Rubia akane Nakai Fruit Extract Improves Obesity and Insulin Sensitivity in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Results
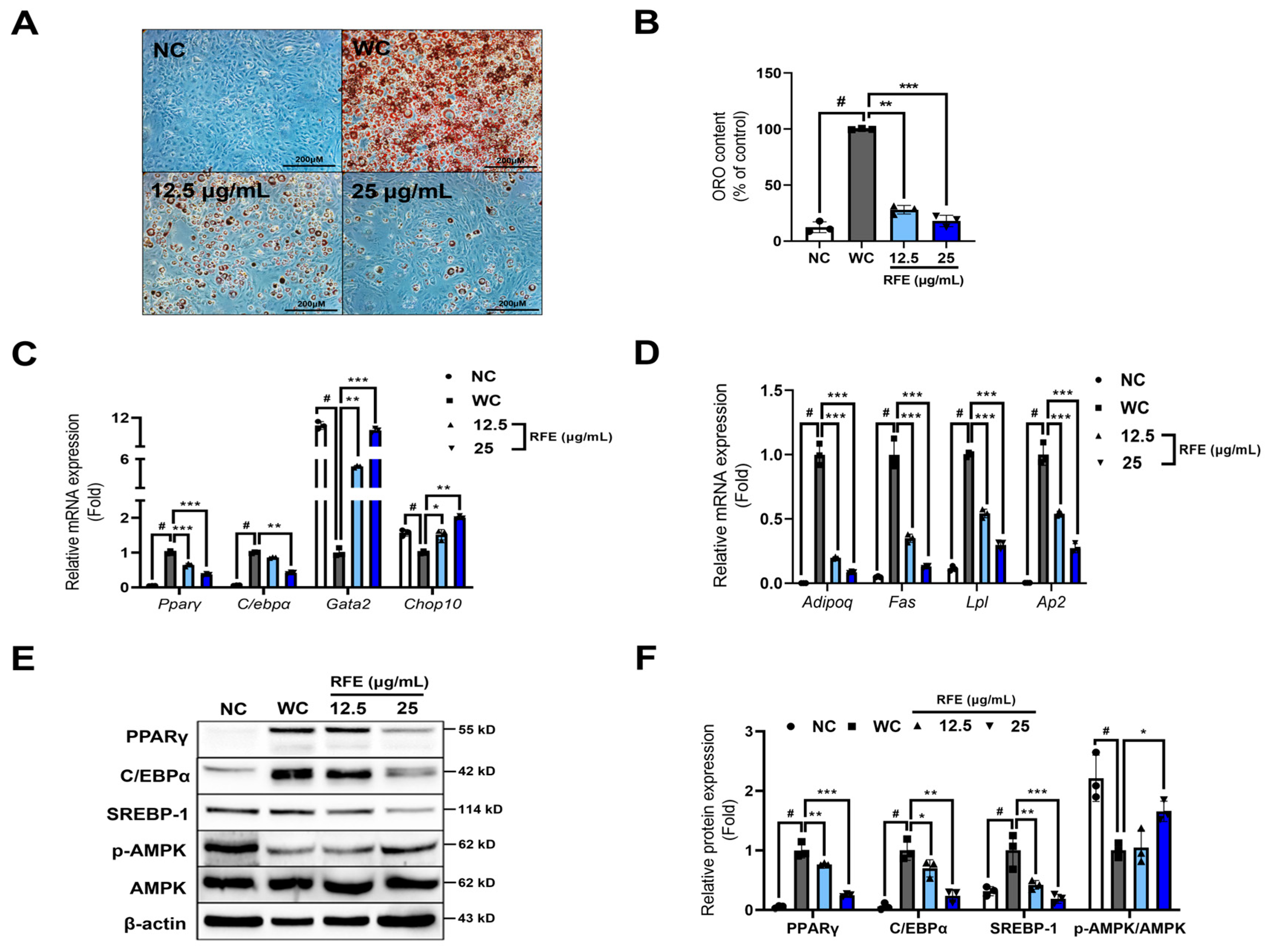
2.1. RFE Inhibits Adipogenic Differentiation and Lipogenesis in 3T3-L1 Adipocytes
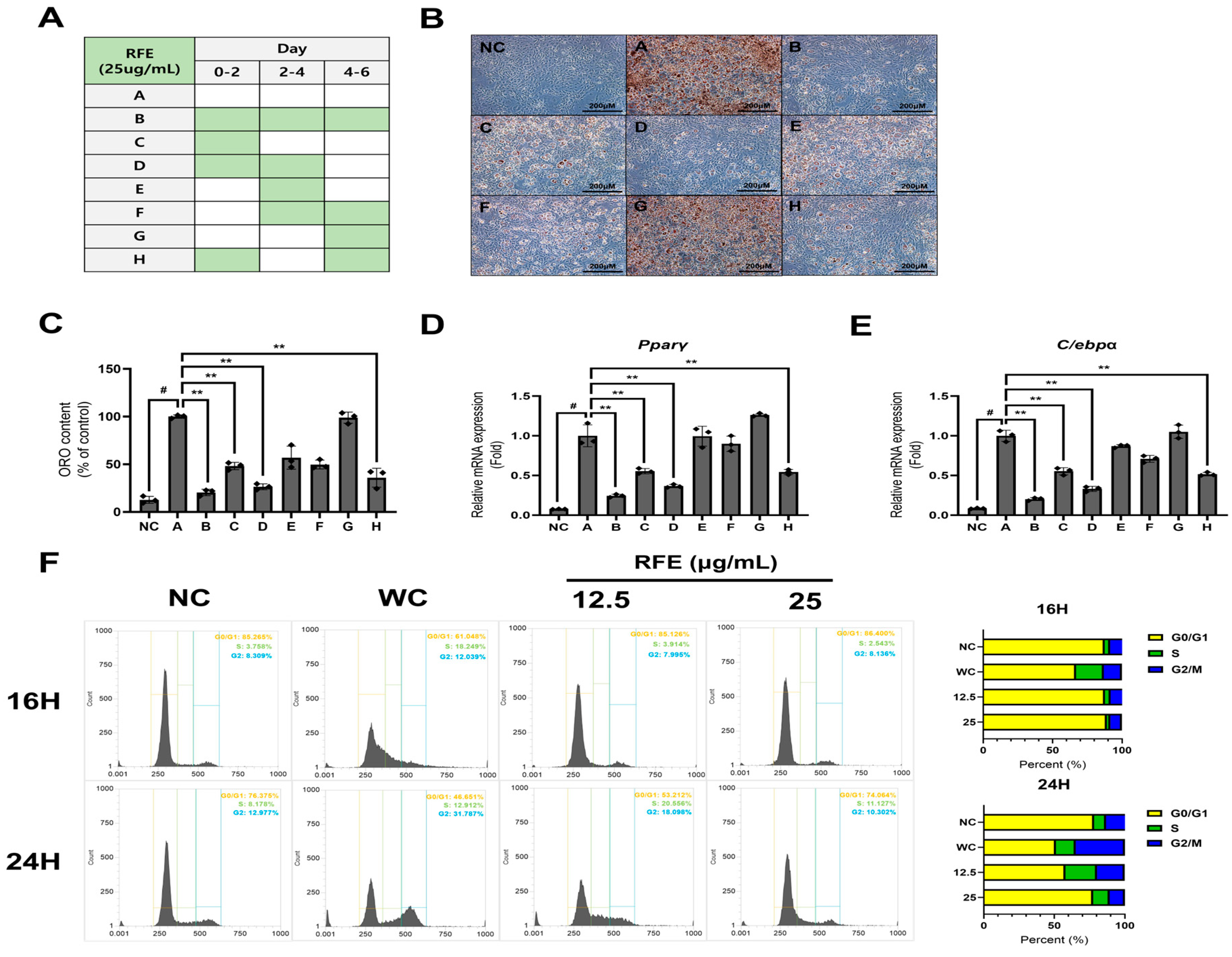
2.2. RFE Inhibits Adipogenesis in the Early Phase of Differentiation
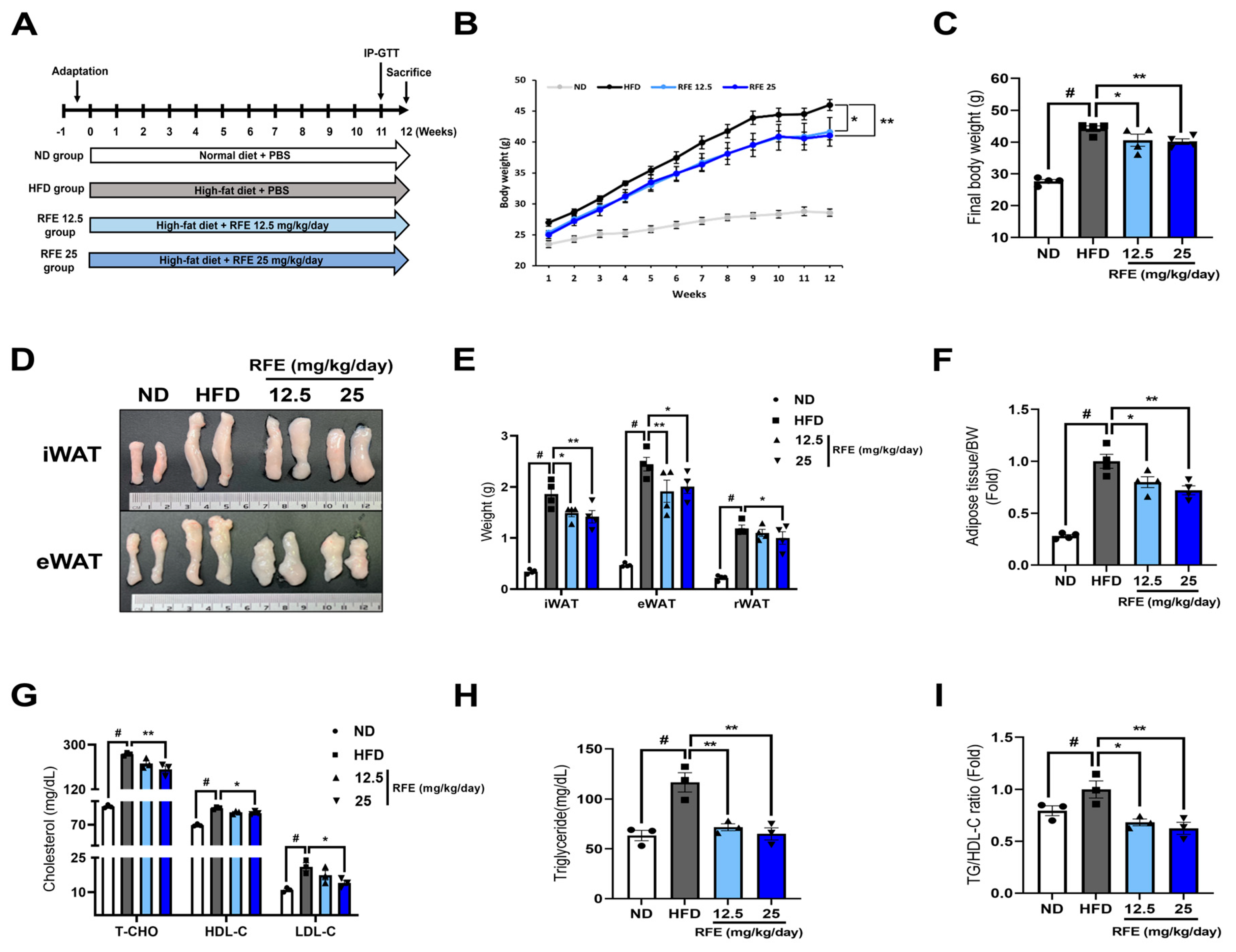
2.3. RFE Improves High-Fat Diet-Induced Obesity and Dyslipidemia
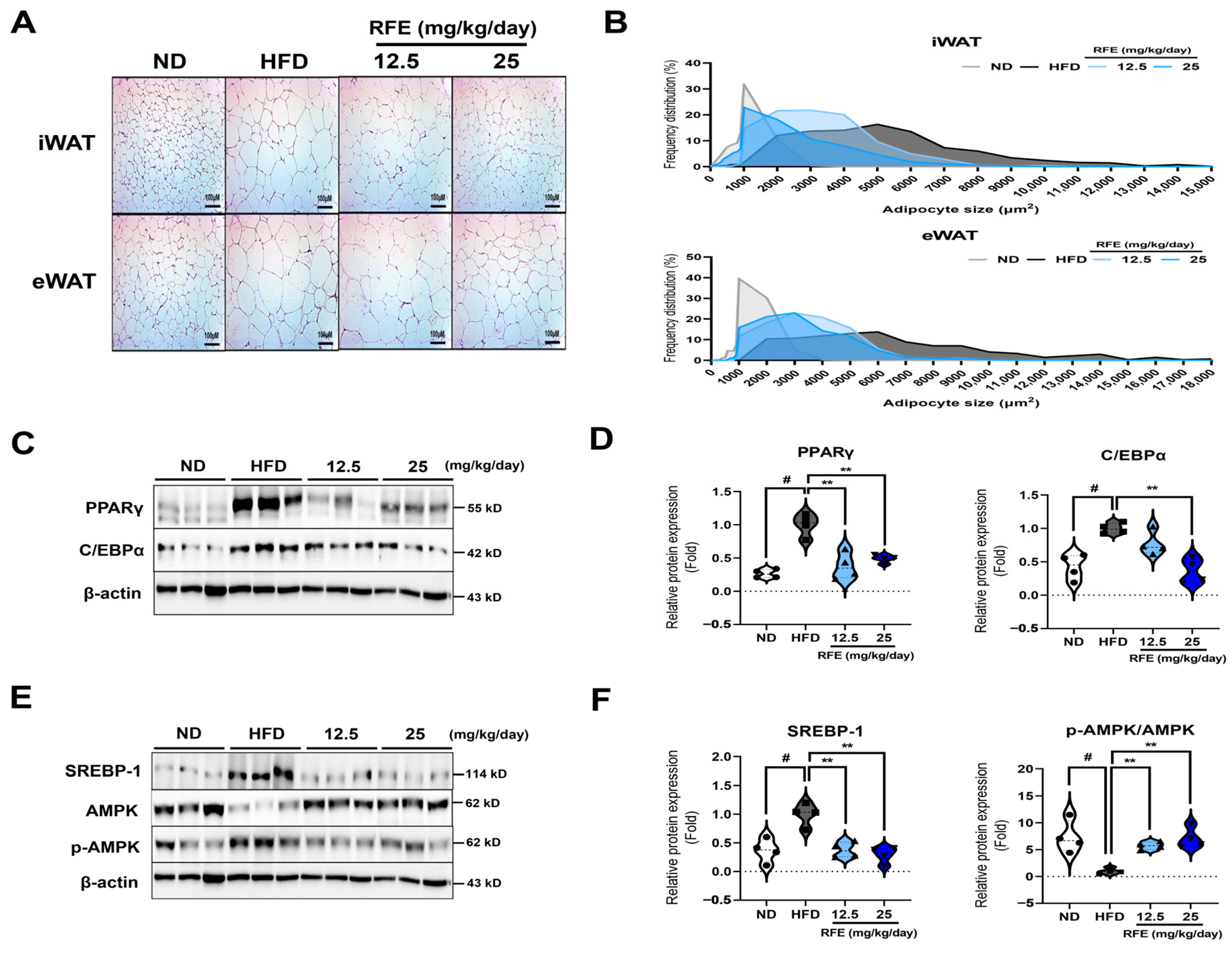
2.4. RFE Inhibits Adipogenic Differentiation and Lipogenesis in White Adipose Tissue
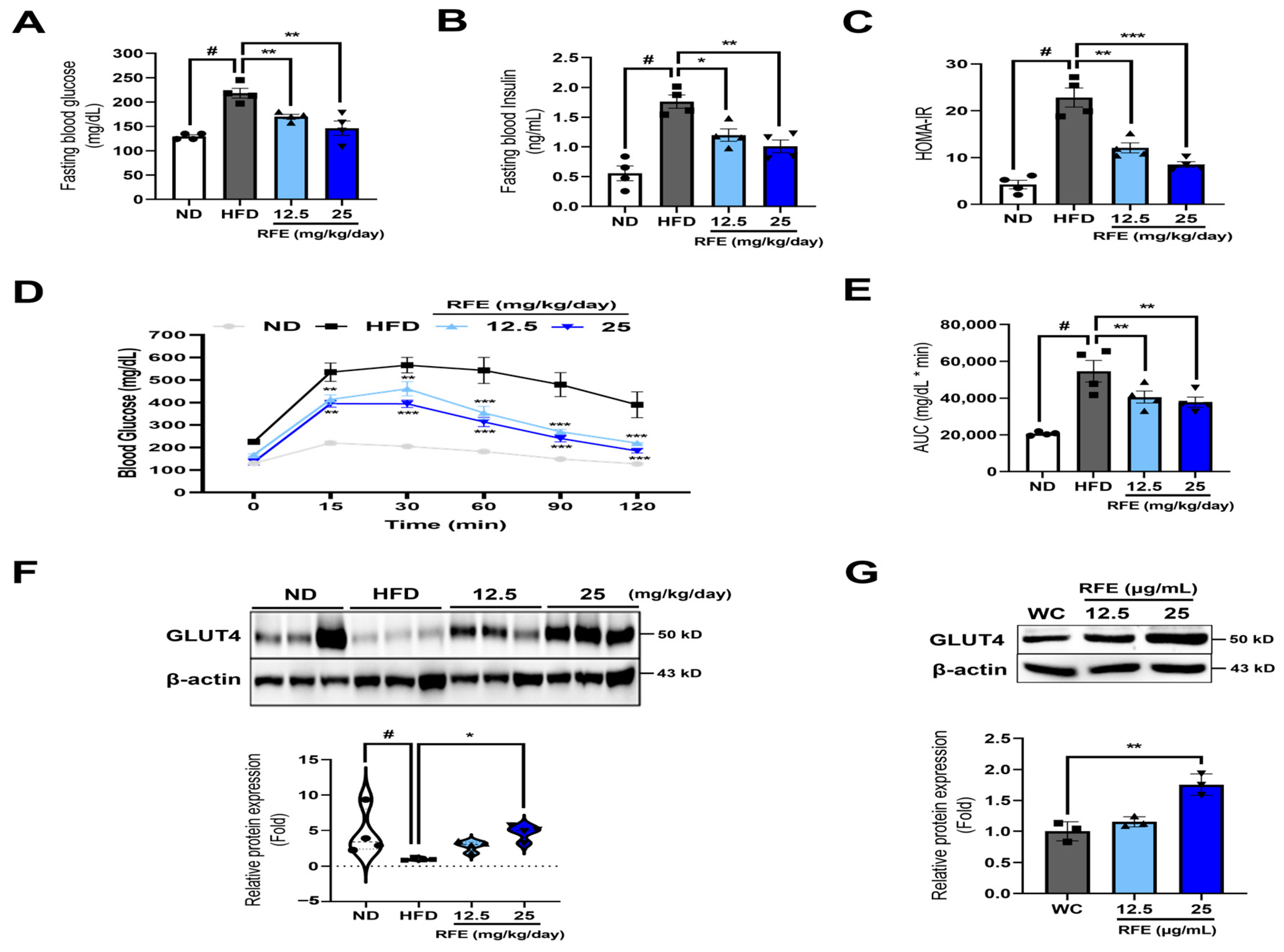
2.5. RFE Improves Systematic Insulin Sensitivity in Obese Mice and 3T3-L1 Adipocytes
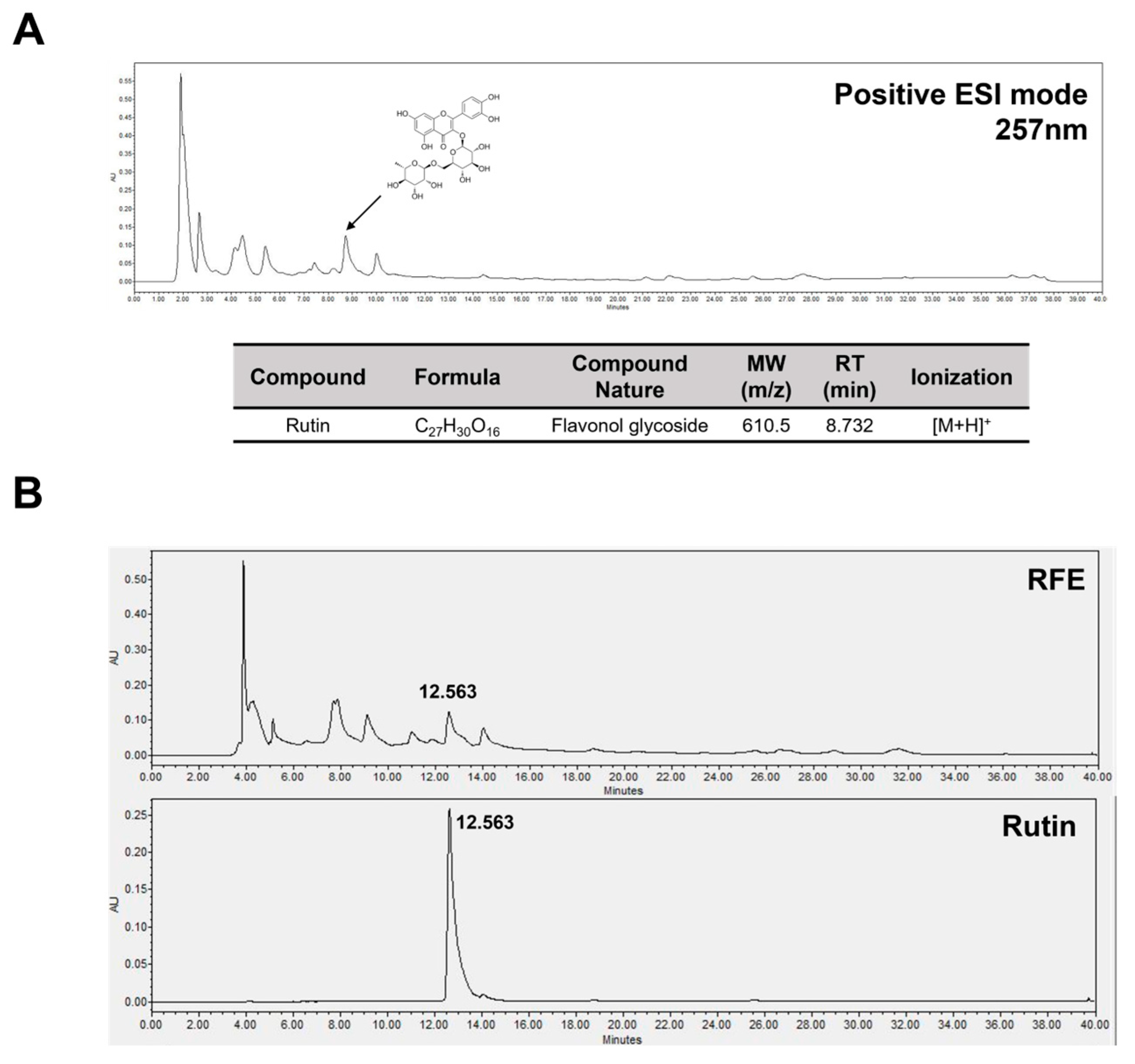
2.6. Rutin Is a Bioactive Component in RFE That Has Anti-Obesity Effects and Improves Insulin Sensitivity
3. Discussion
4. Materials and Methods
4.1. Preparation of RFE
4.2. Cell Culture and Differentiation
4.3. Animal Experiments
4.4. Cell Viability Assays
4.5. Oil Red O Staining
4.6. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Western Blot Analysis
4.8. Flow Cytometry
4.9. Blood Biochemical Analysis
4.10. Hematoxylin and Eosin Staining
4.11. Intraperitoneal Glucose Tolerance Tests (IP-GTTs)
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. LC-MS Analysis
4.14. HPLC Analysis
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and its risk factors: A systematic review. BMC Public Health 2023, 23, 1018. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Obesity Day 2022—Accelerating Action to Stop Obesity; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J. Current State of Pharmacotherapy in Obesity. Korean J. Gastroenterol. 2024, 83, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Bazarbashi, A.N.; Lo, A.; Smith, B.N. Liraglutide-induced hemorrhagic pancreatitis in a nondiabetic patient. ACG Case Rep. J. 2020, 7, e00380. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Eskandarzadeh, M.; Esmaeili, A.; Nikbakht, M.R.; Hitotsuyanagi, Y.; Shkryl, Y.N.; Ghasemian Yadegari, J.; Rezazadeh, H.; Khalilifard, J. Genus Rubia: Therapeutic Effects and Toxicity: A Review. Herb. Med. J. 2023, 8, 34–48. [Google Scholar]
- Hu, Y.-Y.; Zhang, X.-J.; Zhang, Z.-H.; Wang, Z.; Tan, N.-H. Qualitative and quantitative analyses of quinones in multi-origin Rubia species by ultra-performance liquid chromatography-tandem mass spectrometry combined with chemometrics. J. Pharm. Biomed. Anal. 2020, 189, 113471. [Google Scholar] [CrossRef]
- Xu, K.; Wang, P.; Wang, L.; Liu, C.; Xu, S.; Cheng, Y.; Wang, Y.; Li, Q.; Lei, H. Quinone derivatives from the genus Rubia and their bioactivities. Chem. Biodivers. 2014, 11, 341–363. [Google Scholar] [CrossRef]
- Nam, W.; Nam, S.H.; Kim, S.P.; Levin, C.; Friedman, M. Anti-adipogenic and anti-obesity activities of purpurin in 3T3-L1 preadipocyte cells and in mice fed a high-fat diet. BMC Complement. Altern. Med. 2019, 19, 364. [Google Scholar] [CrossRef]
- Xu, L.; Xing, M.; Xu, X.; Saadeldeen, F.S.; Liu, Z.; Wei, J.; Kang, W. Alizarin increase glucose uptake through PI3K/Akt signaling and improve alloxan-induced diabetic mice. Future Med. Chem. 2019, 11, 395–406. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Li, Z.-H.; Wang, Z.-J.; Liu, L.-L.; Sun, T.-T.; Ma, J.-Z.; Zhang, Y. Ultrasound-assisted extraction of total anthocyanins from Rubia sylvatica Nakai fruit and radical scavenging activity of the extract. Ind. Crops Prod. 2020, 150, 112420. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Yang, S.; Zhang, Y. Rubia sylvatica anthocyanins protect retinal pigment epithelial cells from H2O2-induced oxidative damage. Food Biosci. 2023, 56, 103088. [Google Scholar] [CrossRef]
- Zohra, H.F.; Ramazan, E.; Ahmed, H. Biological Activities and Chemical Composition of Rubia tinctorum (L) Root and Aerial Part Extracts Thereof. Acta Biol. Colomb. 2022, 27, 403–414. [Google Scholar]
- Choi, I.; Park, Y.; Choi, H.; Lee, E.H. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors 2006, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, C.Y. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Lee, E.; Park, J.; Nam, J.-O. Euscaphis japonica Kanitz Fruit Exerts Antiobesity Effects by Inhibiting the Early Stage of Adipogenic Differentiation. Nutrients 2023, 15, 3078. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Yuge, H.; Okada, H.; Hamaguchi, M.; Kurogi, K.; Murata, H.; Ito, M.; Fukui, M. Triglycerides/HDL cholesterol ratio and type 2 diabetes incidence: Panasonic Cohort Study 10. Cardiovasc. Diabetol. 2023, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.; Segal, T.; Lichtarowicz-Krynska, E.; Hindmarsh, P. Prevalence of the insulin resistance syndrome in obesity. Arch. Dis. Child. 2005, 90, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Ganjayi, M.S.; Karunakaran, R.S.; Gandham, S.; Meriga, B. Quercetin-3-O-rutinoside from Moringa oleifera downregulates adipogenesis and lipid accumulation and improves glucose uptake by activation of AMPK/Glut-4 in 3T3-L1 cells. Rev. Bras. Farmacogn. 2023, 33, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.F.; Farmer, S.R. Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 2000, 130, 3116S–3121S. [Google Scholar] [CrossRef]
- Feng, S.; Reuss, L.; Wang, Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef]
- Suwa, A.; Kurama, T.; Shimokawa, T. Adipocyte hyperplasia and RMI1 in the treatment of obesity. FEBS J. 2011, 278, 565–569. [Google Scholar] [CrossRef]
- Lee, H.-W.; Rhee, D.-K.; Kim, B.-O.; Pyo, S. Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed. Pharmacother. 2018, 102, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Nam, J.-O. Anti-Obesity and Anti-Diabetic Effects of Ostericum koreanum (Ganghwal) Extract. Int. J. Mol. Sci. 2024, 25, 4908. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, N.; Sugahara, K. A survey of anthocyanins in fruits of some angiosperms, II. J. Plant Res. 1979, 92, 157–161. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Li, Z.-H.; Liu, L.-L.; Wang, H.; Yang, S.-H.; Zhang, J.-S.; Zhang, Y. Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. LWT 2021, 148, 111708. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Nafie, M.S.; Khodeer, D.M.; El-Tanahy, A.H.; Abdel-Kader, M.S.; Badr, J.M.; Abdelhameed, R.F. Rubia tinctorum root extracts: Chemical profile and management of type II diabetes mellitus. RSC Adv. 2020, 10, 24159–24168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, E.; Nam, J.-O. Rubia akane Nakai Fruit Extract Improves Obesity and Insulin Sensitivity in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2025, 26, 1833. https://doi.org/10.3390/ijms26051833
Park J, Lee E, Nam J-O. Rubia akane Nakai Fruit Extract Improves Obesity and Insulin Sensitivity in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2025; 26(5):1833. https://doi.org/10.3390/ijms26051833
Chicago/Turabian StylePark, Juhye, Eunbi Lee, and Ju-Ock Nam. 2025. "Rubia akane Nakai Fruit Extract Improves Obesity and Insulin Sensitivity in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice" International Journal of Molecular Sciences 26, no. 5: 1833. https://doi.org/10.3390/ijms26051833
APA StylePark, J., Lee, E., & Nam, J.-O. (2025). Rubia akane Nakai Fruit Extract Improves Obesity and Insulin Sensitivity in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice. International Journal of Molecular Sciences, 26(5), 1833. https://doi.org/10.3390/ijms26051833






