An Update of Salivary Biomarkers for the Diagnosis of Alzheimer’s Disease
Abstract
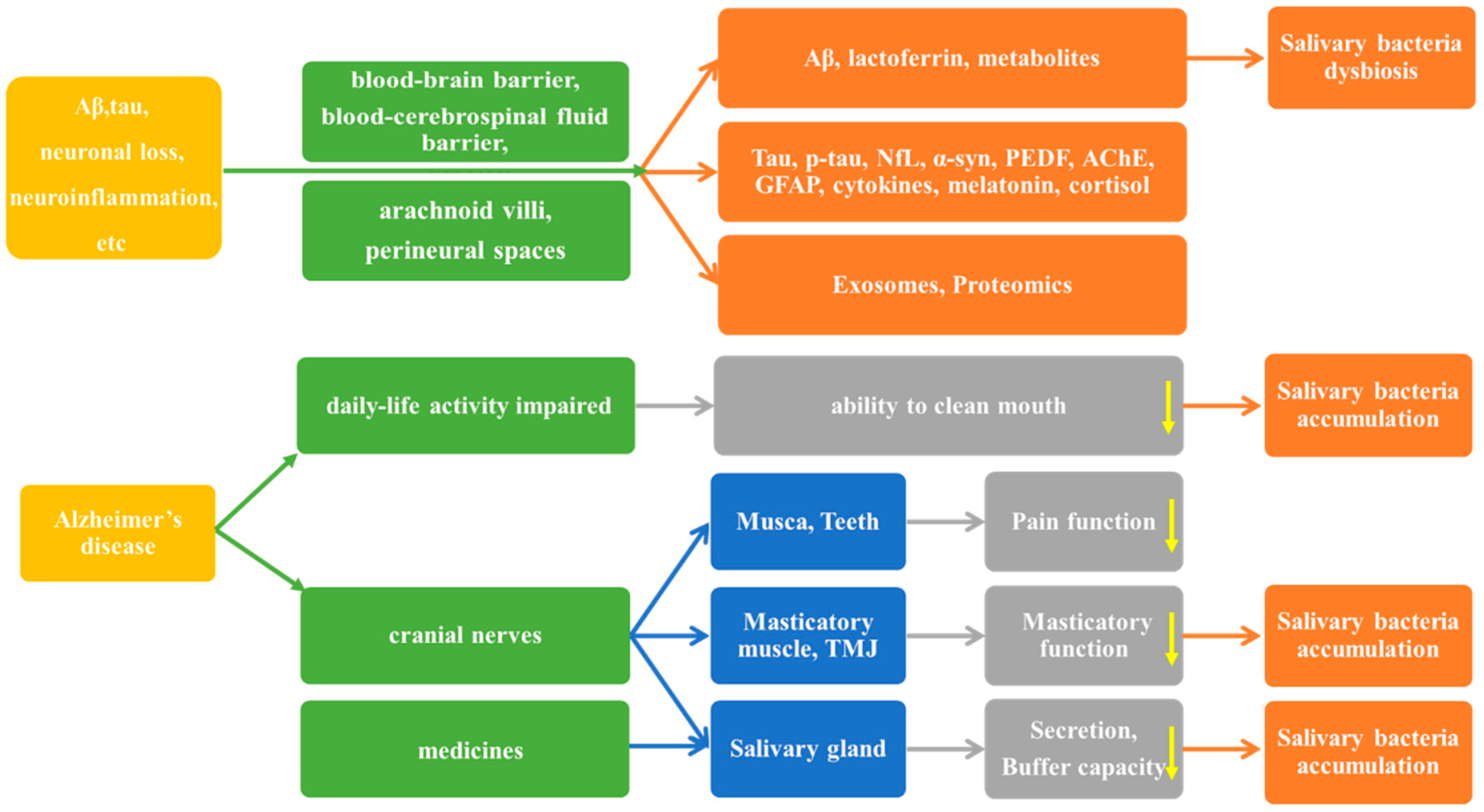
1. Introduction
2. Results
2.1. Direct Biomarkers of the Pathological Changes of AD
2.1.1. Aβ
2.1.2. Tau Protein
2.1.3. The Salivary Biomarkers of Neurodegeneration or Neuronal Injury
2.1.4. Acetylcholinesterase (AChE) Activity in Saliva
2.1.5. Neuroinflammation Markers in Saliva
2.2. The Indirect Biomarkers of AD in Saliva
2.2.1. Lactoferrin
2.2.2. Salivary Melatonin
2.2.3. Salivary Cortisol
2.2.4. Oxidative Stress Markers in Saliva
2.2.5. The Biomarkers of AD in Salivary Exosomes
2.2.6. Salivary Proteomics
2.2.7. Salivary Metabolites
2.2.8. Potential AD Biomarkers in the Salivary Microbiome
α and β Diversity of the Salivary Microbiome
Significant Bacteria of the Salivary Microbiome Between Groups
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β peptide Aβ |
| AChE | Acetylcholinesterase |
| AchE-I | Acetylcholinesterase inhibitors |
| AD | Alzheimer’s disease |
| ADA | Adenosine deaminase |
| AGE | Advanced glycation end products |
| aMCI | Mild Cognitive Impairment due to AD |
| AOPP | Advanced oxidation protein products |
| APP | Amyloid precursor protein |
| ASV | Amplicon sequence variants (ASV) |
| CAT | Catalase |
| CC4 | Complement C4; |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| CST-C | Cystatin-C |
| ELISA | Enzyme-linked immunosorbent assay |
| FIA-MS/MS | Flow injection analysis-tandem mass spectrometry |
| FRAP | Ferric reducing ability of plasma |
| FUPLC-MS | Faster ultra-high performance liquid chromatography-mass spectrometry |
| GC-MS | Gas chromatograph-mass spectrometry |
| GFAP | Glial fibrillary acidic protein |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| Hp | Haptoglobin |
| HPLC-ESI-IT-MS | High-performance liquid chromatography separation coupled to electrospray ion trap mass spectrometry |
| IL-1 | Interleukin-1 |
| IL-1RN | Interleukin-1 receptor antagonist |
| IMA | Immunology multiplex assay |
| LC-MS/MS | Liquid-chromatography/mass spectroscopy |
| MDA | Malondialdehyde |
| MIP-4 | Macrophage inflammatory protein-4 |
| MMP-9 | Matrix metalloproteinase 9 |
| MMSE | Mini Mental State Examination |
| MNI | Magnetic nanoparticle immunoassay |
| NA | Not applicable |
| ncRNAs | non-coding RNAs |
| NfL | Neurofilament light chain |
| NIA-AA | National Institute on Aging Alzheimer’s Association |
| NINCDS–ADRDA | National Institute on Neurological Communicative Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association |
| NMR | nuclear magnetic resonance |
| NO | Nitric oxide |
| OSI | Oxidative stress index |
| OTU | Operational taxonomic unit |
| PEDF | Pigment epithelium-derived factor |
| p-tau | Phosphorylated tau |
| Px | Peroxidase |
| SFN | Stratifin |
| Simoa | Single molecule array |
| SOD | Superoxide dismutase |
| 16S rRNA | 16S ribosomal ribosomal RNA |
| α-syn | α-synuclein |
| TAC | Mean total antioxidant capacity |
| TBARS | Thiobarbituric acid reactive substance |
| TNF | Tumor necrosis factor |
| TOS | Mean total oxidant status |
| t-tau | Total tau |
| UPLC-MS/MS | Ultra-performance liquid chromatography coupled to tandem mass spectrometry |
| UA | Uric acid |
| WHO | World Health Organization |
| V3 | Third hypervariable region |
| V4 | Fourth hypervariable region |
Appendix A
| Authors (Year) | AD | Control | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|
| Aβ42 | ||||||
| Bermejo et al. (2010) [43] a | 7.67 ± 16.25 | 2.89 ± 4.96 | 0.043 | 0.16 | 0.93 | 0.547 |
| Boschi et al. (2022) [38] a | 127.11 ± 33.44 | 66.11 ± 24.82 | <0.001 | 0.84 | 0.68 | / |
| Cui et al. (2022) [39] | / | / | <0.05 | / | / | 0.8483 |
| Katsipis et al. (2021) [44] b | 10.43 ± 3.56 | 3.22 ± 1.13 | <0.0001 | / | / | / |
| Lee et al. (2017) [37] a,c | 59.07 ± 6.33 | 22.06 ± 0.4 | <0.001 | / | / | / |
| McGeer et al. (2020) [40] a | 51.70 ± 10.50 | / | <0.05 | / | / | / |
| Sabaei et al. (2023) [42] a,d | 104.3 ± 155.2 | 13.5 ± 21.5 | <0.001 | 0.625 | 0.91 | 0.81 |
| Sabbagh et al. (2018) [36] a | 51.7 ±1.6 | 21.1 ±0.3 | <0.05 | / | / | / |
| Tvarijonaviciute et al. (2020) [45] a | 3.15 ± 0.72 | 3.57 ± 0.93 | 0.041 | / | / | / |
| Aβ40 | ||||||
| Bermejo et al. (2010) [43] a | 21.87 ± 5.7 | 20.82 ± 5.55 | >0.05 | / | / | / |
| Cui et al. (2022) [39] | / | / | >0.05 | / | / | 0.5311 |
| Tvarijonaviciute et al. (2020) [45] a | 21.98 ± 16.94 | 19.97 ± 6.35 | 0.515 | / | / | / |
| Authors (Year) | AD | Control | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|
| p-tau | ||||||
| Cui et al. (2022) [39] | / | / | >0.05 | / | / | 0.5831 |
| Katsipis et al. (2021) [44] a | 33.87 ± 4.86 | 18.16 ± 5.67 | <0.0001 | / | / | / |
| Marksteiner et al. (2022) [48] a | 22.5 ± 3.6 | 9.7 ± 1.3 | >0.05 | / | / | / |
| Sabaei et al. (2023) [42] b,c | 9.2 ± 10.9 | 4.2 ± 6.1 | 0.001 | 0.917 | 0.638 | 0.78 |
| Tvarijonaviciute et al. (2020) [45] b | 40.33 ± 42.95 | 42.5 ± 38.35 | 0.813 | / | / | / |
| t-tau | ||||||
| Cui et al. (2022) [39] | / | / | >0.05 | / | / | 0.505 |
| Marksteiner et al. (2022) [48] a | 260 ± 53 | 577 ± 134 | <0.05 | / | / | / |
| Tvarijonaviciute et al. (2020) [45] b | 21.57 ± 22.11 | 21.15 ±16.58 | 0.923 | / | / | / |
| p-tau/t-tau | ||||||
| Cui et al. (2022) [39] | / | / | >0.05 | / | / | 0.6344 |
| Marksteiner et al. (2022) [48] d | 41 ± 17 | 78 ± 17 | >0.05 | / | / | / |
| Pekeles et al. (2019) [54] | / | / | <0.05 | 0.73 | 0.5 | / |
| Authors (Year) | Biomarker | AD | Control | Unit | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|---|
| Gleerup et al. (2021) [58] | NfL | 2.1 ± 1.6 | 2.3 ± 2.0 | pg/mL | >0.05 | / | / | / |
| Sabaei et al. (2023) [42] | α-syn | 7.8 ± 6.6 | 12.5 ± 6.3 | pg/mg | <0.001 | 0.667 | 0.682 | 0.71 |
| Tvarijonaviciute et al. (2020) [45] | PEDF | 31.41 ± −64.38 | 22.86 ± −49.21 | pg/mL | 0.52 | / | / | / |
| Authors (Year) | AD | Control | Unit | p-Value |
|---|---|---|---|---|
| Ahmadi et al. (2019) [64] | 20.99 ± 10.99 | 13.08 ± 7.23 | Unreported | 0.002 |
| Boston et al. (2008) [66] | 0.039 ± 0.3 | 0.040 ± 0.044 | a.u./50 μg | >0.05 |
| Authors (Year) | Biomarker | AD | Control | Unit | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|---|
| Katsipis et al. (2021) [44] | IL-1 | 18.08± 29.03 | 281.2± 75.13 | pg/mg | <0.001 | / | / | / |
| IL-6 | 14.58 ± 5.88 | 33.60 ± 3.56 | pg/mg | <0.001 | / | / | / | |
| TNF-α | 2.44 ±1.69 | 10.01 ± 2.82 | pg/mg | <0.001 | / | / | / | |
| COX-2 | 81.06 ± 15.65 × 103 | 50.28 ± 7.70 × 103 | pg/mg | <0.001 | / | / | / | |
| Caspase-8 | 4.29 ± 1.53 × 103 | 1.58 ± 0.77 × 103 | pg/mg | <0.001 | / | / | / | |
| GFAP a | 3.56 ± 2.24 | 13.35 ± 3.03 | ng/mg | <0.0001 | 0.75 | 1 | / | |
| GFAP b | 4.57 ± 1.69 | 11.88 ± 2.42 | ng/mg | <0.0001 | 0.85 | 0.75 | / | |
| Tvarijonaviciute et al. (2020) [45] | CRP | 73.59 ± −64.1 | 57.38 ± −66.75 | pg/mL | 0.311 | / | / | / |
| α1 Antitrypsin | 28.71 ± −108.62 | 17.89 ± −34.01 | pg/mL | 0.583 | / | / | / | |
| MIP−4 | 0.49 ± −0.55 | 0.4 ± −0.57 | pg/mL | 0.496 | / | / | / | |
| CC4 | 22.95 ± −17.66 | 15.37 ± −11.22 | pg/mL | 0.048 | / | / | 0.613 | |
| ADA | 7.61 ± −6.31 | 8.62 ± −11.05 | IU/L | 0.529 | / | / | / | |
| Hp | 2098.62 ± −1225.68 | 2252.91 ± −1510.63 | ng/mL | 0.532 | / | / | / | |
| Zalewska et al. (2021) [21] | IL-1β c | 88.47 | 70.58 | ng/mg | <0.0001 | 0.84 | 0.84 | 0.8528 |
| Authors (Year) | AD | Control | Unit | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Carro et al. (2017) [80] | 4.78 ± 1.11 | 10.24 ± 1.96 | μg/mL | <0.001 | 1 | 0.986 | 0.984 |
| Gonzalez et al. (2020) [81] | 67.2 ± 26.3 | / | μg/mL | <0.05 | 0.8696 | 0.9167 | 0.95 |
| Gleerup et al. (2021) [83] | 26.9 ± 26.3 | 16.4 ± 6.6 | μg/mL | >0.05 | / | / | / |
| Zalewska et al. (2021) [21] a | 24.52 | 29.97 | μg/mg | 0.0211 | 0.64 | 0.64 | 0.6896 |
| Authors (Year) | AD | Control | Unit | p-Value |
|---|---|---|---|---|
| Giubilei et al. (2001) [91] | 16.55 ± 12.38 | 10.31 ± 4.14 | μg/dL | <0.05 |
| James et al. (2019) [92] a | 0.82 ± 0.33 | 0.80 ± 0.31 | / | 0.761 |
| Pena-Bautista et al. (2019) [93] b | 0.9 | 0.51 | ng/mg | >0.05 |
| Authors (Year) | Biomarker | AD | Control | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Tvarijonaviciute et al. (2020) [45] | FRAP | 0.94 ± −0.55 | 1.06 ± −0.74 | 0.274 | / | / | / |
| Zalewska et al. (2021) [21] | SOD | / | / | 0.007 | 0.6957 | 0.68 | 0.7774 |
| CAT | / | / | <0.0001 | 0.8261 | 0.84 | 0.9183 | |
| GPx | / | / | 0.0037 | 0.7391 | 0.72 | 0.7409 | |
| UA | / | / | 0.38955 | 0.5217 | 0.52 | 0.5739 | |
| GSH | / | / | 0.03122 | 0.7273 | 0.72 | 0.6836 | |
| TAC | / | / | 0.838 | 0.5217 | 0.52 | 0.5183 | |
| TOS | / | / | <0.0001 | 0.913 | 0.92 | 0.92 | |
| OSI | / | / | <0.0001 | 0.9 | 0.92 | 0.936 | |
| AGE | / | / | <0.0001 | 0.8696 | 0.88 | 0.9357 | |
| AOPP | / | / | 0.0285 | 0.56 | 0.56 | 0.68 | |
| MDA | / | / | 0.0297 | 0.6667 | 0.68 | 0.6876 | |
| NO | / | / | 0.0371 | 0.56 | 0.56 | 0.672 | |
| Peroxynitrite | / | / | 0.0001 | 0.6364 | 0.7917 | 0.8163 | |
| Nitrotyrosine | / | / | 0.0175 | 0.6364 | 0.64 | 0.7018 |
| Authors (Year) | AD | Control | Unit | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Ryu et al. (2023) [100] | 0.0483 ± 0.0278 | 0.0205 ± 0.0082 | Pg | <0.05 | 0.7407 | 0.9231 | 0.775 |
| Authors (Year) | Biomarker | AD | Control | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Contini et al. (2021) [103] a | α-defensins | 3.9 ± 4.0 × 105 | 1.2 ± 1.5 × 105 | 0.0005 | / | / | / |
| thymosin β4 | 0.7 ± 0.8 × 105 | 0.2 ± 0.4 × 105 | 0.0005 | / | / | / | |
| cystatin B | 1.7 ± 1.9 × 105 | 0.6 ± 0.6 × 105 | 0.002 | / | / | / | |
| Eldem et al. (2022) [101] b,c | TTR | 0.519 ± 0.107 | 0.99 ± 0.149 | <0.05 | / | / | / |
| Authors (Year) | Biomarker | AD/Control Fold Change | p-Value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|
| Huan et al. (2018) [108] and Sapkota et al. (2018) [110] | Methylguanosine | 4.28 | <0.05 | 0.9852 | 0.9655 | 0.997 |
| Histidinyl- Phenylalanine | 5.06 | <0.05 | ||||
| Choline-cytidine | 4.39 | <0.05 | ||||
| Liang et al. (2015) [109] | sphinganine-1- phosphate | 12.11 | <0.05 | 0.994 | 0.982 | 0.998 |
| ornithine | 3.94 | <0.05 | 0.819 | 0.907 | 0.927 | |
| phenyllactic | 3.44 | <0.05 | 0.795 | 0.843 | 0.831 | |
| Yilmaz et al. (2017) [107] | propionate and acetone | / | <0.05 | 0.9 | 0.944 | 0.897 |
| Authors (Year) | Biomarker | AD | Control | Unit | p-Value |
|---|---|---|---|---|---|
| Marksteiner et al. (2019) [106] | PCae C34:(1 + 2) | 358 ± 80 | 985 ± 233 | μM | 0.008 |
| PCae C36:(1 + 2 + 3) | 224 ± 34 | 593 ± 108 | μM | 0.0011 | |
| PCae C38:(1 + 3) | 57 ± 10 | 135 ± 27 | μM | 0.009 | |
| PCae C40:(2 + 3) | 53 ± 11 | 128 ± 27 | μM | 0.011 |
References
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 7 August 2024).
- Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Rafii, M.S.; Aisen, P.S. Detection and treatment of Alzheimer’s disease in its preclinical stage. Nature Aging. 2023, 3, 520–531. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef]
- Hrubešová, K.; Fousková, M.; Habartová, L.; Fišar, Z.; Jirák, R.; Raboch, J.; Setnička, V. Search for biomarkers of Alzheimer’s disease: Recent insights, current challenges and future prospects. Clin. Biochem. 2019, 72, 39–51. [Google Scholar] [CrossRef]
- Gunes, S.; Aizawa, Y.; Sugashi, T.; Sugimoto, M.; Rodrigues, P.P. Biomarkers for Alzheimer’s Disease in the Current State: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 4962. [Google Scholar] [CrossRef]
- Counts, S.E.; Ikonomovic, M.D.; Mercado, N.; Vega, I.E.; Mufson, E.J. Biomarkers for the Early Detection and Progression of Alzheimer’s Disease. Neurotherapeutics 2017, 14, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Balasa, A.F.; Chircov, C.; Grumezescu, A.M. Body Fluid Biomarkers for Alzheimer’s Disease-An Up-To-Date Overview. Biomedicines 2020, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frolich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Robinson, R.A.; Amin, B.; Guest, P.C. Multiplexing Biomarker Methods, Proteomics and Considerations for Alzheimer’s Disease. Adv. Exp. Med. Biol. 2017, 974, 21–48. [Google Scholar] [CrossRef] [PubMed]
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef]
- Solorzano, A.; Brady, M.; Bhatt, N.; Johnson, A.; Burgess, B.; Leyva, H.; Puangmalai, N.; Jerez, C.; Wood, R.; Kayed, R.; et al. Central and peripheral tau retention modulated by an anti-tau antibody. bioRxiv 2023. [Google Scholar] [CrossRef]
- Liu, Z.H.; Wang, Y.J.; Bu, X.L. Alzheimer’s disease: Targeting the peripheral circulation. Mol. Neurodegener. 2023, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Park, T.J.; Huang, X.; Kim, M.O. Abnormal amyloid beta metabolism in systemic abnormalities and Alzheimer’s pathology: Insights and therapeutic approaches from periphery. Ageing Res. Rev. 2021, 71, 101451. [Google Scholar] [CrossRef]
- Floden, A.M.; Sohrabi, M.; Nookala, S.; Cao, J.J.; Combs, C.K. Salivary Abeta Secretion and Altered Oral Microbiome in Mouse Models of AD. Curr. Alzheimer Res. 2020, 17, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Singhrao, S.K. Low levels of salivary lactoferrin may affect oral dysbiosis and contribute to Alzheimer’s disease: A hypothesis. Med. Hypotheses 2021, 146, 110393. [Google Scholar] [CrossRef]
- Zalewska, A.; Klimiuk, A.; Zięba, S.; Wnorowska, O.; Rusak, M.; Waszkiewicz, N.; Szarmach, I.; Dzierżanowski, K.; Maciejczyk, M. Salivary gland dysfunction and salivary redox imbalance in patients with Alzheimer’s disease. Sci. Rep. 2021, 11, 23904. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Allsop, D.; Karim, S.; Martin, F.L.; Crean, S. Diagnostic Biomarkers for Alzheimer’s Disease Using Non-Invasive Specimens. J. Clin. Med. 2020, 9, 1673. [Google Scholar] [CrossRef]
- Aragón, F.; Zea-Sevilla, M.A.; Montero, J.; Sancho, P.; Corral, R.; Tejedor, C.; Frades-Payo, B.; Paredes-Gallardo, V.; Albaladejo, A. Oral health in Alzheimer’s disease: A multicenter case-control study. Clin. Oral. Investig. 2018, 22, 3061–3070. [Google Scholar] [CrossRef]
- Campos, C.H.; Ribeiro, G.R.; Costa, J.L.; Rodrigues Garcia, R.C. Correlation of cognitive and masticatory function in Alzheimer’s disease. Clin. Oral. Investig. 2017, 21, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Chu, C.H.; Young, F.Y.F. Oral Health and Care for Elderly People with Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2020, 17, 5713. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Barrios, R.; Sánchez-Lara, I.; Carnero-Pardo, C.; Fornieles-Rubio, F.; Montes, J.; Gonzalez-Moles, M.A.; Bravo, M. Prevalence of Drug-Induced Xerostomia in Older Adults with Cognitive Impairment or Dementia: An Observational Study. Drugs Aging 2016, 33, 611–618. [Google Scholar] [CrossRef]
- Zheng, H.; Koo, E.H. Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 2011, 6, 27. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Xia, W.; Zhang, Y.; Wang, C. Targeting Amyloidogenic Processing of APP in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 137. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Q.; Chen, Y.X.; Zhao, Y.F.; Li, Y.M. Abeta42 and Abeta40: Similarities and differences. J. Pept. Sci. 2015, 21, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, S.J.; Hong, S.; Kim, Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scherber, A.; Richter, K.; Schaps, P. Distribution of antiepileptic drugs between plasma, plasma water, cerebrospinal fluid, saliva and brain. Monogr. Neural. Sci. 1980, 5, 208–212. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Shi, J.; Lee, M.; Arnold, L.; Al-Hasan, Y.; Heim, J.; McGeer, P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 2018, 18, 155. [Google Scholar] [CrossRef]
- Lee, M.; Guo, J.P.; Kennedy, K.; McGeer, E.G.; McGeer, P.L. A Method for Diagnosing Alzheimer’s Disease Based on Salivary Amyloid-beta Protein 42 Levels. J. Alzheimer’s Dis. 2017, 55, 1175–1182. [Google Scholar] [CrossRef]
- Boschi, S.; Roveta, F.; Grassini, A.; Marcinnò, A.; Cermelli, A.; Ferrandes, F.; Rainero, I.; Rubino, E. Aβ42 as a Biomarker of Alzheimer’s Disease: Is Saliva a Viable Alternative to Cerebrospinal Fluid? Brain. Sci. 2022, 12, 1729. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Zhu, J.; Liao, Z.; Wang, S.; Liu, W. Investigation of Whole and Glandular Saliva as a Biomarker for Alzheimer’s Disease Diagnosis. Brain Sci. 2022, 12, 595. [Google Scholar] [CrossRef]
- McGeer, P.L.; Lee, M.; Kennedy, K.; McGeer, E.G. Saliva Diagnosis as a Disease Predictor. J. Clin. Med. 2020, 9, 377. [Google Scholar] [CrossRef]
- Kim, C.B.; Choi, Y.Y.; Song, W.K.; Song, K.B. Antibody-based magnetic nanoparticle immunoassay for quantification of Alzheimer’s disease pathogenic factor. J. Biomed. Opt. 2014, 19, 051205. [Google Scholar] [CrossRef]
- Sabaei, M.; Rahimian, S.; Haj Mohamad Ebrahim Ketabforoush, A.; Rasoolijazi, H.; Zamani, B.; Hajiakhoundi, F.; Soleimani, M.; Shahidi, G.; Faramarzi, M. Salivary levels of disease-related biomarkers in the early stages of Parkinson’s and Alzheimer’s disease: A cross-sectional study. IBRO Neurosci. Rep. 2023, 14, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Antequera, D.; Vargas, T.; Molina, J.A.; Carro, E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: A pilot study. BMC Neurol. 2010, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Katsipis, G.; Tzekaki, E.E.; Tsolaki, M.; Pantazaki, A.A. Salivary GFAP as a potential biomarker for diagnosis of mild cognitive impairment and Alzheimer’s disease and its correlation with neuroinflammation and apoptosis. J. Neuroimmunol. 2021, 361, 577744. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Zamora, C.; Ceron, J.J.; Bravo-Cantero, A.F.; Pardo-Marin, L.; Valverde, S.; Lopez-Jornet, P. Salivary biomarkers in Alzheimer’s disease. Clin. Oral Investig. 2020, 24, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sui, Y.T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary tau species are potential biomarkers of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 299–305. [Google Scholar] [CrossRef]
- Lau, H.C.; Lee, I.K.; Ko, P.W.; Lee, H.W.; Huh, J.S.; Cho, W.J.; Lim, J.O. Non-invasive screening for Alzheimer’s disease by sensing salivary sugar using Drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EG-ISFET) biosensor. PLoS ONE 2015, 10, e0117810. [Google Scholar] [CrossRef]
- Marksteiner, J.; Defrancesco, M.; Humpel, C. Saliva tau and phospho-tau-181 measured by Lumipulse in patients with Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1014305. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Bull, C.F.; Fenech, M.F.; Leifert, W.R. Current State of Saliva Biomarkers for Aging and Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 56–66. [Google Scholar] [CrossRef]
- Xin, S.H.; Tan, L.; Cao, X.; Yu, J.T.; Tan, L. Clearance of Amyloid Beta and Tau in Alzheimer’s Disease: From Mechanisms to Therapy. Neurotox. Res. 2018, 34, 733–748. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Scholl, M.; Blennow, K.; Lovestone, S.; Hye, A.; Zetterberg, H. No association of salivary total tau concentration with Alzheimer’s disease. Neurobiol. Aging 2018, 70, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Pekeles, H.; Qureshi, H.Y.; Paudel, H.K.; Schipper, H.M.; Gornistky, M.; Chertkow, H. Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheimer’s Dement. 2019, 11, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef]
- Dhiman, K.; Gupta, V.B.; Villemagne, V.L.; Eratne, D.; Graham, P.L.; Fowler, C.; Bourgeat, P.; Li, Q.X.; Collins, S.; Bush, A.I.; et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimer’s Dement. 2020, 12, e12005. [Google Scholar] [CrossRef]
- Sahrai, H.; Norouzi, A.; Hamzehzadeh, S.; Majdi, A.; Kahfi-Ghaneh, R.; Sadigh-Eteghad, S. SIMOA-based analysis of plasma NFL levels in MCI and AD patients: A systematic review and meta-analysis. BMC Neurol. 2023, 23, 331. [Google Scholar] [CrossRef]
- Gleerup, H.S.; Sanna, F.; Høgh, P.; Simrén, J.; Blennow, K.; Zetterberg, H.; Hasselbalch, S.G.; Ashton, N.J.; Simonsen, A.H. Saliva Neurofilament Light Chain Is Not a Diagnostic Biomarker for Neurodegeneration in a Mixed Memory Clinic Population. Front. Aging Neurosci. 2021, 13, 659898. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Youn, Y.C.; An, S.S.A.; Kim, S. Alpha-synuclein: A pathological factor with Aβ and tau and biomarker in Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Korff, A.; Shaw, L.M.; Trojanowski, J.Q.; Zhang, J. CSF α-synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol. 2013, 126, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Qi, W.; Fang, S.; Jiang, P.; Yang, C.; Mo, Y.; Dong, C.; Li, Y.; Zhong, J.; Cai, W.; et al. Pigment Epithelium-Derived Factor Plays a Role in Alzheimer’s Disease by Negatively Regulating Aβ42. Neurotherapeutics 2018, 15, 728–741. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Sayer, R.; Law, E.; Connelly, P.J.; Breen, K.C. Association of a salivary acetylcholinesterase with Alzheimer’s disease and response to cholinesterase inhibitors. Clin. Biochem. 2004, 37, 98–104. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Tarazi, S.; Vahabian, M. Evaluation of salivary acetylcholinesterase and pseudocholinesterase in patients with Alzheimer’s disease: A case-control study. Spec. Care Dent. 2019, 39, 39–44. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Moghadam, N.B.; Ehsani, M.; Mortazavi, H.; Sabour, S.; Bakhshi, M. Can Salivary Acetylcholinesterase be a Diagnostic Biomarker for Alzheimer? J. Clin. Diagn. Res. JCDR 2017, 11, Zc58–Zc60. [Google Scholar] [CrossRef]
- Boston, P.F.; Gopalkaje, K.; Manning, L.; Middleton, L.; Loxley, M. Developing a simple laboratory test for Alzheimer’s disease: Measuring acetylcholinesterase in saliva—A pilot study. Int. J. Geriatr. Psychiatry 2008, 23, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Si, Z.Z.; Zou, C.J.; Mei, X.; Li, X.F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [CrossRef]
- McNicholas, K.; François, M.; Liu, J.W.; Doecke, J.D.; Hecker, J.; Faunt, J.; Maddison, J.; Johns, S.; Pukala, T.L.; Rush, R.A.; et al. Salivary inflammatory biomarkers are predictive of mild cognitive impairment and Alzheimer’s disease in a feasibility study. Front. Aging Neurosci. 2022, 14, 1019296. [Google Scholar] [CrossRef] [PubMed]
- Ganne, A.; Balasubramaniam, M.; Griffin, W.S.T.; Shmookler Reis, R.J.; Ayyadevara, S. Glial Fibrillary Acidic Protein: A Biomarker and Drug Target for Alzheimer’s Disease. Pharmaceutics 2022, 14, 1354. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Cheng, J.B.; Wang, J.Q.; Bu, D.P.; Liu, G.L.; Zhang, C.G.; Wei, H.Y.; Zhou, L.Y.; Wang, J.Z. Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; Gonzalez, N.; Ramon, D.; Serrano, G.; Torrens, A.; Serrano, J.M.; Navarro, M.; Genoves, S. A nutritional supplement containing lactoferrin stimulates the immune system, extends lifespan, and reduces amyloid beta peptide toxicity in Caenorhabditis elegans. Food Sci. Nutr. 2017, 5, 255–265. [Google Scholar] [CrossRef]
- Wang, L.; Sato, H.; Zhao, S.; Tooyama, I. Deposition of lactoferrin in fibrillar-type senile plaques in the brains of transgenic mouse models of Alzheimer’s disease. Neurosci. Lett. 2010, 481, 164–167. [Google Scholar] [CrossRef]
- Naidu, S.A.G.; Wallace, T.C.; Davies, K.J.A.; Naidu, A.S. Lactoferrin for Mental Health: Neuro-Redox Regulation and Neuroprotective Effects across the Blood-Brain Barrier with Special Reference to Neuro-COVID-19. J. Diet. Suppl. 2021, 20, 218–253. [Google Scholar] [CrossRef]
- Antequera, D.; Moneo, D.; Carrero, L.; Bartolome, F.; Ferrer, I.; Proctor, G.; Carro, E. Salivary Lactoferrin Expression in a Mouse Model of Alzheimer’s Disease. Front. Immunol. 2021, 12, 749468. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Del Ser, T.; Valentí, M.; de la Fuente, M.; Bartolome, F.; Carro, E. Salivary lactoferrin as biomarker for Alzheimer’s disease: Brain-immunity interactions. Alzheimer’s Dement. 2020, 16, 1196–1204. [Google Scholar] [CrossRef]
- Tsatsanis, A.; McCorkindale, A.N.; Wong, B.X.; Patrick, E.; Ryan, T.M.; Evans, R.W.; Bush, A.I.; Sutherland, G.T.; Sivaprasadarao, A.; Guennewig, B.; et al. The acute phase protein lactoferrin is a key feature of Alzheimer’s disease and predictor of Abeta burden through induction of APP amyloidogenic processing. Mol. Psychiatry 2021, 26, 5516–5531. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Bartolome, F.; Bermejo-Pareja, F.; Villarejo-Galende, A.; Molina, J.A.; Ortiz, P.; Calero, M.; Rabano, A.; Cantero, J.L.; Orive, G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimer’s Dement. 2017, 8, 131–138. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martin, V.; Gonzalez, P.; Gomez-Grande, A.; Llamas-Velasco, S.; Herrero-San Martin, A.; Perez-Martinez, D.; Villarejo-Galende, A.; et al. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. EBioMedicine 2020, 57, 102834. [Google Scholar] [CrossRef] [PubMed]
- Antequera, D.; Carrero, L.; Gonzalez-Sanchez, M.; Cantero, J.L.; Orive, G.; Municio, C.; Carro, E. Reduced Salivary Lactoferrin Levels in Early-Onset Alzheimer’s Disease. Aging Dis. 2024, 15, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Gleerup, H.S.; Jensen, C.S.; Hogh, P.; Hasselbalch, S.G.; Simonsen, A.H. Lactoferrin in cerebrospinal fluid and saliva is not a diagnostic biomarker for Alzheimer’s disease in a mixed memory clinic population. EBioMedicine 2021, 67, 103361. [Google Scholar] [CrossRef]
- Li, P.; Gao, L.; Gaba, A.; Yu, L.; Cui, L.; Fan, W.; Lim, A.S.P.; Bennett, D.A.; Buchman, A.S.; Hu, K. Circadian disturbances in Alzheimer’s disease progression: A prospective observational cohort study of community-based older adults. Lancet Healthy Longev. 2020, 1, e96–e105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.N.; Liu, R.Y.; Kamphorst, W.; Hofman, M.A.; Swaab, D.F. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 2003, 35, 125–130. [Google Scholar] [CrossRef]
- Vincent, B. Protective roles of melatonin against the amyloid-dependent development of Alzheimer’s disease: A critical review. Pharmacol. Res. 2018, 134, 223–237. [Google Scholar] [CrossRef]
- Manni, R.; Cremascoli, R.; Perretti, C.; De Icco, R.; Picascia, M.; Ghezzi, C.; Cerri, S.; Sinforiani, E.; Terzaghi, M. Evening melatonin timing secretion in real life conditions in patients with Alzheimer disease of mild to moderate severity. Sleep Med. 2019, 63, 122–126. [Google Scholar] [CrossRef]
- Weissova, K.; Bartos, A.; Sladek, M.; Novakova, M.; Sumova, A. Moderate Changes in the Circadian System of Alzheimer’s Disease Patients Detected in Their Home Environment. PLoS ONE 2016, 11, e0146200. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Ouanes, S.; Clark, C.; Richiardi, J.; Marechal, B.; Lewczuk, P.; Kornhuber, J.; Kirschbaum, C.; Popp, J. Cerebrospinal Fluid Cortisol and Dehydroepiandrosterone Sulfate, Alzheimer’s Disease Pathology, and Cognitive Decline. Front. Aging Neurosci. 2022, 14, 892754. [Google Scholar] [CrossRef] [PubMed]
- Giubilei, F.; Patacchioli, F.R.; Antonini, G.; Sepe Monti, M.; Tisei, P.; Bastianello, S.; Monnazzi, P.; Angelucci, L. Altered circadian cortisol secretion in Alzheimer’s disease: Clinical and neuroradiological aspects. J. Neurosci. Res. 2001, 66, 262–265. [Google Scholar] [CrossRef]
- James, K.A.; Grace, L.K.; Pan, C.Y.; Combrinck, M.I.; Thomas, K.G.F. Psychosocial stress associated with memory performance in older South African adults. Aging Neuropsychol. Cogn. 2019, 27, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Pena-Bautista, C.; Baquero, M.; Ferrer, I.; Hervas, D.; Vento, M.; Garcia-Blanco, A.; Chafer-Pericas, C. Neuropsychological assessment and cortisol levels in biofluids from early Alzheimer’s disease patients. Exp. Gerontol. 2019, 123, 10–16. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, T.; Zhao, C.; Li, G. The Regulation of Exosome Generation and Function in Physiological and Pathological Processes. Int. J. Mol. Sci. 2023, 25, 255. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z. Unveiling the Complex Role of Exosomes in Alzheimer’s Disease. J. Inflamm. Res. 2024, 17, 3921–3948. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Nikolajeff, F.; Kumar, S. Employing nanoparticle tracking analysis of salivary neuronal exosomes for early detection of neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zheng, J.; Lu, Y.; Lin, P.; Lin, Y.; Zheng, Y.; Xu, R.; Mai, Z.; Guo, B.; Zhao, X. New frontiers in salivary extracellular vesicles: Transforming diagnostics, monitoring, and therapeutics in oral and systemic diseases. J. Nanobiotechnol. 2024, 22, 171. [Google Scholar] [CrossRef]
- Rani, K.; Rastogi, S.; Vishwakarma, P.; Bharti, P.S.; Sharma, V.; Renu, K.; Modi, G.P.; Vishnu, V.Y.; Chatterjee, P.; Dey, A.B.; et al. A novel approach to correlate the salivary exosomes and their protein cargo in the progression of cognitive impairment into Alzheimer’s disease. J. Neurosci. Methods 2021, 347, 108980. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I.S.; Kim, D.H.; Ro, J.Y.; Park, B.G.; Kim, S.H.; Im, J.Y.; Lee, J.Y.; Yoon, S.J.; Kang, H.; Iwatsubo, T.; et al. The microRNA-485-3p concentration in salivary exosome-enriched extracellular vesicles is related to amyloid β deposition in the brain of patients with Alzheimer’s disease. Clin. Biochem. 2023, 118, 110603. [Google Scholar] [CrossRef]
- Eldem, E.; Barve, A.; Sallin, O.; Foucras, S.; Annoni, J.M.; Schmid, A.W.; Alberi Auber, L. Salivary Proteomics Identifies Transthyretin as a Biomarker of Early Dementia Conversion. J. Alzheimer’s Dis. Rep. 2022, 6, 31–41. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Karpe, A.; Liu, J.-W.; Beale, D.; Hor, M.; Hecker, J.; Faunt, J.; Maddison, J.; Johns, S.; Doecke, J.; et al. Salivaomics as a Potential Tool for Predicting Alzheimer’s Disease During the Early Stages of Neurodegeneration. J. Alzheimer’s Dis. 2021, 82, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Olianas, A.; Serrao, S.; Deriu, C.; Iavarone, F.; Boroumand, M.; Bizzarro, A.; Lauria, A.; Faa, G.; Castagnola, M.; et al. Top-Down Proteomics of Human Saliva Highlights Anti-inflammatory, Antioxidant, and Antimicrobial Defense Responses in Alzheimer Disease. Front. Neurosci. 2021, 15, 668852. [Google Scholar] [CrossRef]
- Contini, C.; Serrao, S.; Manconi, B.; Olianas, A.; Iavarone, F.; Guadalupi, G.; Messana, I.; Castagnola, M.; Masullo, C.; Bizzarro, A.; et al. Characterization of Cystatin B Interactome in Saliva from Healthy Elderly and Alzheimer’s Disease Patients. Life 2023, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Marksteiner, J.; Oberacher, H.; Humpel, C. Acyl-Alkyl-Phosphatidlycholines are Decreased in Saliva of Patients with Alzheimer’s Disease as Identified by Targeted Metabolomics. J. Alzheimer’s Dis. 2019, 68, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Geddes, T.; Han, B.; Bahado-Singh, R.O.; Wilson, G.D.; Imam, K.; Maddens, M.; Graham, S.F. Diagnostic Biomarkers of Alzheimer’s Disease as Identified in Saliva using 1H NMR-Based Metabolomics. J. Alzheimer’s Dis. 2017, 58, 355–359. [Google Scholar] [CrossRef]
- Huan, T.; Tran, T.; Zheng, J.; Sapkota, S.; MacDonald, S.W.; Camicioli, R.; Dixon, R.A.; Li, L. Metabolomics Analyses of Saliva Detect Novel Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 65, 1401–1416. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, H.; Zhang, T.; Jiang, Y.; Xing, H.; Zhang, A.-h. Metabolomics-based screening of salivary biomarkers for early diagnosis of Alzheimer’s disease. RSC Adv. 2015, 5, 96074–96079. [Google Scholar] [CrossRef]
- Sapkota, S.; Huan, T.; Tran, T.; Zheng, J.; Camicioli, R.; Li, L.; Dixon, R.A. Alzheimer’s Biomarkers From Multiple Modalities Selectively Discriminate Clinical Status: Relative Importance of Salivary Metabolomics Panels, Genetic, Lifestyle, Cognitive, Functional Health and Demographic Risk Markers. Front. Aging Neurosci. 2018, 10, 296. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2021, 29, 1341–1355. [Google Scholar] [CrossRef]
- Liu, X.X.; Jiao, B.; Liao, X.X.; Guo, L.N.; Yuan, Z.H.; Wang, X.; Xiao, X.W.; Zhang, X.Y.; Tang, B.S.; Shen, L. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 633–640. [Google Scholar] [CrossRef]
- Fu, K.L.; Chiu, M.J.; Wara-Aswapati, N.; Yang, C.N.; Chang, L.C.; Guo, Y.L.; Ni, Y.H.; Chen, Y.W. Oral microbiome and serological analyses on association of Alzheimer’s disease and periodontitis. Oral Dis. 2022, 29, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Bathini, P.; Foucras, S.; Dupanloup, I.; Imeri, H.; Perna, A.; Berruex, J.L.; Doucey, M.A.; Annoni, J.M.; Auber Alberi, L. Classifying dementia progression using microbial profiling of saliva. Alzheimer’s Dement. 2020, 12, e12000. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Bozyel, R. The relationship between the diagnostic value of salivary cortisol levels and behavioral symptoms in patients with Alzheimer’s disease. Dusunen Adam J. Psychiatry Neurol. Sci. 2024, 37, 34–43. [Google Scholar] [CrossRef]
- Caumo, W.; Hidalgo, M.P.; Souza, A.; Torres, I.L.S.; Antunes, L.C. Melatonin is a biomarker of circadian dysregulation and is correlated with major depression and fibromyalgia symptom severity. J. Pain Res. 2019, 12, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Leira, Y.; Carballo, Á.; Orlandi, M.; Aldrey, J.M.; Pías-Peleteiro, J.M.; Moreno, F.; Vázquez-Vázquez, L.; Campos, F.; D’Aiuto, F.; Castillo, J.; et al. Periodontitis and systemic markers of neurodegeneration: A case-control study. J. Clin. Periodontol. 2020, 47, 561–571. [Google Scholar] [CrossRef]
- Komine, K.; Kuroishi, T.; Ozawa, A.; Komine, Y.; Minami, T.; Shimauchi, H.; Sugawara, S. Cleaved inflammatory lactoferrin peptides in parotid saliva of periodontitis patients. Mol. Immunol. 2007, 44, 1498–1508. [Google Scholar] [CrossRef]
- Ji, S.; Kook, J.K.; Park, S.N.; Lim, Y.K.; Choi, G.H.; Jung, J.S. Characteristics of the Salivary Microbiota in Periodontal Diseases and Potential Roles of Individual Bacterial Species To Predict the Severity of Periodontal Disease. Microbiol. Spectr. 2023, 11, e0432722. [Google Scholar] [CrossRef]
- Kawamata, T.; Tooyama, I.; Yamada, T.; Walker, D.G.; McGeer, P.L. Lactotransferrin immunocytochemistry in Alzheimer and normal human brain. Am. J. Pathol. 1993, 142, 1574–1585. [Google Scholar] [PubMed]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar]
- Strazdins, L.; Meyerkort, S.; Brent, V.; D’Souza, R.M.; Broom, D.H.; Kyd, J.M. Impact of saliva collection methods on sIgA and cortisol assays and acceptability to participants. J. Immunol. Methods 2005, 307, 167–171. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Sodium Azide | Thioflavin S | Assessment Method | Main Findings | |
|---|---|---|---|---|---|---|---|---|
| Aβ42 | Aβ40 | |||||||
| Bermejo et al. (2010) [43] | AD: 29 Control: 56 | Unstimulated whole saliva | Spit | Yes | Unreported | ELISA | AD > Control | No difference |
| Boschi et al. (2022) [38] | AD: 18 Control: 18 | Unstimulated whole saliva | Spit | Yes | Yes | ELISA | AD > Control | NA |
| Cui et al. (2022) [39] | AD: 30 Control: 30 | Unstimulated parotid saliva | Swab | Unreported | Unreported | ELISA | AD > Control | No difference |
| Katsipis et al. (2021) [44] | AD: 20 Control: 20 | Unstimulated whole saliva | Spit | Unreported | Unreported | ELISA | AD > Control | NA |
| Kim et al. (2014) [41] | AD: 28 Control: 17 | Unstimulated whole saliva | Spit | Yes | Unreported | MNI | AD > Control | AD > Control |
| Lee et al. (2017) [37] | AD: 7 Control: 26 | Unstimulated whole saliva | Spit | Yes | Yes | ELISA | AD > Control | NA |
| McGeer et al. (2020) [40] | AD: 30 Control: 237 | Unreported | Unreported | Yes | Yes | ELISA | AD > Control | NA |
| Sabaei et al. (2023) [42] | AD: 24 Control: 22 | Unstimulated whole saliva | Cotton | Unreported | Unreported | ELISA | AD > Control | NA |
| Sabbagh et al. (2018) [36] | AD: 15 Control: 7 | Unstimulated whole saliva | Spit | Yes | Yes | ELISA | AD > Control | NA |
| Tvarijonaviciute et al. (2020) [45] | AD: 69 Control: 83 | Unstimulated whole saliva | Spit | Unreported | Unreported | IMA | AD < Control | NA |
| Lau et al. (2015) [47] | AD: 20 Control: 20 | Unstimulated whole saliva | Spit | Unreported | Unreported | ELISA | Not detected | NA |
| Marksteiner et al. (2022) [48] | AD: 44 Control: 27 | Unstimulated whole saliva | Spit | Unreported | Unreported | Lumipulse Assay | Not detected | Not detected |
| Shi et al. (2011) [46] | AD: 21 Control: 38 | Unstimulated whole saliva | Cotton | Unreported | Unreported | Mass spectrometry | Not detected | NA |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Inhibitor | Assessment Method | Main Findings | ||
|---|---|---|---|---|---|---|---|---|
| p-tau | t-tau | p-tau/t-tau | ||||||
| Cui et al. (2022) [39] | AD: 30 Control: 30 | Unstimulated parotid saliva | Swab | Unreported | ELISA | No difference | No difference | No difference |
| Katsipis et al. (2021) [44] | AD: 20 Control: 20 | Unstimulated whole saliva | Spit | Unreported | ELISA | AD > Control | NA | NA |
| Marksteiner et al. (2022) [48] | AD: 44 Control: 27 | Unstimulated whole saliva | Spit | Unreported | Lumipulse assay | NA | AD < Control | NA |
| Pekeles et al. (2019) [54] | AD: 46 Control: 47 | Unstimulated whole saliva | Spit | Yes | Western Blot | NA | NA | AD > Control |
| Sabaei et al. (2023) [42] | AD: 24 Control: 22 | Unstimulated whole saliva | Cotton | Unreported | ELISA | AD > Control | NA | NA |
| Shi et al. (2011) [46] | AD: 21 Control: 38 | Unstimulated whole saliva | Cotton | Unreported | Mass spectrometry | No difference | No difference | AD > Control |
| Ashton et al. (2018) [53] | AD: 53 Control: 160 | Unstimulated whole saliva | Spit | Unreported | Simoa | NA | No difference | NA |
| Lau et al. (2015) [47] | AD: 20 Control: 20 | Unstimulated whole saliva | Spit | Yes | ELISA | No difference | No difference | NA |
| Tvarijonaviciute et al. (2020) [45] | AD: 69 Control: 83 | Unstimulated whole saliva | Spit | Unreported | IMA | No difference | No difference | NA |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | No Smoking | Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Gleerup et al. (2021) [58] | AD: 49 Control: 17 | Unstimulated whole saliva | Spit | Yes | Simoa | NfL: No difference |
| Sabaei et al. (2023) [42] | AD: 24 Control: 22 | Unstimulated whole saliva | Cotton | Unreported | ELISA | α-syn: AD < Control |
| Tvarijonaviciute et al. (2020) [45] | AD: 69 Control: 83 | Unstimulated whole saliva | Spit | Yes | Immunoassays | PEDF: No difference |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Medicine Using | Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Ahmadi et al. (2019) [64] | AD: 30 Control: 30 | Unstimulated whole saliva | Spit | Unreported | Ellman colorimetric | AD > Control |
| Sayer et al. (2004) [63] | AD: 14 Control: 11 | Unstimulated whole saliva | Spit | AchE-I | Ellman colorimetric | AD < Control |
| Bakhtiari et al. (2017) [65] | AD: 15 Control: 15 | Unstimulated whole saliva | Spit | Memantine | Ellman colorimetric | No difference |
| Boston et al. (2008) [66] | AD: 15 Control: 13 | Unstimulated whole saliva | Spit | Anticholinergics | Ellman colorimetric | No difference |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Protein Stabilizing | Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Katsipis et al. (2021) [44] | AD: 20 Control: 20 | Unstimulated whole saliva | Spit | Unreported | ELISA | AD < Control: IL-1, IL-6, TNF-α, GFAP AD > Control: COX-2, Caspase-8 |
| McNicholas et al. (2022) [69] | AD: 16 Control: 29 | Unreported | Absorbent pad | Yes | ELISA | AD < Control: IL-1RN AD > Control: MMP-9 |
| Tvarijonaviciute et al. (2020) [45] | AD: 69 Control: 83 | Unstimulated whole saliva | Spit | Unreported | Immunoassays | AD > Control: CC4 No difference: MIP-4, CRP |
| Zalewska et al. (2021) [21] | AD: 25 Control: 25 | Stimulated whole saliva | Suction | Unreported | ELISA | AD > Control: IL-1β |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Sodium Azide | Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Antequera et al. (2024) [82] | EOAD: 28 LOAD: 25 YC: 59 OC: 45 | Unstimulated whole saliva | Spit | Yes | ELISA | AD < Control EOAD > LOAD YC vs OC: No difference |
| Carro et al. (2017) [80] | AD: 80 Control: 91 | Unstimulated whole saliva | Spit | Yes | ELISA | AD < Control |
| Gonzalez et al. (2020) [81] | AD: 25 Control: 118 | Unstimulated whole saliva | Spit | Yes | ELISA | AD < Control |
| Gleerup et al. (2021) [83] | AD: 71 Control: 20 | Unstimulated whole saliva | Spit | Unreported | ELISA | No difference |
| Zalewska et al. (2021) [21] | AD: 25 Control: 25 | Stimulated whole saliva | Suction | Unreported | ELISA | AD < Control |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Assessment Method | Main Findings |
|---|---|---|---|---|---|
| Manni et al. (2019) [87] | AD: 21 Control: 17 | Unreported | Unreported | ELISA | Dim light melatonin: AD < Control |
| Weissová et al. (2014) [88] | AD: 13 Control: 13 | Unstimulated whole saliva | Spit | Radioimmunoassay | Daily melatonin: No difference |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Assessment Method | Main Findings |
|---|---|---|---|---|---|
| Giubilei et al. (2001) [91] | AD: 18 Control: 18 | Stimulated whole saliva | Polyester wool swab | Radioimmunoassay | AD > Control |
| James et al. (2019) [92] | AD: 65 Control: 69 | Unstimulated whole saliva | Cotton | ELISA | No difference |
| Pena-Bautista et al. (2019) [93] | AD: 97 Control: 86 | Unstimulated whole saliva | Spit | UPLC-MS/MS | No difference |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Assessment Method | Main Findings |
|---|---|---|---|---|---|
| Tvarijonaviciute et al. (2020) [45] | AD: 69 Control: 83 | Unstimulated whole saliva | Spit | Colorimetric method | No difference: FRAP |
| Zalewska et al. (2021) [21] | AD: 25 Control: 25 | Stimulated whole saliva | Suction | Colorimetric method | AD > Control: NO, TOS, OSI, Peroxynitrite |
| AD < Control: GSH, UA | |||||
| No difference: TAC | |||||
| Spectrophotometric method | AD > Control: AGE, AOPP | ||||
| AD < Control: SOD, CAT, Px/GPx | |||||
| TBARS assay | AD > Control: MDA | ||||
| ELISA | AD > Control: Nitrotyrosine |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Assessment Method | Main Findings |
|---|---|---|---|---|---|
| Rani et al. (2021) [99] | AD: 5 Control: 12 | Unstimulated whole saliva | Spit | Western Blot | AD > Control: oligomeric Aβ, p-tau AD < Control: Aβ monomer |
| Ryu et al. (2023) [100] | AD: 27 Control: 13 | Unreported | Oral swab | qPCR | AD > Control: miRNA-485-3p |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Amylase Depletion | Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Contini et al. (2021) [103] | AD: 35 Control: 35 | Unstimulated whole saliva | Suction | Unreported | HPLC-ESI-IT-MS; Dot blotting | AD > Control: α-defensins, thymosin β4, cystatin B |
| Eldem et al. (2022) [101] | AD: 17 Control: 19 | Unstimulated whole saliva | Unreported | Yes | LC-MS Western Blot | AD < Control: transthyretin |
| François et al. (2021) [102] | AD: 20 Control: 40 | Unreported | Suction | Unreported | LC-MS | AD > Control: PKM, PGAM1, HSPA1A, MYL12B AD < Control: ALDH3 |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Refrain Smoking | Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Huan et al. (2018) [108] and Sapkota et al. (2018) [110] | AD: 22 Control: 35 | Unstimulated whole saliva | Spit | Unreported | LC-MS | AD > Control: methylguanosine, histidinyl-phenylalanine, choline-cytidine |
| Marksteiner et al. (2019) [106] | AD: 25 Control: 25 | Unstimulated whole saliva | Spit | Yes | FIA-MS/MS | AD < Control: acyl-alkyl phosphatidylcholines |
| Yilmaz et al. (2017) [107] | AD: 9 Control: 12 | Unstimulated whole saliva | Spit | Yes | NMR spectroscopy | AD > Control: propionate and acetone |
| Liang et al. (2015) [109] | AD: 256 Control: 218 | Unstimulated whole saliva | Spit | Yes | FUPLC-MS | AD > Control: sphinganine-1-phosphate, ornithine, phenyllactic acid |
| François et al. (2021) [102] | AD: 20 Control: 40 | Unreported | Unreported | Unreported | GC-MS | AD < Control: succinate, fumarate, L-lactate |
| Authors (Year) | Group: No. | Saliva Type | Collection Method | Antibiotics | Saliva Buffer | Dental Treatment | Teeth Number | Oral Health |
|---|---|---|---|---|---|---|---|---|
| Bathini et al. (2020) [114] | AD: 17 Control: 43 | Unstimulated whole saliva | Spit | Unreported | Unreported | Unreported | Unreported | Unreported |
| Fu et al. (2022) [113] | AD: 20 Control: 20 | Unstimulated whole saliva | Spit | 3 months | TE | 6 months | Unreported | No difference |
| Guo et al. (2021) [111] | AD: 26 Control: 26 | Stimulated whole saliva | Spit | 3 months | Saliva stabilizer | 6 months | 7 | No difference |
| Liu et al. (2019) [112] | AD: 39 Control: 39 | Unstimulated whole saliva | Spit | 1 month | Unreported | 2 months | Unreported | Unreported |
| Author (Year) | Assessment Method | Platform | Algorithm | Main Findings | ||
|---|---|---|---|---|---|---|
| α Diversity | β Diversity | Significant Bacteria | ||||
| Bathini et al. (2020) [114] | V3-V4 16S rRNA sequencing | Illumina MiSeq | Unreported | Shannon: No difference | Unreported | AD < Control: Filifactor villosus |
| Fu et al. (2022) [113] | V3-V4 16S rRNA sequencing | Illumina MiSeq | out | Unreported | Difference | AD > Control: Eubacterium infirmum, Prevotella buccae, Selenomonas artemidis |
| Guo et al. (2021) [111] | 16S rRNA full- length sequencing | PacBio platform | ASV | Unreported | No difference | AD > Control: Veillonella parvula |
| Liu et al. (2019) [112] | V3-V4 16S rRNA sequencing | Illumina Hiseq | OTU | Chao1: AD < Control Shannon: AD < Control | No difference | AD > Control: Moraxella, Leptotrichia, Sphaerochaeta AD < Control: Rothia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Yang, R.; Cheng, W.; Li, Q.; Du, M. An Update of Salivary Biomarkers for the Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 2059. https://doi.org/10.3390/ijms26052059
Guo H, Yang R, Cheng W, Li Q, Du M. An Update of Salivary Biomarkers for the Diagnosis of Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(5):2059. https://doi.org/10.3390/ijms26052059
Chicago/Turabian StyleGuo, Haiying, Ruihuan Yang, Weigao Cheng, Qiwen Li, and Minquan Du. 2025. "An Update of Salivary Biomarkers for the Diagnosis of Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 5: 2059. https://doi.org/10.3390/ijms26052059
APA StyleGuo, H., Yang, R., Cheng, W., Li, Q., & Du, M. (2025). An Update of Salivary Biomarkers for the Diagnosis of Alzheimer’s Disease. International Journal of Molecular Sciences, 26(5), 2059. https://doi.org/10.3390/ijms26052059






