The Role of Calprotectin in the Diagnosis and Treatment of Inflammatory Bowel Disease
Abstract
1. Introduction
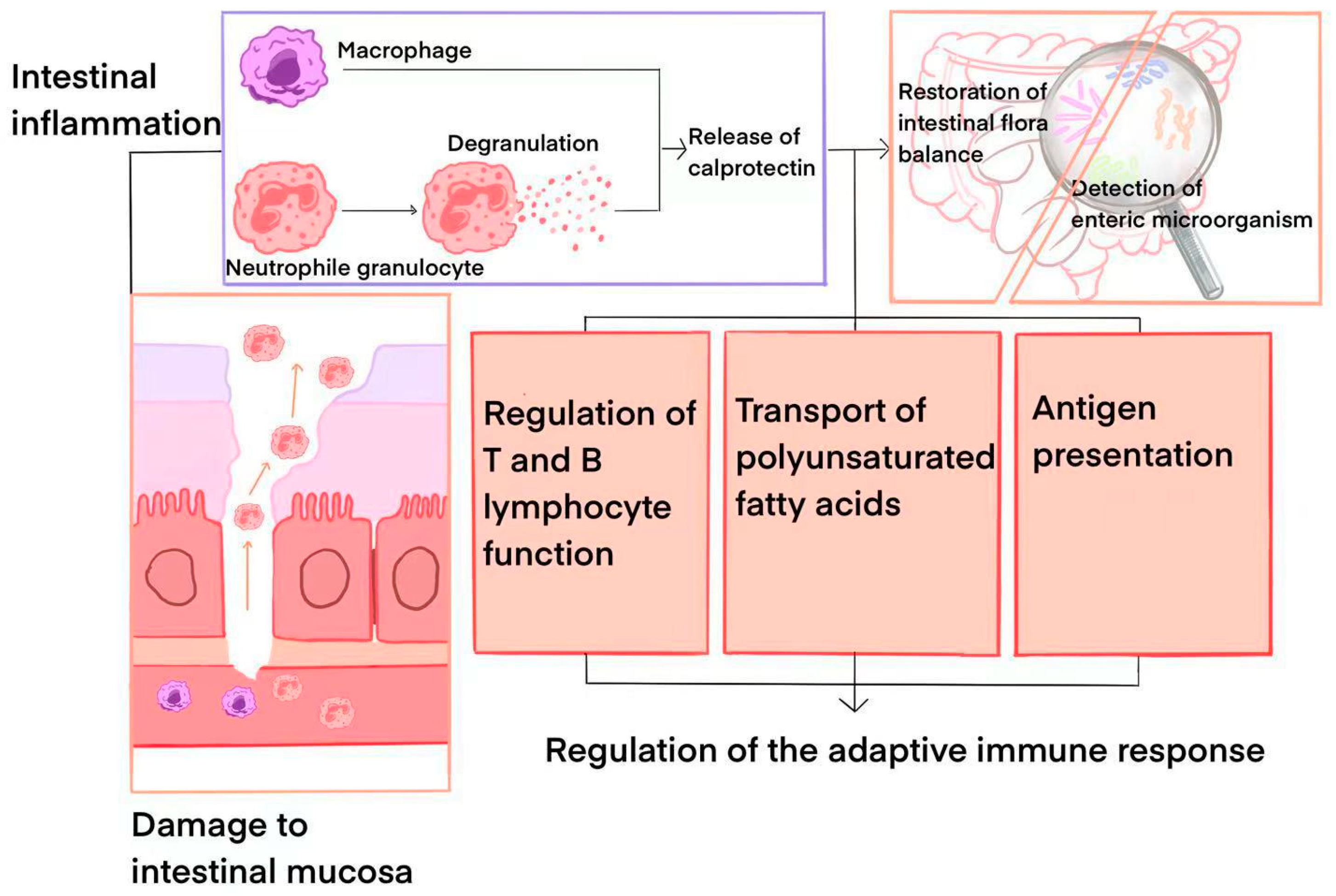
2. Calprotectin: Physicochemical Properties and Pathophysiological Data
3. Calprotectin in the Diagnosis of IBD
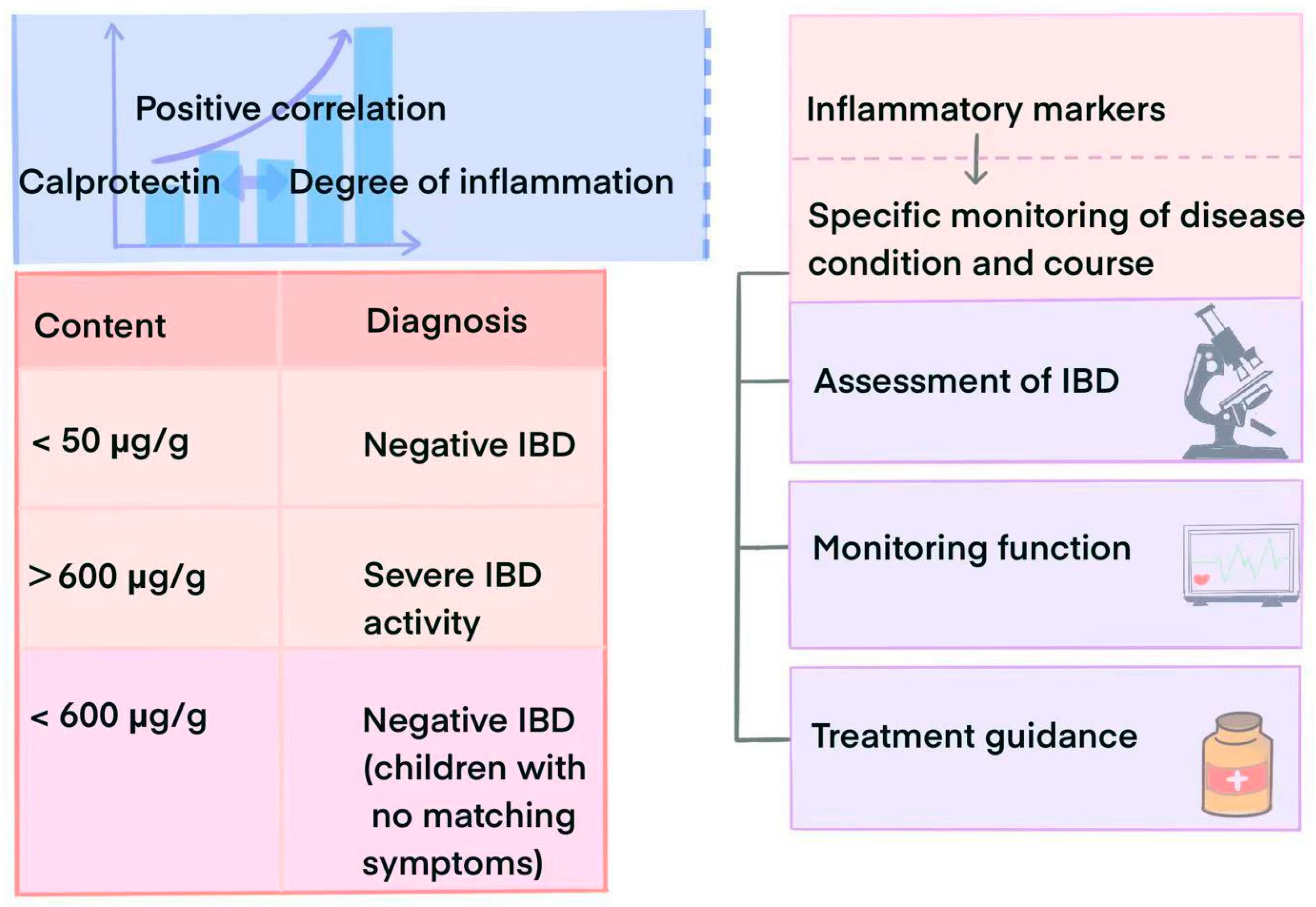
3.1. Diagnostic Value
3.2. Role in Disease Surveillance and Prognostic Assessment
4. The Potential Value of Calprotectin in the Treatment of IBD
4.1. Potential as a Therapeutic Target
4.2. Evaluation of Treatment Effects
5. Relationship Between Calprotectin and Microbiota in IBD
6. Application of Calprotectin in Regard to Other Gastrointestinal Diseases
7. Conclusions and Outlook
- Search Strategy:
- 2.
- Screening Criteria:
- (i)
- Review articles, meta-analyses, and commentaries with prior synthesized evidence to avoid redundancy (n = 298 excluded);
- (ii)
- Studies lacking primary data on calprotectin–IBD interactions (n = 378 excluded);
- (iii)
- Publications that were not peer reviewed (e.g., conference abstracts, preprints).
- 3.
- Data Extraction:
- 4.
- Limitations:
Funding
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| FC | Fecal calprotectin |
| SC | Serum calprotectin |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| Lf | Lactoferrin |
| IBS | Irritable bowel syndrome |
| MPO | Myeloperoxidase |
| fMPO | Fecal myeloperoxidase |
| CRP | C-reactive protein |
| CTM | Calcium tungstate functional microgel |
References
- Hodson, R. Inflammatory bowel disease. Nature 2016, 11, 97. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokari, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Zografos, C.G.; Konstadoulakis, M.M.; Papalois, A.E. Combination treatment of inflammatory bowel disease: Present status and future perspectives. World J. Gastroenterol. 2024, 4, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Balderramo, D.; Quaresma, A.B.; Olivera, P.A.; Savio, M.C.; Villamil MP, G.; Panaccione, R.; Ng, S.C.; Kaplan, G.G.; Kotze, P.G. Challenges in the diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol. Hepatol. 2024, 9, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.S.; Algarni, S.S.; Althubaiti, A.Q.; Alzubaidi, M.A.; Alghamdi, J.A.; Almalki, G.A. Fecal Calprotectin and Organic Gastrointestinal Disease: A Systematic Review. Cureus 2023, 15, e45019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, M.; Qian, W.; Ling, F.; Chen, Y.; Li, S.; Cheng, Y.; Zhu, L. Clinical value of fecal calprotectin for evaluating disease activity in patients with Crohn’s disease. Front. Physiol. 2023, 14, 1186665. [Google Scholar] [CrossRef] [PubMed]
- Ayling, R.M.; Kok, K. Fecal Calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. [Google Scholar]
- Inciarte-Mundo, J.; Frade-Sosa, B.; Sanmartí, R. From bench to bedside: Calprotectin (S100A8/S100A9) as a biomarker in rheumatoid arthritis. Front. Immunol. 2022, 13, 1001025. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; Elbeltagi, R. Fecal calprotectin in pediatric gastrointestinal diseases: Pros and cons. World J. Clin. Pediatr. 2024, 13, 93341. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães Costa, M.H.; Sassaki, L.Y.; Chebli, J.M.F. Fecal calprotectin and endoscopic scores: The cornerstones in clinical practice for evaluating mucosal healing in inflammatory bowel disease. World J. Gastroenterol. 2024, 30, 3022–3035. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 11, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kapel, N.; Ouni, H.; Benahmed, N.A.; Barbot-Trystram, L. Fecal Calprotectin for the Diagnosis and Management of Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2023, 14, e00617. [Google Scholar] [CrossRef]
- Deputy, M.; Devanaboina, R.; Al Bakir, I.; Burns, E.; Faiz, O. The role of faecal calprotectin in the diagnosis of inflammatory bowel disease. BMJ 2023, 380, e068947. [Google Scholar] [CrossRef]
- Dulai, P.S.; Feagan, B.G.; Sands, B.E.; Chen, J.; Lasch, K.; Lirio, R.A. Prognostic Value of Fecal Calprotectin to Inform Treat-to-Target Monitoring in Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2023, 21, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Safroneeva, E.; Romero, Y.; Armstrong, D.; Schmidt, C.; Trummler, M.; Pittet, V.; Vavricka, S.R. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm. Bowel Dis. 2013, 19, 332–341. [Google Scholar] [CrossRef]
- Orfei, M.; Gasparetto, M.; Hensel, K.O.; Zellweger, F.; Heuschkel, R.B.; Zilbauer, M. Guidance on the interpretation of faecal calprotectin levels in children. PLoS ONE 2021, 16, e0246091. [Google Scholar] [CrossRef]
- Cesaro, A.; Anceriz, N.; Plante, A.; Pagé, N.; Tardif, M.R.; Tessier, P.A. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS ONE 2012, 7, e45478. [Google Scholar] [CrossRef] [PubMed]
- Carnazzo, V.; Redi, S.; Basile, V.; Natali, P.; Gulli, F.; Equitani, F.; Marino, M.; Basile, U. Calprotectin: Two sides of the same coin. Rheumatology 2024, 63, 26–33. [Google Scholar] [CrossRef]
- Kerkhoff, C.; Vogl, T.; Nacken, W.; Sopalla, C.; Sorg, C. Zinc binding reverses the calcium-induced arachidonic acid-binding capacity of the S100A8/A9 protein complex. FEBS Lett. 1999, 460, 134–138. [Google Scholar] [CrossRef]
- Willers, M.; Ulas, T.; Völlger, L.; Vogl, T.; Heinemann, A.S.; Pirr, S.; Pagel, J.; Fehlhaber, B.; Halle, O.; Schöning, J.; et al. S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants. Gastroenterology 2020, 159, 2130–2145.e5. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.; Xu, Y.; Yong, J.; Li, X.; Zhang, K.; Gan, T.; Yang, J.; Rao, N. A systematic review on diagnosis and treatment of gastrointestinal diseases by magnetically controlled capsule endoscopy and artificial intelligence. Ther. Adv. Gastroenterol. 2023, 16, 17562848231206991. [Google Scholar] [CrossRef]
- Chowdhury, M.F.K.; Ghosh, C.K.; Miah, M.S.A.; Uddin, M.A.; Rassell, M.; Rashid, M.H.; Pal, A.K.; Mahabub-Uz-Zaman, K.; Saha, K.P.; Miah, M.A.R. Clinical Significance of Faecal Calprotectin in Differentiating Inflammatory Bowel Disease from Irritable Bowel Syndrome. Mymensingh Med. J. 2024, 33, 1149–1156. [Google Scholar] [PubMed]
- Ding, N.S.; Lee, T.; Bettenworth, D.; Cleynen, I.; Yassin, N.A.; Burisch, J.; Armuzzi, A.; Ferrante, M.; Zagorowicz, E.; Mansfield, J.; et al. Assessing aCCess to Investigations in Inflammatory Bowel Disease (ACCID): Results from an international survey. Eur. J. Gastroenterol. Hepatol. 2021, 33 (Suppl. S1), e837–e842. [Google Scholar] [CrossRef]
- Khan, H.H.; Munden, M.M.; Spence, L.H.; Jones, R.H.; Whatley, J.; Suppa, C. Intestinal ultrasound at diagnosis of pediatric inflammatory bowel disease compared to endoscopy. J. Pediatr. Gastroenterol. Nutr. 2024, 12, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, T.; Miyake, T.; Yokoyama, Y.; Kazama, T.; Hayashi, Y.; Hirayama, D.; Yoshii, S.; Yamano, H.; Takahashi, S.; Nakase, H. Clinical performance of fecal calprotectin, lactoferrin, and hemoglobin for evaluating the disease activity of IBD and detecting colorectal tumors. JGH Open 2024, 8, e13077. [Google Scholar] [CrossRef] [PubMed]
- Dajti, E.; Frazzoni, L.; Iascone, V.; Secco, M.; Vestito, A.; Fuccio, L.; Eusebi, L.H.; Fusaroli, P.; Rizzello, F.; Calabrese, C.; et al. Systematic review with meta-analysis: Diagnostic performance of faecal calprotectin in distinguishing inflammatory bowel disease from irritable bowel syndrome in adults. Aliment. Pharmacol. Ther. 2023, 58, 1120–1131. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Spencer, E.A.; Dolinger, M.T.; Suskind, D.L.; Mitrova, K.; Hradsky, O.; Conrad, M.A.; Kelsen, J.R.; Uhlig, H.H.; Tzivinikos, C.; et al. Upadacitinib for Induction of Remission in Pediatric Ulcerative Colitis: An International Multi-center Study. J. Crohns Colitis 2024, jjae182. [Google Scholar] [CrossRef] [PubMed]
- Koureta, E.; Karatzas, P.; Kanellopoulos, P.N.; Papapanagiotou, A.; Lekakis, V.; Bamias, G.; Karamanolis, G.; Vlachogiannakos, J.; Papavassiliou, A.G.; Papatheodoridis, G.V. The importance of growth differentiation factor 15 and interleukin 6 serum levels in inflammatory bowel diseases. J. Physiol. Biochem. 2024, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Temido, M.J.; Peixinho, M.; Cunha, R.; Silva, A.; Lopes, S.; Mendes, S.; Ferreira, A.M.; Ferreira, M.; Figueiredo, P.; Portela, F. Plasma calprotectin as a biomarker of inflammatory activity in ulcerative colitis. Med. Clin. 2025, 164, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Jergens, A.E.; Ackermann, M.R.; Barr, J.W.; Suchodolski, J.S.; Steiner, J.M. Serum calprotectin concentrations in dogs with idiopathic inflammatory bowel disease. Am. J. Vet. Res. 2012, 73, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Amara, J.; Saliba, Y.; Hajal, J.; Smayra, V.; Bakhos, J.-J.; Sayegh, R.; Fares, N. Circadian Rhythm Disruption Aggravates DSS-Induced Colitis in Mice with Fecal Calprotectin as a Marker of Colitis Severity. Dig. Dis. Sci. 2019, 64, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Adler, J.; Chachu, K.A.; Nguyen, N.H.; Siddique, S.M.; Weiss, J.M.; Sultan, S.; Velayos, F.S.; Cohen, B.L.; Singh, S. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease. Gastroenterology 2023, 165, 1367–1399. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Spencer, E.A.; Helmus, D.S.; Yang, J.C.; Lagishetty, V.; Bongers, G.; Britton, G.; Gettler, K.; Reyes-Mercedes, P.; Hu, J.; et al. Age-related patterns of microbial dysbiosis in multiplex inflammatory bowel disease families. Gut 2024, 73, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ma, L. Inflammatory markers and physical frailty: Towards clinical application. Immun. Ageing 2024, 21, 4. [Google Scholar] [CrossRef]
- Ishida, N.; Ito, T.; Takahashi, K.; Asai, Y.; Miyazu, T.; Higuchi, T.; Tamura, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; et al. Comparison of fecal calprotectin levels and endoscopic scores for predicting relapse in patients with ulcerative colitis in remission. World J. Gastroenterol. 2023, 29, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, N.; Valvano, M.; Monaco, S.; Stefanelli, G.; Fabiani, S.; Vernia, F.; Necozione, S.; Viscido, A.; Latella, G. The role of new inflammatory indices in the prediction of endoscopic and histological activity in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2025, 37, 24–32. [Google Scholar] [CrossRef]
- Runde, J.; Ryan, K.; Hirst, J.; Lebowitz, J.; Chen, W.; Brown, J.; Strople, J. Upadacitinib is associated with clinical response and steroid-free remission for children and adolescents with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 133–140. [Google Scholar] [CrossRef]
- Edwards, T.S.; Ho, S.S.C.; Brown, S.C.; Appleton, L.; Smith, B.R.; Borichevsky, G.M.; Swaminathan, A.; Frampton, C.M.A.; Gearry, R.B.; Kettle, A.J.; et al. Fecal Myeloperoxidase Levels Reflect Disease Activity in Children With Crohn’s Disease. Inflamm. Bowel Dis. 2024, 11, izae262. [Google Scholar] [CrossRef] [PubMed]
- Caenepeel, C.; Falony, G.; Machiels, K.; Verstockt, B.; Goncalves, P.J.; Ferrante, M.; Sabino, J.; Raes, J.; Vieira-Silva, S.; Vermeire, S. Dysbiosis and Associated Stool Features Improve Prediction of Response to Biological Therapy in Inflammatory Bowel Disease. Gastroenterology 2024, 166, 483–495. [Google Scholar] [CrossRef]
- Liu, D.; Saikam, V.; Skrada, K.A.; Merlin, D.; Iyer, S.S. Inflammatory bowel disease biomarkers. Med. Res. Rev. 2022, 42, 1856–1887. [Google Scholar] [CrossRef]
- Ling Lundström, M.; Peterson, C.; Hedin, C.R.; Bergemalm, D.; Lampinen, M.; Magnusson, M.K.; Keita, Å.V.; Kruse, R.; Lindqvist, C.M.; Repsilber, D.; et al. Faecal biomarkers for diagnosis and prediction of disease course in treatment-naïve patients with IBD. Aliment. Pharmacol. Ther. 2024, 60, 765–777. [Google Scholar] [CrossRef]
- Rendek, Z.; Falk, M.; Grodzinsky, E.; Kechagias, S.; Hjortswang, H. Diagnostic value of fecal calprotectin in primary care patients with gastrointestinal symptoms: A retrospective Swedish cohort study. JGH Open 2023, 7, 708–716. [Google Scholar] [CrossRef]
- Biedermann, L.; Doulberis, M.; Schreiner, P.; Nielsen, O.H.; The, F.O.; Brand, S.; Burk, S.; Hruz, P.; Juillerat, P.; Krieger-Grübel, C.; et al. Efficacy and Safety of Anthocyanin-Rich Extract in Patients with Ulcerative Colitis: A Randomized Controlled Trial. Nutrients 2024, 16, 4197. [Google Scholar] [CrossRef] [PubMed]
- Constantine-Cooke, N.; Monterrubio-Gómez, K.; Plevris, N.; Derikx, L.A.; Gros, B.; Jones, G.R.; Marioni, R.E.; Lees, C.W.; Vallejos, C.A. Longitudinal Fecal Calprotectin Profiles Characterize Disease Course Heterogeneity in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 2918–2927.e6. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Kok, K.B.; Ayling, R.M. Fecal Calprotectin in Gastrointestinal Disease. Clin. Chem. 2023, 69, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Yzet, C.; Meudjo, E.; Brazier, F.; Hautefeuille, V.; Moreau, C.; Robert, C.; Decrombecque, C.; Sarba, R.; Pichois, R.; Richard, N.; et al. Intestinal Ultrasound, Fecal Calprotectin, and Their Combination to Predict Endoscopic Mucosal Healing in Ulcerative Colitis: A Real-Life Cross-Sectional Study. Inflamm. Bowel Dis. 2024, 7, izae145. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zhang, L.; Li, J.; Sun, C.; Zhou, G.; Wan, H.; Lu, W.; Dong, H. Zinc pyrithione ameliorates colitis in mice by interacting on intestinal epithelial TRPA1 and TRPV4 channels. Life Sci. 2024, 358, 123090. [Google Scholar] [CrossRef]
- Reenaers, C.; Enea, D.; Nachury, M.; Laharie, D.; Bouhnik, Y.; Fumery, M.; Gornet, J.-M.; Amiot, A.; Altwegg, R.; de Vos, M.; et al. Impact of histological remission for predicting clinical relapse in Crohn’s disease: A post-hoc analysis of the prospective STORI cohort. J. Crohns Colitis 2024, 11, jjae167. [Google Scholar] [CrossRef]
- Hayashida, M.; Miyoshi, J.; Mitsui, T.; Miura, M.; Saito, D.; Sakuraba, A.; Kawashima, S.; Ikegaya, N.; Fukuoka, K.; Karube, M.; et al. Elevated fecal calprotectin and lactoferrin associated with small intestinal lesions in patients with Behçet disease. J. Gastroenterol. Hepatol. 2020, 35, 1340–1346. [Google Scholar] [CrossRef]
- Kalla, R.; Kennedy, N.A.; Ventham, N.T.; Boyapati, R.K.; Adams, A.T.; Nimmo, E.R.; Visconti, M.R.; Drummond, H.; Ho, G.-T.; Pattenden, R.J.; et al. Serum Calprotectin: A Novel Diagnostic and Prognostic Marker in Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2016, 111, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Slezak, T.; Pu, J.; Zinkus-Boltz, J.; Adhikari, S.; Pekow, J.R.; Taneja, V.; Zuniga, J.; Gómez-García, I.A.; Regino-Zamarripa, N.; et al. Development of Luminescent Biosensors for Calprotectin. ACS Chem. Biol. 2024, 19, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Pu, W.; Zhang, J. Functional Microgel Enables Effective Delivery and Colonization of Probiotics for Treating Colitis. ACS Cent. Sci. 2023, 9, 1260–1262. [Google Scholar] [CrossRef]
- González, R.; Ceacero-Heras, D.; Tena-Garitaonaindia, M.; Álvarez-Mercado, A.; Gámez-Belmonte, R.; Chazin, W.; de Medina, F.S.; Martínez-Augustin, O. Intestinal inflammation marker calprotectin regulates epithelial intestinal zinc metabolism and proliferation in mouse jejunal organoids. Biomed. Pharmacother. 2024, 174, 116555. [Google Scholar] [CrossRef]
- Roblin, X.; Nancey, S.; Papamichael, K.; Duru, G.; Flamand, M.; Kwiatek, S.; Cheifetz, A.; Fabien, N.; Barrau, M.; Paul, S. Higher Serum Infliximab Concentrations Following Subcutaneous Dosing are Associated with Deep Remission in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2024, 18, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Melas, N.; Bergqvist, V.; Ekholm, N.-P.; Olén, O.; Ludvigsson, J.F.; Hjortswang, H.; Marsal, J.; Eriksson, C.; Halfvarson, J.; et al. Real-World Outcomes of Patients Starting Intravenous and Transitioning to Subcutaneous Vedolizumab in Inflammatory Bowel Disease. Dig. Dis. Sci. 2024, 69, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ning, J.; Lu, J.; Zhang, J.; Zu, M.; Han, X.; Zheng, H.; Gong, Y.; Hao, X.; Xiong, Y.; et al. Cmtm4 deficiency exacerbates colitis by inducing gut dysbiosis and S100a8/9 expression. J. Genet. Genom. 2024, 51, 811–823. [Google Scholar] [CrossRef]
- Catalán-Serra, I.; Thorsvik, S.; Beisvag, V.; Bruland, T.; Underhill, D.; Sandvik, A.K.; Granlund, A.V.B. Fungal Microbiota Composition in Inflammatory Bowel Disease Patients: Characterization in Different Phenotypes and Correlation With Clinical Activity and Disease Course. Inflamm. Bowel Dis. 2024, 30, 1164–1177. [Google Scholar] [CrossRef]
- Preda, C.M.; Istratescu, D.; Nitescu, M.; Manuc, T.; Manuc, M.; Stroie, T.; Tieranu, C.; Meianu, C.G.; Andrei, A.; Ciora, C.A.; et al. Diet Optimization in Inflammatory Bowel Disease: Impact on Disease Relapse and Inflammatory Markers. A 1-year Prospective Trial. J. Gastrointestin. Liver Dis. 2024, 33, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Beheshti-Maal, A.; Shahrokh, S.; Ansari, S.; Mirsamadi, E.S.; Yadegar, A.; Mirjalali, H.; Zali, M.R. Gut mycobiome: The probable determinative role of fungi in IBD patients. Mycoses 2021, 64, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Yang, F.; Fu, Z.; Dong, Y.; Zhang, Z.; Ju, J. The role of enteric dysbacteriosis and modulation of gut microbiota in the treatment of inflammatory bowel disease. Microb. Pathog. 2022, 165, 105381. [Google Scholar] [CrossRef] [PubMed]
- Amoedo Cibeira, J.; Ramió-Pujol, S.; Serra-Pagès, M.; Bahí, A.; Puig-Amiel, C.; Oliver, L.; Gilabert, P.; Clos, A.; Mañosa, M.; Cañete, F.; et al. P835 Correlation between microbial markers and faecal calprotectin in IBD patients. J. Crohn’s Colitis 2020, 14, S648. [Google Scholar] [CrossRef]
- Van Thiel, I.; Maasland, T.; van Wassenaer, E.; Hoekman, D.; Spooren, C.; Hakvoort, T.; Admiraal, I.; Theelen, B.; Levin, E.; Benninga, M.; et al. P076 Abdominal pain severity for IBD in remission correlates with genetic clustering and enzymatic activity of faeces-derived Candida albicans strains. J. Crohn’s Colitis 2023, 17, i241–i242. [Google Scholar] [CrossRef]
- Breton, J.; Tanes, C.; Kyle, B.; Kachelries, K.; Crisci, M.; Compher, C.; Baldassano, R.; Albenberg, L. The modulatory effect of prebiotic inulin-type fructans on the microbiome profile of children with inflammatory bowel disease: A double-blind randomized controlled trial. Inflamm. Bowel Dis. 2024, 30 (Suppl. S1), S10. [Google Scholar] [CrossRef]
- Baston, I.; Sueiro, R.; Calviño, C.; De la Iglesia, D.; Ferreiro-Iglesias, R.; Leiro, J.M.; Dominguez-Munoz, J.E.; Acosta, M.B.-D. P861 Differences in bacteroidal genotypes between newly diagnosed ulcerative colitis patients and healthy controls. J. Crohn’s Colitis 2019, 13 (Suppl. S1), S554–S555. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Cheesbrough, J.; Rimmer, P.; Mullish, B.H.; Sharma, N.; Efstathiou, E.; Acharjee, A.; Gkoutus, G.; Patel, A.; Marchesi, J.R.; et al. Open Label Vancomycin in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Improved Colonic Disease Activity and Associations With Changes in Host-Microbiome-Metabolomic Signatures. J. Crohn’s Colitis 2025, 19, jjae189. [Google Scholar] [CrossRef]
- Carlsen, K.; Thingholm, L.B.; Dempfle, A.; Malham, M.; Bang, C.; Franke, A.; Wewer, V. Gut microbiota diversity repeatedly diminishes over time following maintenance infliximab infusions in paediatric IBD patients. PLoS ONE 2024, 19, e0311604. [Google Scholar] [CrossRef] [PubMed]
- Miquel Cusachs, J.O.; Bahí Saavedra, A.; Vila Currius, M.; Lliròs Dupre, M.; Leal Validivieso, C.; Aldeguer Manté, X. P107 Monitoring inflammatory bowel disease in clinical practice using gut microbial markers in faecal samples. Prospective study in a clinical cohort. J. Crohn’s Colitis 2021, 15 (Suppl. S1), S200–S201. [Google Scholar] [CrossRef]
- Swaminathan, A.; Borichevsky, G.M.; Frampton, C.M.; Day, A.S.; Hampton, M.B.; Kettle, A.J.; Gearry, R.B. Comparison of Fecal Calprotectin and Myeloperoxidase in Predicting Outcomes in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2025, 31, 28–36. [Google Scholar] [CrossRef]
- Moubayed, D.; Piché-Renaud, P.; Provost, C.; Faure, C.; Taddeo, D.; Jamoulle, O.; Frappier, J.; Stheneur, C. Faecal calprotectin: Marker of intestinal inflammatory process in anorexia nervosa? A preliminary study. Eur. Eat. Disord. Rev. 2023, 31, 709–716. [Google Scholar] [CrossRef]
- Lanas, Á.; Balaguer, F.; Sánchez-Luengo, M.; Hijos-Mallada, G.; Hernández-Mesa, G.; Piñero, M.; Castillo, J.; Ocaña, T.; Cubiella, J.; Crespo, A.; et al. Fecal occult blood and calprotectin testing to prioritize primary care patients for colonoscopy referral: The advantage study. United Eur. Gastroenterol. J. 2023, 11, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lv, X.; Tang, W. Fecal calprotectin as a non-invasive marker for the prediction of post-necrotizing enterocolitis stricture. Pediatr. Surg. Int. 2023, 39, 250. [Google Scholar] [CrossRef]
- Jothimani, D.; Paramasivam, R.; Manoharan, M.; Ramachandran, H.; Muthusamy, S.; Simon, E.; Ravichandran, J.; Rela, M. Fecal calprotectin in patients with liver cirrhosis. Indian J. Gastroenterol. 2023, 42, 818–823. [Google Scholar] [CrossRef]
- Sirchak, Y.S.; Kornash, V.V.; Dutko, O.O.; Lopit, M.M.; Ustych, O.V.; Griga, V.I.G.I. Differentiated approach to management of patients with irritable bowel syndrome and ulcerative colitis in non-alcoholic fatty liver disease. Wiadomosci Lek. 2024, 77, 2002–2007. [Google Scholar] [CrossRef]
- Parke, Å.; Unge, C.; Yu, D.; Sundén-Cullberg, J.; Strålin, K. Plasma calprotectin as an indicator of need of transfer to intensive care in patients with suspected sepsis at the emergency department. BMC Emerg. Med. 2023, 23, 16. [Google Scholar] [CrossRef]
- Saviano, A.; Migneco, A.; Brigida, M.; Petruzziello, C.; Zanza, C.; Savioli, G.; Franceschi, F.; Ojetti, V. Serum Calprotectin in the Evaluation of Gastrointestinal Diseases: An Ace up Your Sleeve? Medicina 2024, 60, 762. [Google Scholar] [CrossRef]
- Waldecker-Gall, S.; Waldecker, C.B.; Babel, N.; Baraliakos, X.; Seibert, F.; Westhoff, T.H. Urinary calprotectin as a diagnostic tool for detecting significant bacteriuria. Sci. Rep. 2024, 14, 12230. [Google Scholar] [CrossRef]
- Zhou, Z.; Ouboter, L.F.; Peeters, K.C.M.J.; Hawinkels, L.J.A.C.; Holman, F.; Pascutti, M.F.; Barnhoorn, M.C.; Jong, A.E.v.d.M.-D. Crohn’s Disease-Associated and Cryptoglandular Fistulas: Differences and Similarities. J. Clin. Med. 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, E.S.; Vasco, J.F.d.M.; Führ, F.; Riedi, C.A.; Filho, N.A.R. Systematic review on fecal calprotectin in cystic fibrosis. J. Pediatr. 2022, 99, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Ribaldone, D.G.; Rosso, C.; Saracco, G.M.; Astegiano, M.; Pellicano, R. Fecal calprotectin: Beyond intestinal organic diseases. Panminerva Medica 2018, 60, 29–34. [Google Scholar] [CrossRef]
- Becker, M.A.J.; Stevens, T.W.; de Voogd, F.A.E.; Wildenberg, M.E.; D’haens, G.R.A.M.; Gecse, K.B.; Buskens, C.J. Clinical relevance of calprotectin in patients with perianal fistulas in Crohn’s disease and cryptoglandular fistulas. United Eur. Gastroenterol. J. 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Riggers, D.S.; Trewin, I.; Köller, G.; Kathrani, A. Treatment success in cats with chronic enteropathy is associated with a decrease in fecal calprotectin concentrations. Front. Vet. Sci. 2024, 11, 1390681. [Google Scholar] [CrossRef]
- Ukashi, O.; Kopylov, U.; Ungar, B.; Talan Asher, A.; Shachar, E.; Engel, T.; Albshesh, A.; Yablecovitch, D.; Lahat, A.; Eliakim, R.; et al. Fecal Calprotectin Diagnostic Level Gradient Along the Small Bowel in Patients With Crohn’s Disease. J. Crohns Colitis 2025, 19, jjae123. [Google Scholar] [CrossRef]
- Schiepatti, A.; Cappellini, A.; Maimaris, S.; Minerba, P.; Retrosi, M.; Mantica, G.; Scarcella, C.; Delogu, C.; Arpa, G.; Bianchi, P.I.; et al. Fecal calprotectin measurement as a biomarker of severe disease phenotype in celiac disease and non-celiac enteropathies. Dig. Liver Dis. 2024, 57, 308–314. [Google Scholar] [CrossRef]
- Acharya, K.; Bhardwaj, V.; Chuahan, I.; Mushfiq, S.; Bhatt, S.; Lamba, B.M. Comparison of Fecal Calprotectin with Different Endoscopic Scores in the Assessment of Ulcerative Colitis (UC) Activity and Its Utility in Differentiating IBS from IBD. Euroasian J. Hepato-Gastroenterol. 2023, 13, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Saruta, M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef]
| Name | Level (μg/g, Median) | Contribution | Ref. | Calprotectin | Species |
|---|---|---|---|---|---|
| Anorexia Nervosa | 273.5 (124–423) | Dina Moubayed et al. | [74] | FC | Human |
| Colorectal Cancer | 117.3 (26.5–390.8) | Ángel Lanas et al. | [75] | FC | Human |
| Colorectal Tumors | 279 (28–1536) | Tsukasa Yamakawa et al. | [30] | FC | Human |
| Stenosis Post-necrotizing Enterocolitis | 1237.55 (741.25–1378.80) | Guanglin Chen et al. | [76] | FC | Human |
| Cirrhosis | 543.5 (207.09–879.91) | Dinesh Jothimani et al. | [77] | FC | Human |
| Sepsis | 2.2 mg/L | Åsa Parke et al. | [79] | SC | Human |
| Severe Bacteriuria | 1758.5 (242.4–6260.4) | Sabina Waldecker-Gall et al. | [81] | Urinary calprotectin | Human |
| Active Luminal Disease | 1746.0 (741.8–1800.0) | Zhou Z et al. | [82] | FC | Human |
| Chronic Enteropathy | 61 (3–1066) | Romy M Heilmann et al. | [86] | FC | Cat |
| Celiac Disease | 250.0 mg/kg (111.9–416.0) | Annalisa Schiepatti et al. | [88] | FC | Human |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Cao, W.; Zhang, S.; Chen, D.; Liu, L. The Role of Calprotectin in the Diagnosis and Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 1996. https://doi.org/10.3390/ijms26051996
Wang W, Cao W, Zhang S, Chen D, Liu L. The Role of Calprotectin in the Diagnosis and Treatment of Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2025; 26(5):1996. https://doi.org/10.3390/ijms26051996
Chicago/Turabian StyleWang, Wenqian, Wenfu Cao, Shenyun Zhang, Dapeng Chen, and Lihong Liu. 2025. "The Role of Calprotectin in the Diagnosis and Treatment of Inflammatory Bowel Disease" International Journal of Molecular Sciences 26, no. 5: 1996. https://doi.org/10.3390/ijms26051996
APA StyleWang, W., Cao, W., Zhang, S., Chen, D., & Liu, L. (2025). The Role of Calprotectin in the Diagnosis and Treatment of Inflammatory Bowel Disease. International Journal of Molecular Sciences, 26(5), 1996. https://doi.org/10.3390/ijms26051996






