Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective
Abstract
1. Introduction
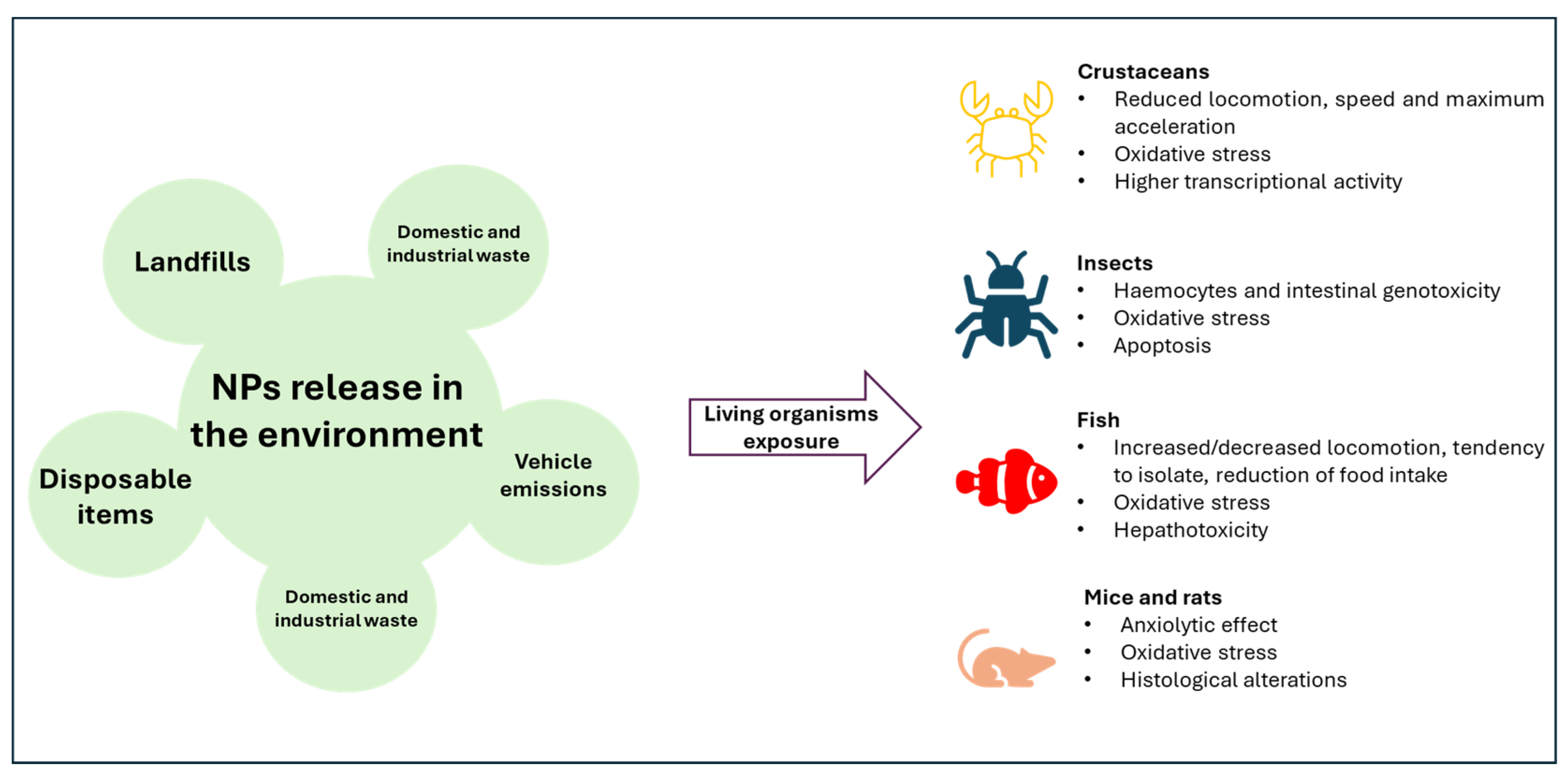
Biological Effects
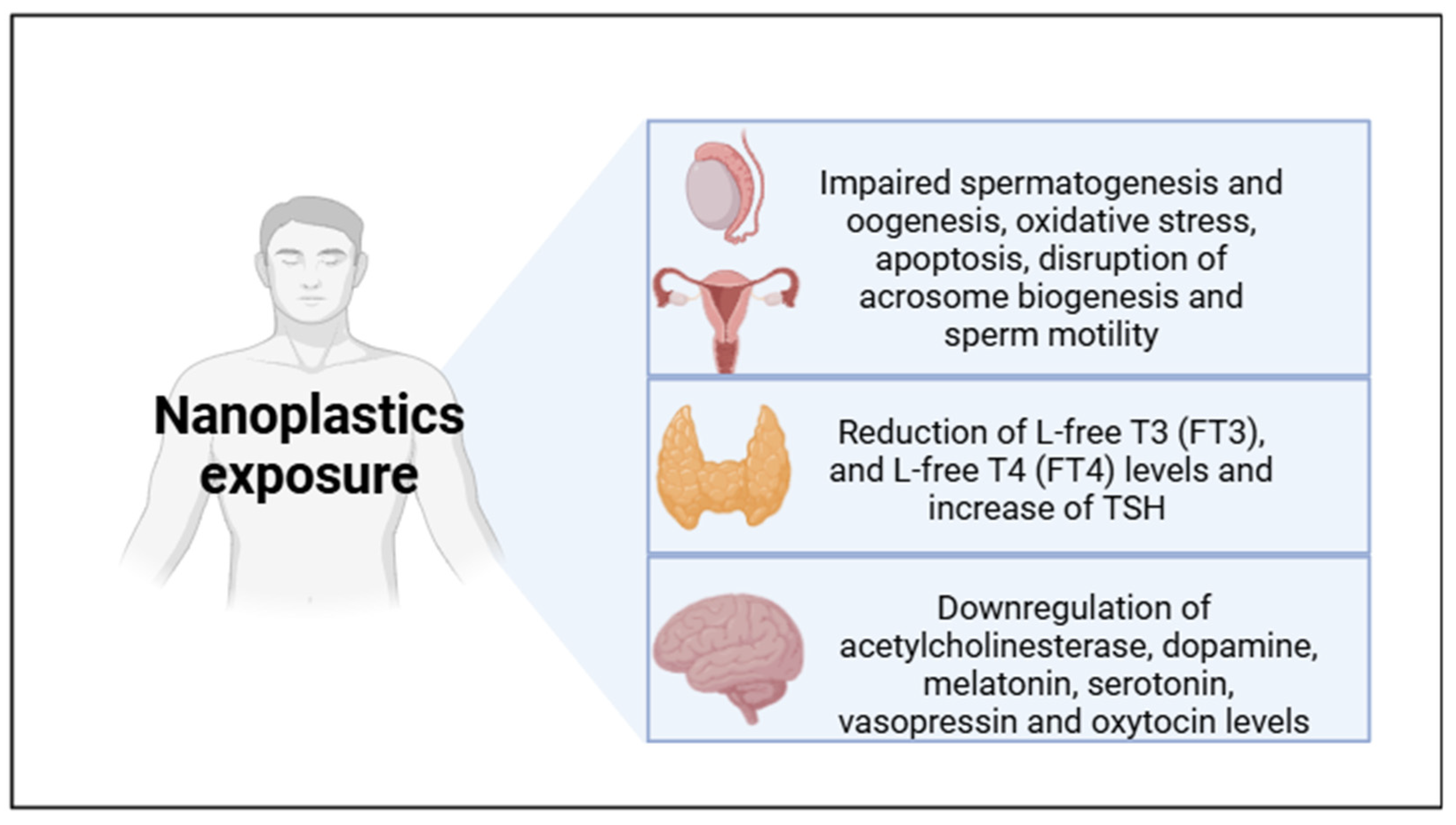
2. Nanoplastics as Endocrine Disruptors
2.1. NPs and Reproduction
2.2. NPs and Human Breast Epithelial Cells
2.3. NPs and Behavior
2.4. NPs and Thyroid Function
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 2019, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Tran, T.V.; Jalil, A.A.; Nguyen, T.M.; Nguyen, T.T.T.; Nabgan, W.; Nguyen, D.T.C. A review on the occurrence, analytical methods, and impact of microplastics in the environment. Environ. Toxicol. Pharmacol. 2023, 102, 104248. [Google Scholar] [CrossRef] [PubMed]
- Bajt, O. From plastics to microplastics and organisms. FEBS Open Bio 2021, 11, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Wang, B.; Chen, M.; Lee, X.; Zhang, X.; Wang, S.; Yu, Z.; Wu, P. Environmental behaviors and degradation methods of microplastics in different environmental media. Chemosphere 2022, 299, 134354. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.H. Recent Advancements in Polythiophene-Based Materials and their Biomedical, Geno Sensor and DNA Detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef]
- Monfared, M.; Taghizadeh, S.; Zare-Hoseinabadi, A.; Mousavi, S.M.; Hashemi, S.A.; Ranjbar, S.; Amani, A.M. Emerging frontiers in drug release control by core-shell nanofibers: A review. Drug Metab. Rev. 2019, 51, 589–611. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Behbudi, G.; Gholami, A.; Hashemi, S.A.; Nejad, Z.M.; Bahrani, S.; Chiang, W.H.; Wei, L.C.; Omidifar, N. Shape-controlled synthesis of zinc nanostructures mediating macromolecules for biomedical applications. Biomater. Res. 2022, 26, 4. [Google Scholar] [CrossRef]
- Forster, N.A.; Wilson, S.C.; Tighe, M.K. Examining sampling protocols for microplastics on recreational trails. Sci. Total Environ. 2022, 818, 151813. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Y.; Lai, Y.J.; Yu, S.J.; Li, Q.C.; Zhou, Q.X.; Liu, J.F. Quantitation of Atmospheric Suspended Polystyrene Nanoplastics by Active Sampling Prior to Pyrolysis-Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2023, 57, 10754–10762. [Google Scholar] [CrossRef]
- Cerasa, M.; Teodori, S.; Pietrelli, L. Searching Nanoplastics: From Sampling to Sample Processing. Polymers 2021, 13, 3658. [Google Scholar] [CrossRef]
- Askham, C.; Pauna, V.H.; Boulay, A.M.; Fantke, P.; Jolliet, O.; Lavoie, J.; Booth, A.M.; Coutris, C.; Verones, F.; Weber, M.; et al. Generating environmental sampling and testing data for micro- and nanoplastics for use in life cycle impact assessment. Sci. Total Environ. 2023, 859 Pt 2, 160038. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. IJEST 2023, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Su, J.; Dong, J.; Liang, M.; Xiao, J.; Liu, J.; Zeng, Q.; Li, Y.; Huang, W.; Chen, C. Aggregation kinetics of polystyrene nanoplastics in gastric environments: Effects of plastic properties, solution conditions, and gastric constituents. Environ. Int. 2022, 170, 107628. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H. The Trojan Horse Goes Wild: The Effect of Drug Loading on the Behavior of Nanoparticles. Angew. Chem. Int. Ed. Engl. 2021, 60, 2202–2206. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Parvathi, V.D.; Yoghalakshmi, N.; Kumar, S.M.; Athulya, P.A.; Mukherjee, A.; Chandrasekaran, N. Plastic particles in medicine: A systematic review of exposure and effects to human health. Chemosphere 2022, 303 Pt 3, 135227. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef] [PubMed]
- Symeonides, C.; Aromataris, E.; Mulders, Y.; Dizon, J.; Stern, C.; Barker, T.H.; Whitehorn, A.; Pollock, D.; Marin, T.; Dunlop, S. An Umbrella Review of Meta-Analyses Evaluating Associations between Human Health and Exposure to Major Classes of Plastic-Associated Chemicals. Ann. Glob. Health 2024, 90, 52. [Google Scholar] [CrossRef] [PubMed]
- Camerano Spelta Rapini, C.; Di Berardino, C.; Peserico, A.; Capacchietti, G.; Barboni, B. Can Mammalian Reproductive Health Withstand Massive Exposure to Polystyrene Micro- and Nanoplastic Derivatives? A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12166. [Google Scholar] [CrossRef]
- Schwarzenberger, A. Negative Effects of Cyanotoxins and Adaptative Responses of Daphnia. Toxins 2022, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Choi, B.S.; Kim, M.S.; Park, J.C.; Jeong, C.B.; Han, J.; Lee, J.S. The genome of the freshwater water flea Daphnia magna: A potential use for freshwater molecular ecotoxicology. Aquat. Toxicol. 2019, 210, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Jiang, R.; Hu, S.; Xiao, X.; Wu, J.; Wei, S.; Xiong, Y.; Ouyang, G. Investigating the toxicities of different functionalized polystyrene nanoplastics on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 180, 509–516. [Google Scholar] [CrossRef]
- De Felice, B.; Sugni, M.; Casati, L.; Parolini, M. Molecular, biochemical and behavioral responses of Daphnia magna under long-term exposure to polystyrene nanoplastics. Environ. Int. 2022, 164, 107264. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Bergami, E.; Salvati, A.; Faleri, C.; Cirino, P.; Dawson, K.A.; Corsi, I. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 2014, 48, 12302–12311. [Google Scholar] [CrossRef]
- Alaraby, M.; Abass, D.; Domenech, J.; Hernández, A.; Marcos, R. Hazard Assessment of Ingested Polystyrene Nanoplastics in Drosophila Larvae. Environ. Sci. Nano 2022, 9, 1845–1857. [Google Scholar] [CrossRef]
- Aloisi, M.; Grifoni, D.; Zarivi, O.; Colafarina, S.; Morciano, P.; Poma, A.M.G. Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene. Int. J. Mol. Sci. 2024, 25, 7965. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Villacorta, A.; Abass, D.; Hernández, A.; Marcos, R. The hazardous impact of true-to-life PET nanoplastics in Drosophila. Sci. Total Environ. 2023, 863, 160954. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.H.; Liang, S.T.; Chen, J.R.; Chen, K.H.; Hsiao, C.D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, B.; Yao, Q.; Feng, X.; Shen, T.; Guo, P.; Wang, P.; Bai, Y.; Li, B.; Wang, P.; et al. Toxicological effects of micro/nano-plastics on mouse/rat models: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1103289. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wei, X.; Hu, H.; Zhang, B.; Yang, D.; Du, H.; Zhu, R.; Sun, X.; Oh, Y.; Gu, N. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere 2022, 288 Pt 3, 132607. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Deng, S.; Wang, B.; Zhang, F.; Luo, T.; Kuang, H.; Kuang, X.; Yuan, Y.; Huang, J.; Zhang, D. Exposure to polystyrene nanoplastics induces hepatotoxicity involving NRF2-NLRP3 signaling pathway in mice. Ecotoxicol. Environ. Saf. 2024, 278, 116439. [Google Scholar] [CrossRef]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Vecchiotti, G.; Colafarina, S.; Aloisi, M.; Zarivi, O.; Di Carlo, P.; Poma, A. Genotoxicity and oxidative stress induction by polystyrene nanoparticles in the colorectal cancer cell line HCT116. PLoS ONE 2021, 16, e0255120. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zhang, L.; Han, L.; Wang, J.; Zhang, W.; Liu, Z.; Gao, A. Polystyrene micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut microbiota, metabolites, and cytokines. Environ. Int. 2022, 161, 107131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Niu, J.; Guo, E.; Zhao, C.; Zhang, J.; Liu, X.; Wang, L.; Rao, L.; Chen, X.; et al. Neutrophil Membrane-Camouflaged Polyprodrug Nanomedicine for Inflammation Suppression in Ischemic Stroke Therapy. Adv. Mater. 2024, 36, e2311803. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environ. Health Perspect. 1996, 104 (Suppl. 4), 715–740. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Jarman, W.M.; Ballschmiter, K. From coal to DDT: The history of the development of the pesticide DDT from synthetic dyes till Silent Spring. Endeavour 2012, 36, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C. Neuroendocrine targets of endocrine disruptors. Hormones 2010, 9, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Tabata, M.; Kubo-Irie, M.; Shimizu, T.; Suzuki, K.; Nihei, Y.; Takeda, K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol. In Vitro 2008, 22, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Zakhidov, S.T.; Marshak, T.L.; Malolina, E.A.; Kulibin, A.Y.; Zelenina, I.A.; Pavluchenkova, S.M.; Rudoy, V.M.; Dementeva, O.V.; Skuridin, S.G.; Evdokimov, Y.M. Gold nanoparticles disturb nuclear chromatin decondensation in mouse sperm in vitro. Biol. Membr. 2010, 4, 349–353. [Google Scholar] [CrossRef]
- Makhluf, S.B.D.; Arnon Patra, C.R.; Mukhopadhyay, D.; Gedanken, A.; Mukherjee, P.; Breitbart, H. Labeling of sperm cells via the spontaneous penetration of Eu3+ ions as nanoparticles complexed with PVA or PVP. J. Phys. Chem. C 2008, 112, 12801–12807. [Google Scholar] [CrossRef]
- Zhu, R.R.; Wang, S.L.; Chao, J.; Shi, D.L.; Zhang, R.; Sun, X.Y.; Yao, S.D. Bio-effects of nano-TiO2 on DNA and cellular ultrastructure with different polymorph and size. Mater. Sci. Eng. C 2009, 29, 691–696. [Google Scholar] [CrossRef]
- Hou, J.; Wan, X.Y.; Wang, F.; Xu, G.F.; Liu, Z. Effects of titanium dioxide nanoparticles on development and maturation of rat preantral follicle in vitro. Acad. J. Second Mil. Med. Univ. 2009, 29, 869–873. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, J.; Zhang, X.; Huo, K.; Wong, H.M.; Yeung, K.W.; Zhang, W.; Hu, T.; Chu, P.K. Activation of mitogen-activated protein kinases cellular signal transduction pathway in mammalian cells induced by silicon carbide nanowires. Biomaterials 2010, 31, 7856–7862. [Google Scholar] [CrossRef] [PubMed]
- Hinther, A.; Vawda, S.; Skirrow, R.C.; Veldhoen, N.; Collins, P.; Cullen, J.T.; van Aggelen, G.; Helbing, C.C. Nanometals induce stress and alter thyroid hormone action in amphibia at or below North American water quality guidelines. Environ. Sci. Technol. 2010, 44, 8314–8321. [Google Scholar] [CrossRef] [PubMed]
- Asare, N.; Instanes, C.; Sandberg, W.J.; Refsnes, M.; Schwarze, P.; Kruszewski, M.; Brunborg, G. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology 2012, 291, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sycheva, L.P.; Zhurkov, V.S.; Iurchenko, V.V.; Daugel-Dauge, N.O.; Kovalenko, M.A.; Krivtsova, E.K.; Durnev, A.D. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat. Res. 2011, 726, 8–14. [Google Scholar] [CrossRef]
- Li, W.Q.; Wang, F.; Liu, Z.M.; Wang, Y.C.; Wang, J.; Sun, F. Gold nanoparticles elevate plasma testosterone levels in male mice without affecting fertility. Small 2013, 27, 1708–1714. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Zhang, X.; Zhao, Z.; Liu, H.; George, R.; Wilson-Rawls, J.; Chang, Y.; Chen, Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 2011, 83, 461–467. [Google Scholar] [CrossRef]
- Gao, G.; Ze, Y.; Li, B.; Zhao, X.; Zhang, T.; Sheng, L.; Hu, R.; Gui, S.; Sang, X.; Sun, Q.; et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012, 243, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Zeng, J.; Sun, S.; Deng, H.; Luo, H.; Li, W. Chromium(III) nanoparticles affect hormone and immune responses in heat-stressed rats. Biol. Trace Elem. Res. 2009, 129, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, Q.; Xu, H.; Wang, Y.; Li, M. Chronic nanoplastic exposure induced oxidative and immune stress in medaka gonad. Sci. Total Environ. 2023, 869, 161838. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Yen, P.L.; Kuo, Y.H.; Chang, C.H.; Liao, V.H. Nanoplastic exposure in soil compromises the energy budget of the soil nematode C. elegans and decreases reproductive fitness. Environ. Pollut. 2022, 312, 120071. [Google Scholar] [CrossRef] [PubMed]
- Gartner, A.; Boag, P.R.; Blackwell, T.K. Germline survival and apoptosis. In WormBook; National Institutes of Health (NIH): Bethesda, MD, USA, 2008; pp. 1–20. [Google Scholar] [CrossRef]
- Chatterjee, A.; Maity, S.; Banerjee, S.; Dutta, S.; Adhikari, M.; Guchhait, R.; Biswas, C.; De, S.; Pramanick, K. Toxicological impacts of nanopolystyrene on zebrafish oocyte with insight into the mechanism of action: An expression-based analysis. Sci. Total Environ. 2022, 830, 154796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, Z.; Xia, Y.; Cheng, S.; Gao, J.; Sun, W.; Jiang, X.; Zhang, J.; Mao, L.; Qin, X.; et al. Repression of autophagy leads to acrosome biogenesis disruption caused by a sub-chronic oral administration of polystyrene nanoparticles. Environ. Int. 2022, 163, 107220. [Google Scholar] [CrossRef]
- Shen, H.H.; Zhang, T.; Yang, H.L.; Lai, Z.Z.; Zhou, W.J.; Mei, J.; Shi, J.W.; Zhu, R.; Xu, F.Y.; Li, D.J.; et al. Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis. Theranostics 2021, 11, 3512–3526. [Google Scholar] [CrossRef]
- Leso, V.; Battistini, B.; Vetrani, I.; Reppuccia, L.; Fedele, M.; Ruggieri, F.; Bocca, B.; Iavicoli, I. The endocrine disrupting effects of nanoplastic exposure: A systematic review. Toxicol. Ind. Health 2023, 39, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti MC, A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, M.S.; Lee, Y.; Kim, D.H.; Lee, J.S. Nanoplastics induce epigenetic signatures of transgenerational impairments associated with reproduction in copepods under ocean acidification. J. Hazard. Mater. 2023, 449, 131037. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, S.; Kim, O.H.; Kim, C.H.; Kim, J.; Kim, J.W.; Hong, S.; Lee, H.J. Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 2023, 13, 6252. [Google Scholar] [CrossRef] [PubMed]
- Schnee, M.; Sieler, M.; Dörnen, J.; Dittmar, T. Effects of polystyrene nano- and microplastics on human breast epithelial cells and human breast cancer cells. Heliyon 2024, 10, e38686. [Google Scholar] [CrossRef]
- Bozicevic, L.; Altmann, K.; Hildebrandt, J.; Knigge, X.; Vrcek, V.; Peranic, N.; Kalčec, N.; Vrček, I.V. Estrogenic activity of plastic nanoparticle mixtures under settings. Environ. Sci. Nano 2024, 11, 2112–2126. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Obayemi, A.; Wigestrand, M.B.; Fote, G.M.; Calarco, C.A.; Li, A.M.; Picciotto, M.R. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef]
- Neumann, I.D. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 2008, 20, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Manzanares, P.A.; Isoardi, N.A.; Carrer, H.F.; Molina, V.A. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 2005, 25, 8725–8734. [Google Scholar] [CrossRef] [PubMed]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Linnstaedt, S.D.; Zannas, A.S.; McLean, S.A.; Koenen, K.C.; Ressler, K.J. Literature review and methodological considerations for understanding circulating risk biomarkers following trauma exposure. Mol. Psychiatry 2020, 25, 1986–1999. [Google Scholar] [CrossRef]
- van Zuiden, M.; Geuze, E.; Willemen, H.L.; Vermetten, E.; Maas, M.; Amarouchi, K.; Kavelaars, A.; Heijnen, C.J. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: A prospective study. Biol. Psychiatry 2012, 71, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.S.; Maihofer, A.X.; Glatt, S.J.; Tylee, D.S.; Chandler, S.D.; Tsuang, M.T.; Risbrough, V.B.; Baker, D.G.; O’Connor, D.T.; Nievergelt, C.M.; et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol. Psychiatry 2015, 20, 1538–1545. [Google Scholar] [CrossRef]
- Yu, S.; Chen, C.; Pan, Y.; Kurz, M.C.; Datner, E.; Hendry, P.L.; Velilla, M.A.; Lewandowski, C.; Pearson, C.; Domeier, R.; et al. Genes known to escape X chromosome inactivation predict co-morbid chronic musculoskeletal pain and posttraumatic stress symptom development in women following trauma exposure. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2019, 180, 415–427. [Google Scholar] [CrossRef]
- Linnstaedt, S.D.; Rueckeis, C.A.; Riker, K.D.; Pan, Y.; Wu, A.; Yu, S.; Wanstrath, B.; Gonzalez, M.; Harmon, E.; Green, P.; et al. MicroRNA-19b predicts widespread pain and posttraumatic stress symptom risk in a sex-dependent manner following trauma exposure. Pain 2020, 161, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.R.; van Hage, P.; Hunting, E.R.; Haramis, A.G.; Vink, S.C.; Vijver, M.G.; Schaaf MJ, M.; Tudorache, C. Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun. Biol. 2019, 2, 382. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Hsu, L.F.; Wu, I.L.; Wang, Y.L.; Chen, W.C.; Liu, Y.J.; Yang, L.T.; Tan, C.L.; Luo, Y.H.; Wang, C.C.; et al. Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice. J. Hazard. Mater. 2023, 430, 128431. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Ren, X.; Duan, X. Parental transfer of nanopolystyrene-enhanced tris(1,3-dichloro-2-propyl) phosphate induces transgenerational thyroid disruption in zebrafish. Aquat. Toxicol. 2021, 236, 105871. [Google Scholar] [CrossRef] [PubMed]
- Amereh, F.; Eslami, A.; Fazelipour, S.; Rafiee, M.; Zibaii, M.I.; Babaei, M. Thyroid endocrine status and biochemical stress responses in adult male Wistar rats chronically exposed to pristine polystyrene nanoplastics. Toxicol. Res. 2019, 8, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Hedayati, A.; Taheri Mirghaed, A.; Ghelichpour, M. Toxic effects of copper sulfate and copper nanoparticles on minerals, enzymes, thyroid hormones and protein fractions of plasma and histopathology in common carp Cyprinus carpio. Exp. Toxicol. Pathol. 2016, 68, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.L.; Wang, D.; Luan, Y.L.; Huang, S.N.; Liu, L.Y.; Guo, Y. A review on micro- and nanoplastics in humans: Implication for their translocation of barriers and potential health effects. Chemosphere 2024, 361, 142424. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307 Pt 1, 135662. [Google Scholar] [CrossRef]
- Chen, G.; Shan, H.; Xiong, S.; Zhao, Y.; van Gestel, C.A.M.; Qiu, H.; Wang, Y. Polystyrene nanoparticle exposure accelerates ovarian cancer development in mice by altering the tumor microenvironment. Sci. Total Environ. 2024, 906, 167592. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiriccò, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Huang, G.Y.; Li, X.P.; Lei, D.Q.; Wang, C.S.; Ying, G.G. Endocrine disruptor responses in the embryos of marine medaka (Oryzias melastigma) after exposure to aged plastic leachates. Aquat. Toxicol. 2023, 261, 106635. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fan, M.; Zhan, J.; Zhang, X.; Lu, S.; Chai, M.; Zhang, Y.; Zhao, X.; Li, S.; Zhang, D. In silico bioactivity prediction of proteins interacting with graphene-based nanomaterials guides rational design of biosensor. Talanta 2024, 277, 126397. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, Z.; Saikia, S.; Jha, A.N. Interaction of Nanomaterials with Protein-Peptide. Curr. Protein Pept. Sci. 2022, 23, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, M.; Ortuño Guzmán, F.M.; Han, D.; Stojkovic, P.; Dopazo, J.; Stankovic, K.M. Polystyrene nanoplastics affect transcriptomic and epigenomic signatures of human fibroblasts and derived induced pluripotent stem cells: Implications for human health. Environ. Pollut. 2023, 320, 120849. [Google Scholar] [CrossRef]
- Zingaro, F.; Gianoncelli, A.; Ceccone, G.; Birarda, G.; Cassano, D.; La Spina, R.; Agostinis, C.; Bonanni, V.; Ricci, G.; Pascolo, L. Morphological and lipid metabolism alterations in macrophages exposed to model environmental nanoplastics traced by high-resolution synchrotron techniques. Front. Immunol. 2023, 14, 1247747. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.X. Single-Cell RNA Sequencing Profiling Cellular Heterogeneity and Specific Responses of Fish Gills to Microplastics and Nanoplastics. Environ. Sci. Technol. 2024, 58, 5974–5986. [Google Scholar] [CrossRef]
- Deng, J.; Zeng, X.; Li, J.; Luo, L.; Yang, Y.; Luan, T. Single-cell transcriptomic analysis reveals heterogeneity of the patterns of responsive genes and cell communications in liver cell populations of zebrafish exposed to polystyrene nanoplastics. Sci. Total Environ. 2023, 889, 164082. [Google Scholar] [CrossRef] [PubMed]
| Tissues | Types of Nanoparticles | Size (nm) | Concentrations | Effects | Ref. | |
|---|---|---|---|---|---|---|
| In vitro | Male reproductive system | TiO2 Au Eu2O3 | 25–70 2.5 9.3–15.4 | 0–1000 μg/mL 0.5–1.0 × 1015 particles/mL 2.5 mg/mL | Reduced spermatogenesis, biosynthesis and catabolic pathways of testosterone, DNA damage in sperm, loss of spermatozoa motility | [41,42,43] |
| Female reproductive system | TiO2 SiCNWs | 50–60 25 80 | 0–100 μg/mL 12.5–50 μg/mL 0.5–10 μg/mL | Cytotoxicity, reduced viability, increased genotoxicity, inhibition of oocyte maturation and follicle development | [44,45,46] | |
| Thyroid | Ag, Zn, QDs | 2–15 | 0.1, 5–10 nM | Reduced expression of TH-induced receptor β (TRβ) and TH-repressed Rana larval keratin type I (RLKI) | [47] | |
| In vivo | Male reproductive system | TiO2 mPEG@Au | 20 33 28.2 | 0–100 μg/mL 0–1000 mg/kg 45–225 mg/kg | Cytotoxicity, testicular apoptosis, sperm abnormalities | [48,49,50] |
| Female reproductive system | TiO2 | 240–280 208–330 | 0.1, 1 mg/L 10 mg/Kg | Reduced oocyte and follicular maturation, increase of estradiol levels | [51,52] | |
| Thyroid | Cr | 40–70 | 150, 300, 450 μg/Kg | No effects in THSA, FT3 and FT4 serum levels | [53] |
| Organisms | Cells and Tissues | Genes | Effects | Ref. |
|---|---|---|---|---|
| Copepods | Whole body | Hsp70, CuZn SOD, CALM3, CIDEc and p53 | All hypermethylated | [63] |
| Zebrafish | Oocytes | sod, gpx, nrf2, inos, ucp2, atp6 nfkβ, tnfα, il-10, ikβ, gdf9, bmp15, gadd45, rad51, p53 and bcl2 | All overexpressed | [57] |
| Whole body (larvae) | g6pca and pck1 | Both overexpressed | [78] | |
| Rats | Spermatozoa | Gba2, Pick1, Gopc, Hrb, Zpbp1, Spaca1 and Dpy19l2 | Gopc and Dpy19l2 downregulated | [58] |
| Human | Breast | TMBIM6, AP2M1, PTP4A2 and FTH1 | All overexpressed | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisi, M.; Poma, A.M.G. Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective. Int. J. Mol. Sci. 2025, 26, 2071. https://doi.org/10.3390/ijms26052071
Aloisi M, Poma AMG. Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective. International Journal of Molecular Sciences. 2025; 26(5):2071. https://doi.org/10.3390/ijms26052071
Chicago/Turabian StyleAloisi, Massimo, and Anna Maria Giuseppina Poma. 2025. "Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective" International Journal of Molecular Sciences 26, no. 5: 2071. https://doi.org/10.3390/ijms26052071
APA StyleAloisi, M., & Poma, A. M. G. (2025). Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective. International Journal of Molecular Sciences, 26(5), 2071. https://doi.org/10.3390/ijms26052071






