuzzaman, M. Unraveling the importance of nitric oxide in plant-microbe interaction. Plant Stress 2023, 10, 100258. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.W.; Zhou, J.M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, eaav5870. [Google Scholar] [CrossRef]
- Ma, S.; Lapin, D.; Liu, L.; Sun, Y.; Song, W.; Zhang, X.; Logemann, E.; Yu, D.; Wang, J.; Jirschitzka, J.; et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 2020, 370, eabe3069. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gonzalez-Gordo, S.; Palma, J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J. Exp. Bot. 2021, 72, 830–847. [Google Scholar] [CrossRef]
- Lee, D.; Lal, N.K.; Lin, Z.-J.D.; Ma, S.; Liu, J.; Castro, B.; Toruño, T.; Dinesh-Kumar, S.P.; Coaker, G. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 2020, 11, 1838. [Google Scholar] [CrossRef]
- Cui, B.; Pan, Q.; Cui, W.; Wang, Y.; Loake, V.I.P.; Yuan, S.; Liu, F.; Loake, G.J. S-nitrosylation of a receptor-like cytoplasmic kinase regulates plant immunity. Sci. Adv. 2024, 10, eadk3126. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cang, X.; Yan, H.; Zhang, Z.; Li, W.; He, J.; Zhang, M.; Lou, L.; Wang, R.; Chang, M. Activating plant immunity: The hidden dance of intracellular Ca2+ stores. New Phytol. 2024, 242, 2430–2439. [Google Scholar] [CrossRef]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Palma, J.M. Assessing nitric oxide (NO) in higher plants: An outline. Nitrogen 2020, 1, 12–20. [Google Scholar] [CrossRef]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The function of plant PR1 and other members of the CAP protein superfamily in plant-pathogen interactions. Mol. Plant Pathol. 2023, 24, 651–668. [Google Scholar] [CrossRef]
- Mengel, A.; Ageeva, A.; Georgii, E.; Bernhardt, J.; Wu, K.; Durner, J.; Lindermayr, C. Nitric Oxide Modulates Histone Acetylation at Stress Genes by Inhibition of Histone Deacetylases. Plant Physiol. 2017, 173, 1434–1452. [Google Scholar] [CrossRef]
- Guan, Y.; Gajewska, J.; Sobieszczuk-Nowicka, E.; Floryszak-Wieczorek, J.; Hartman, S.; Arasimowicz-Jelonek, M. The effect of nitrosative stress on histone H3 and H4 acetylation in Phytophthora infestans life cycle. Plant Physiol. Biochem. 2024, 216, 109129. [Google Scholar] [CrossRef]
- Courtois, C.; Besson, A.; Dahan, J.; Bourque, S.; Dobrowolska, G.; Pugin, A.; Wendehenne, D. Nitric oxide signalling in plants: Interplays with Ca2+ and protein kinases. J. Exp. Bot. 2008, 59, 155–163. [Google Scholar] [CrossRef]
- Mulaudzi, T.; Ludidi, N.; Ruzvidzo, O.; Morse, M.; Hendricks, N.; Iwuoha, E.; Gehring, C. Identification of a novel Arabidopsis thaliana nitric oxide-binding molecule with guanylate cyclase activity in vitro. FEBS Lett. 2011, 585, 2693–2697. [Google Scholar] [CrossRef]
- Wendehenne, D.; Pugin, A.; Klessig, D.F.; Durner, J. Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Liu, R.; Kang, Y.; Chen, L. NO binds to the distal site of haem in the fully activated soluble guanylate cyclase. Nitric Oxide 2023, 134–135, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gross, I.; Durner, J. In Search of Enzymes with a Role in 3′, 5′-Cyclic Guanosine Monophosphate Metabolism in Plants. Front. Plant Sci. 2016, 7, 576. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Wang, X.-Y.; Xu, Y.-P.; He, Y.-H.; Cai, X.-Z. Characterization of tomato protein kinases embedding guanylate cyclase catalytic center motif. Sci. Rep. 2020, 10, 4078. [Google Scholar] [CrossRef] [PubMed]
- Sedlářová, M.; Binarová, P.; Lebeda, A. Changes in microtubular alignment in Lactuca spp. (Asteraceae) epidermal cells during early stages of infection by Bremia lactucae (Peronosporaceae). Phyton-Ann. Rei Bot. 2001, 41, 21–33. [Google Scholar]
- Hardham, A.R. Microtubules and biotic interactions. Plant J. 2013, 75, 278–289. [Google Scholar] [CrossRef]
- Li, P.; Day, B. Battlefield cytoskeleton: Turning the tide on plant immunity. Mol. Plant-Microbe Interact 2019, 32, 25–34. [Google Scholar] [CrossRef]
- Kumar, S.; Jeevaraj, T.; Yunus, M.H.; Chakraborty, S.; Chakraborty, N. The plant cytoskeleton takes center stage in abiotic stress responses and resilience. Plant Cell Environ. 2023, 46, 5–22. [Google Scholar] [CrossRef]
- Blume, Y.B.; Krasylenko, Y.A.; Demchuk, O.M.; Yemets, A.I. Tubulin tyrosine nitration regulates microtubule organization in plant cells. Front. Plant Sci. 2013, 4, 530. [Google Scholar] [CrossRef]
- Aslan, M.; Ryan, T.M.; Townes, T.M.; Coward, L.; Kirk, M.C.; Barnes, S.; Alexander, C.B.; Rosenfeld, S.S.; Freeman, B.A. Nitric Oxide-dependent Generation of Reactive Species in Sickle Cell Disease: Actin tyrosine nitration induces defective cytoskeletal polymerization. J. Biol. Chem. 2003, 278, 4194–4204. [Google Scholar] [CrossRef]
- Leontovyčová, H.; Kalachova, T.; Janda, M. Disrupted actin: A novel player in pathogen attack sensing? New Phytol. 2020, 227, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Leontovyčová, H.; Kalachova, T.; Trdá, L.; Pospíchalová, R.; Lamparová, L.; Dobrev, P.I.; Malínská, K.; Burketová, L.; Valentová, O.; Janda, M. Actin depolymerization is able to increase plant resistance against pathogens via activation of salicylic acid signalling pathway. Sci. Rep. 2019, 9, 10397. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Agathokleous, E. Nitric oxide, hormesis and plant biology. Sci. Total Environ. 2023, 866, 161299. [Google Scholar] [CrossRef] [PubMed]
- Krasuska, U.; Ciacka, K.; Andryka-Dudek, P.; Bogatek, R.; Gniazdowska, A. “Nitrosative Door” in Seed Dormancy Alleviation and Germination. Signal. Commun. Plants 2015, 23, 215–237. [Google Scholar] [CrossRef]
- Kasten, D.; Mithöfer, A.; Georgii, E.; Lang, H.; Durner, J.; Gaupels, F. Nitrite is the driver, phytohormones are modulators while NO and H2O2 act as promoters of NO2-induced cell death. J. Exp. Bot. 2016, 67, 6337–6349. [Google Scholar] [CrossRef]
- Kandhol, N.; Singh, V.P.; Pandey, S.; Sharma, S.; Zhao, L.; Corpas, F.J.; Chen, Z.-H.; White, J.C.; Tripathi, D.K. Nanoscale materials and NO-ROS homeostasis in plants: Trilateral dynamics. Trends Plant Sci. 2024, 29, 1310–1318. [Google Scholar] [CrossRef]
- Tan, L.; He, C. Advances in inorganic-based colloidal nanovehicles functionalized for nitric oxide delivery. Colloids Surf. B Biointerfaces 2021, 199, 111508. [Google Scholar] [CrossRef]
- do Carmo, G.C.; Iastrenski, L.F.; Debiasi, T.V.; da Silva, R.C.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Stolf-Moreira, R.; Pimenta, J.A.; Seabra, A.B.; et al. Nanoencapsulation improves the protective effects of a nitric oxide donor on drought-stressed Heliocarpus popayanensis seedlings. Ecotoxicol. Environ. Saf. 2021, 225, 112713. [Google Scholar] [CrossRef]
- Gomes, D.G.; Debiasi, T.V.; Pelegrino, M.T.; Pereira, R.M.; Ondrasek, G.; Batista, B.L.; Seabra, A.B.; Oliveira, H.C. Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress. Plants 2022, 11, 3245. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- da Veiga, J.C.; Silveira, N.M.; Seabra, A.B.; Bron, I.U. Exploring the power of nitric oxide and nanotechnology for prolonging postharvest shelf-life and enhancing fruit quality. Nitric Oxide 2024, 142, 26–37. [Google Scholar] [CrossRef] [PubMed]
- da Veiga, J.C.; Silveira, N.M.; Seabra, A.B.; Pieretti, J.C.; Boza, Y.; Jacomino, A.P.; Filho, J.C.Z.; Campagnoli, V.P.; Cia, P.; Bron, I.U. Spraying with encapsulated nitric oxide donor reduces weight loss and oxidative damage in papaya fruit. Nitric Oxide 2024, 150, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Giraldo, J.P.; Steinmetz, N.F.; Avellan, A.; Demirer, G.S.; Ristroph, K.D.; Wang, G.J.; Hendren, C.O.; Alabi, C.A.; Caparco, A.; et al. Towards realizing nano-enabled precision delivery in plants. Nat. Nanotechnol. 2024, 19, 1255–1269. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Seabra, A.B.; Kondak, S.; Adedokun, O.P.; Kolbert, Z. Multilevel approach to plant-nanomaterial relationships: From cells to living ecosystems. J. Exp. Bot. 2023, 74, 3406–3424. [Google Scholar] [CrossRef]
- Singh, A.; Rajput, V.D.; Varshney, A.; Ghazaryan, K.; Minkina, T. Small Tech, Big Impact: Agri-nanotechnology Journey to Optimize Crop Protection and Production for Sustainable Agriculture. Plant Stress 2023, 10, 100253. [Google Scholar] [CrossRef]
- Wang, H.; Jafir, M.; Irfan, M.; Ahmad, T.; Zia-ur-Rehman, M.; Usman, M.; Rizwan, M.; Hamoud, Y.A.; Shaghaleh, H. Emerging trends to replace pesticides with nanomaterials: Recent experiences and future perspectives for ecofriendly environment. J. Environ. Manag. 2024, 360, 121178. [Google Scholar] [CrossRef]
- Prats, E.; Carver, T.L.; Mur, L.A. Pathogen-derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis. Res. Microbiol. 2008, 159, 476–480. [Google Scholar] [CrossRef]
- Turrion-Gomez, J.L.; Benito, E.P. Flux of nitric oxide between the necrotrophic pathogen Botrytis cinerea and the host plant. Mol. Plant Pathol. 2011, 12, 606–616. [Google Scholar] [CrossRef]
- Samalova, M.; Johnson, J.; Illes, M.; Kelly, S.; Fricker, M.; Gurr, S. Nitric oxide generated by the rice blast fungus Magnaporthe oryzae drives plant infection. New Phytol. 2013, 197, 207–222. [Google Scholar] [CrossRef]
- Sedlářová, M.; Kubienová, L.; Drábková Trojanová, Z.; Luhová, L.; Lebeda, A.; Petřivalský, M. Chapter Thirteen—The Role of Nitric Oxide in Development and Pathogenesis of Biotrophic Phytopathogens—Downy and Powdery Mildews. Adv. Bot. Res. 2016, 77, 263–283. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, N.; Acharya, K. Chitosan nanoparticles mitigate Alternaria leaf spot disease of chilli in nitric oxide dependent way. Plant Physiol. Biochem. 2022, 180, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, X.; Zhang, L.; Zhang, Y.; Wei, L.; Liu, Y. The roles of nitric oxide in improving postharvest fruits quality: Crosstalk with phytohormones. Food Chem. 2024, 455, 139977. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlářová, M.; Jedelská, T.; Lebeda, A.; Petřivalský, M. Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection. Int. J. Mol. Sci. 2025, 26, 2087. https://doi.org/10.3390/ijms26052087
Sedlářová M, Jedelská T, Lebeda A, Petřivalský M. Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection. International Journal of Molecular Sciences. 2025; 26(5):2087. https://doi.org/10.3390/ijms26052087
Chicago/Turabian StyleSedlářová, Michaela, Tereza Jedelská, Aleš Lebeda, and Marek Petřivalský. 2025. "Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection" International Journal of Molecular Sciences 26, no. 5: 2087. https://doi.org/10.3390/ijms26052087
APA StyleSedlářová, M., Jedelská, T., Lebeda, A., & Petřivalský, M. (2025). Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection. International Journal of Molecular Sciences, 26(5), 2087. https://doi.org/10.3390/ijms26052087





