A Review of Talin- and Integrin-Dependent Molecular Mechanisms in Cancer Invasion and Metastasis
Abstract
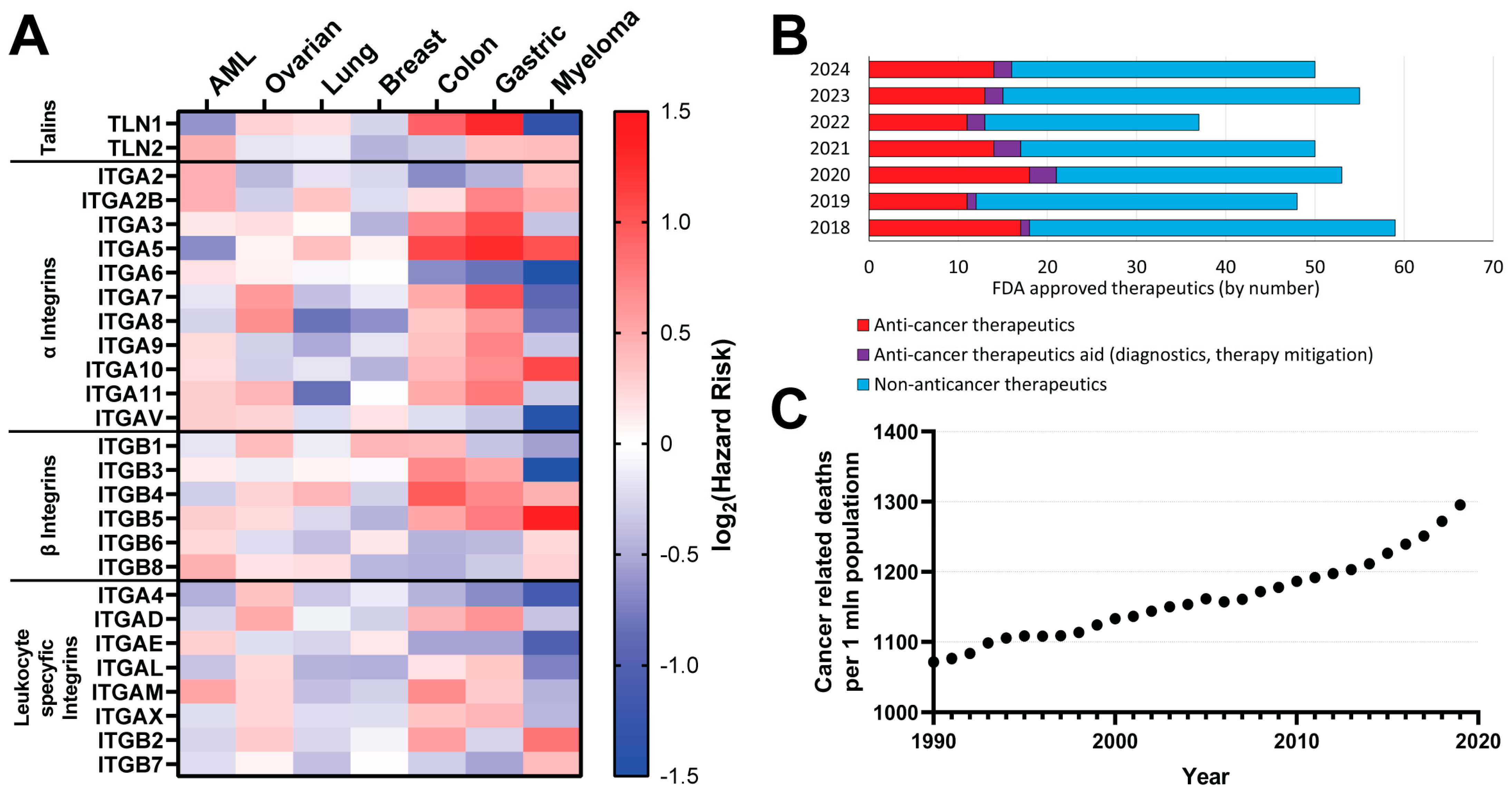
1. Introduction
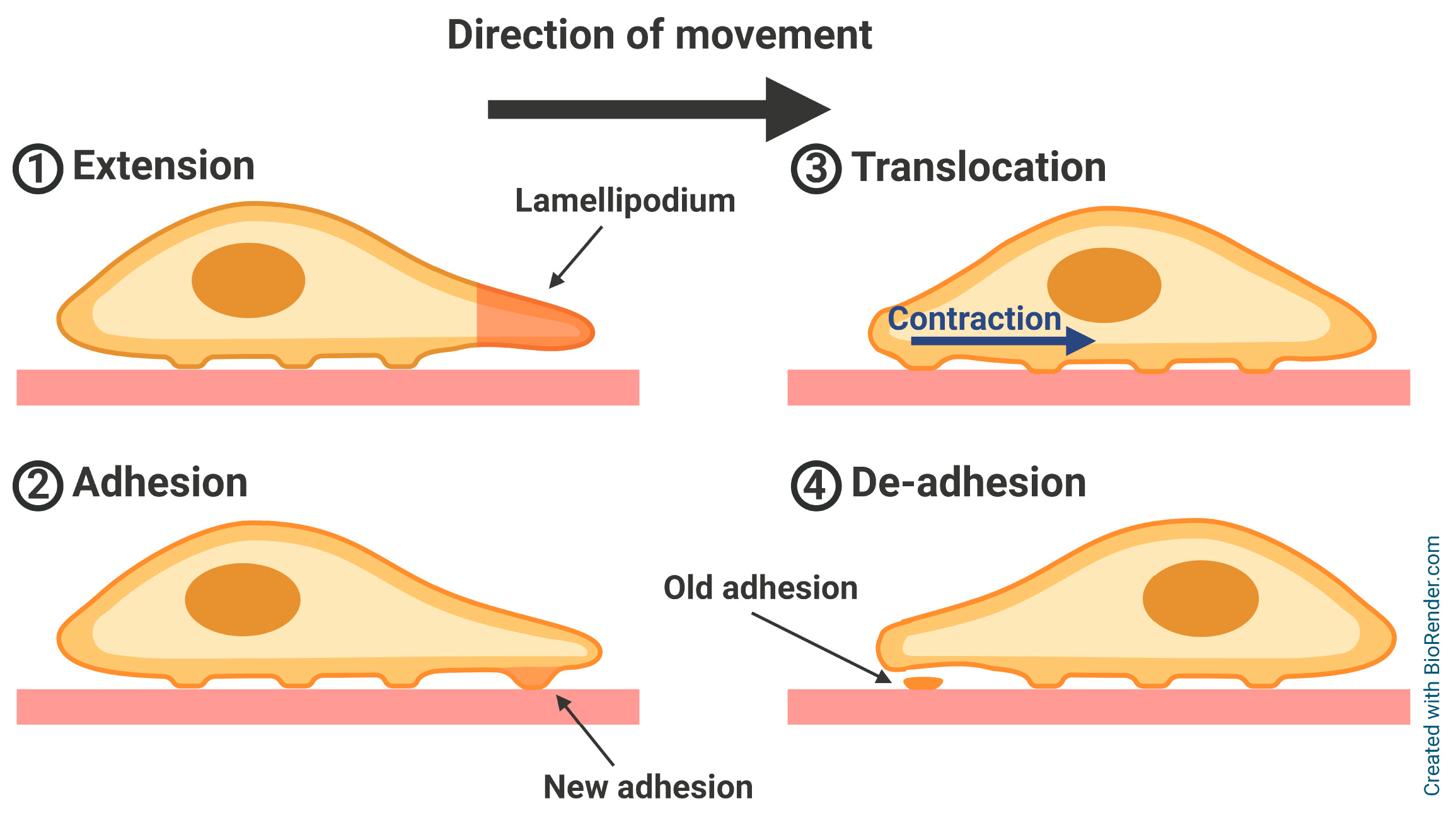
2. Adhesion-Related Proteins in Cell Motility
2.1. Talins
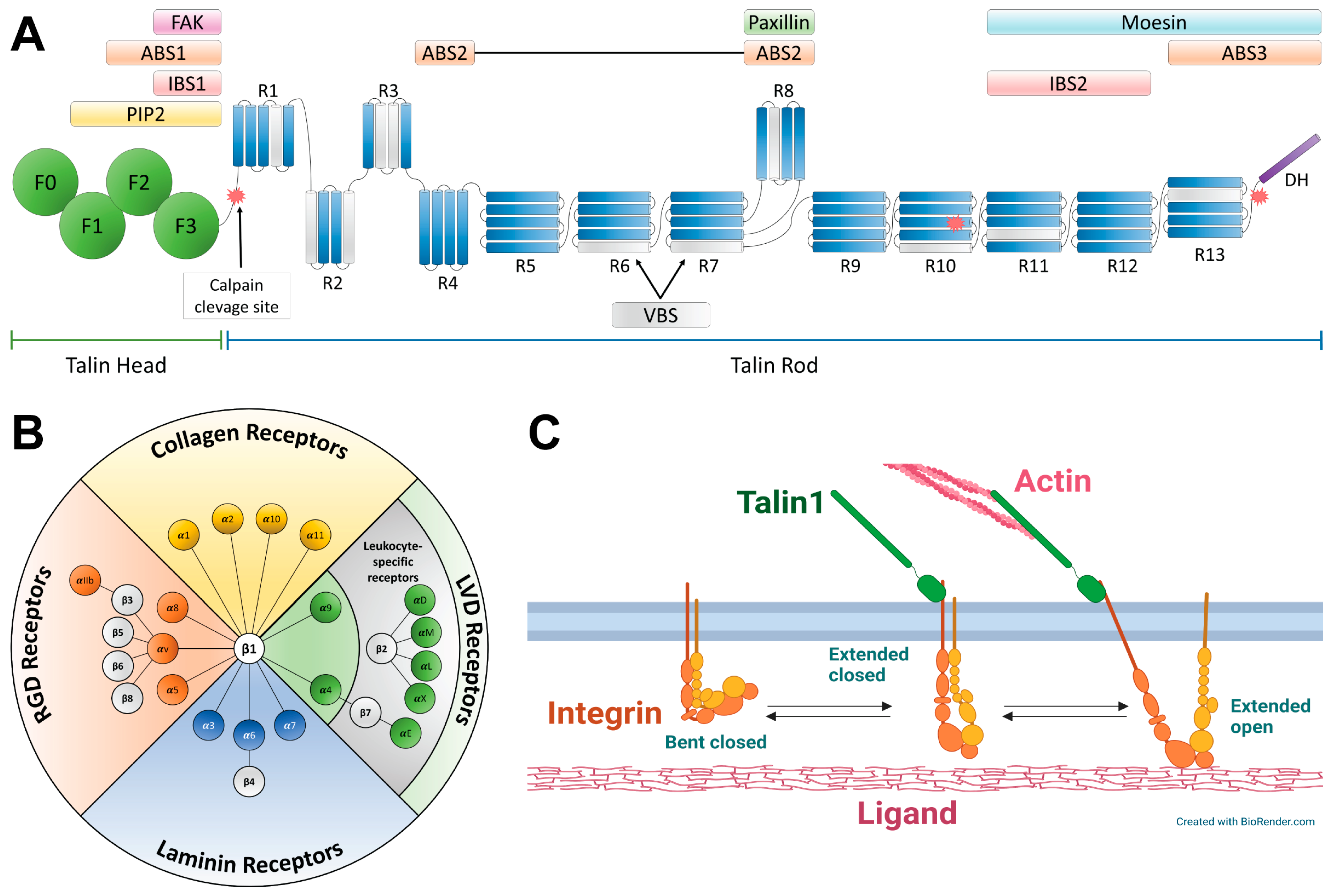
2.2. Integrins
2.3. Molecular Basis of Talin–Integrin Interaction
2.4. Talins and Integrins in the Epithelial–Mesenchymal Transition
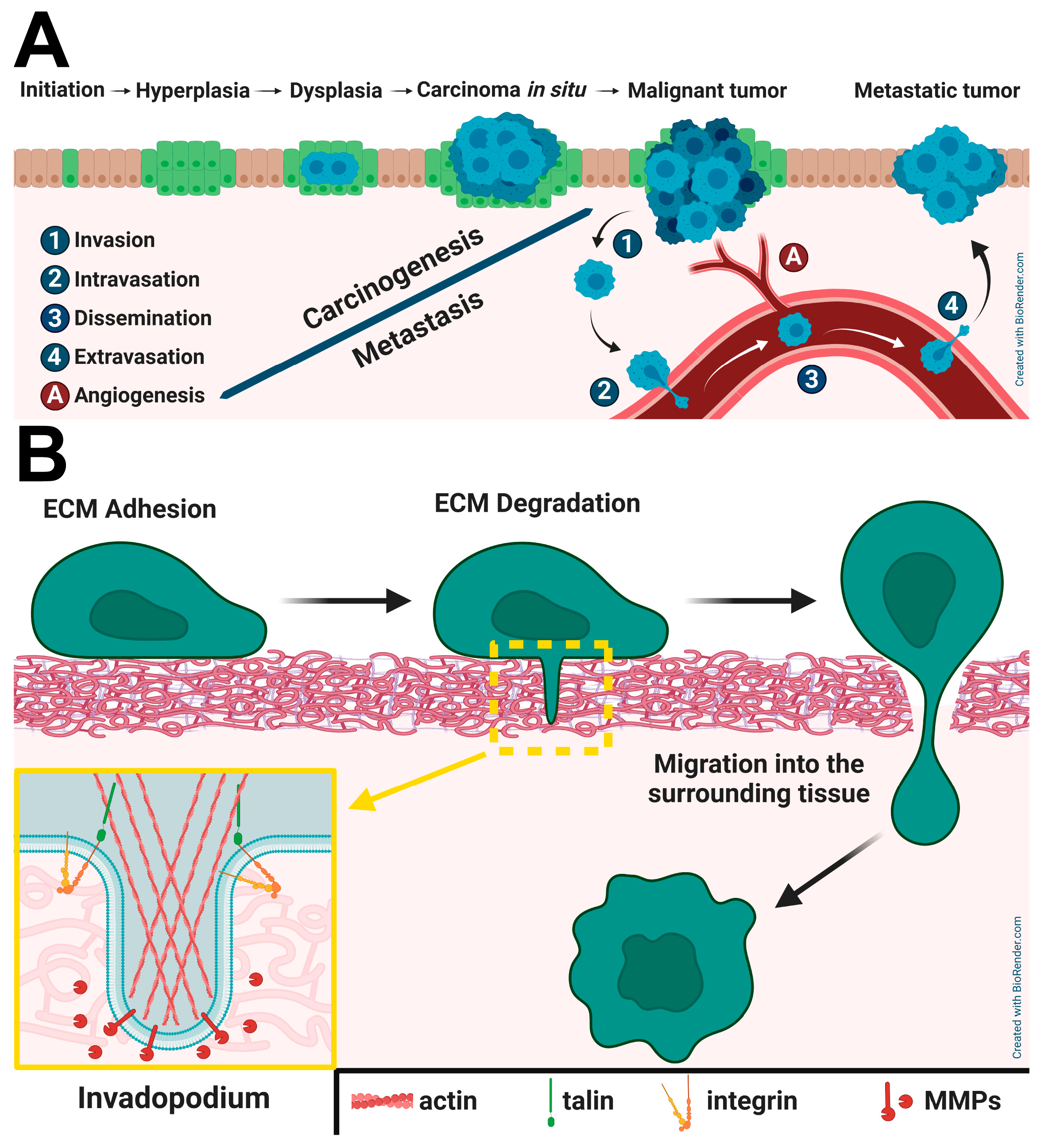
2.5. Interplay Between Talins and β1–Integrin in Invadopodia Formation and Maturation
2.6. Talins and Integrins in Cancer Cells–Tumor Microenvironment Interaction
3. Clinical Aspects of Talin and Integrin in Cancer Development
3.1. Talin- and Integrin-Based Cancer Prognosis
3.2. Integrin-Related Immune Evasion and Anti-Cancer Drug Resistance
3.3. Migrastatics
3.4. Talin and Integrin as Targets for Anti-Cancer Therapies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Extracellular Matrix
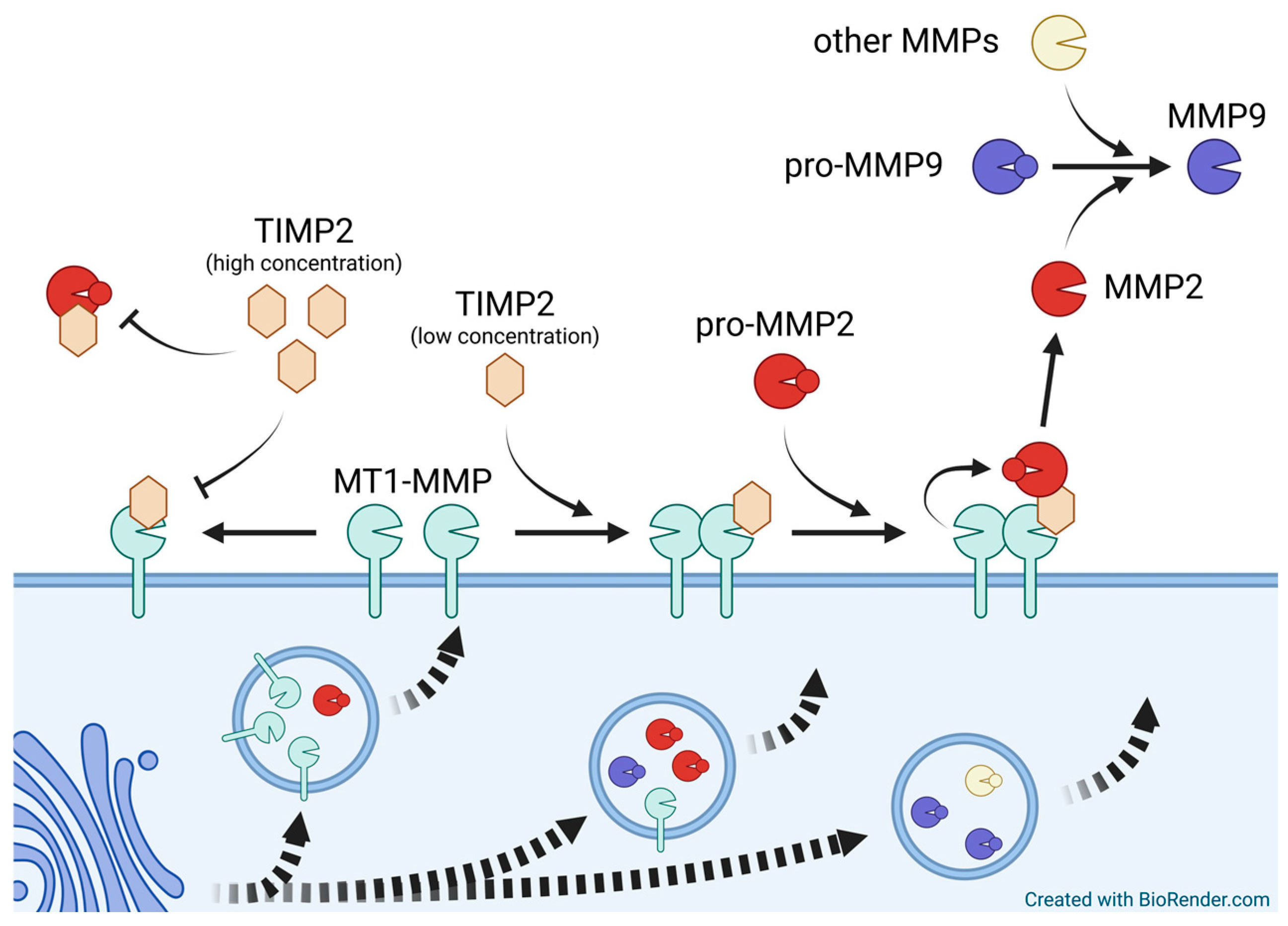
Appendix B. Matrix Metallopeptidases
Appendix C. Novel Anticancer Treatments
| 2024 | ||||
|---|---|---|---|---|
| Cancer Treatment Drug | ||||
| No. | Drug Name | Active Ingredient | Approval Date | FDA-Approved Use on Approval Date |
| 1 | Ensacove | ensartinib | 18 December 2024 | To treat non-small-cell lung cancer |
| 2 | Unloxcyt | cosibelimab-ipdl | 13 December 2024 | To treat classic congenital adrenal hyperplasia |
| 3 | Bizengri | zenocutuzumab-zbco | 4 December 2024 | To treat cutaneous squamous cell carcinoma |
| 4 | Ziihera | zanidatamab-hrii | 20 November 2024 | To treat non-small-cell lung cancer and pancreatic adenocarcinoma |
| 5 | Revuforj | revumenib | 15 November 2024 | To treat unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer |
| 6 | Vyloy | zolbetuximab-clzb | 18 October 2024 | To treat relapsed or refractory acute leukemia |
| 7 | Itovebi | inavolisib | 10 October 2024 | To treat gastric or gastroesophageal junction adenocarcinoma |
| 8 | Lazcluze | lazertinib | 19 August 2024 | To treat locally advanced or metastatic breast cancer |
| 9 | Voranigo | vorasidenib | 6 August 2024 | To treat non-small-cell lung cancer |
| 10 | Rytelo | imetelstat | 6 June 2024 | To treat Grade 2 astrocytoma or oligodendroglioma |
| 11 | Imdelltra | tarlatamab-dlle | 16 May 2024 | To treat low- to intermediate-1 risk myelodysplastic syndromes |
| 12 | Ojemda | tovorafenib | 23 April 2024 | To treat extensive stage small-cell lung cancer |
| 13 | Anktiva | nogapendekin alfa inbakicept-pmln | 22 April 2024 | To treat relapsed or refractory pediatric low-grade glioma |
| 14 | Tevimbra | tislelizumab-jsgr | 13 March 2024 | To treat bladder cancer |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Iomervu | iomeprol | 27 November 2024 | For use as a radiographic contrast agent |
| 2 | Lumisight | pegulicianine | 17 April 2024 | To use as an optical imaging agent for the detection of cancerous tissue |
| 2023 | ||||
| Cancer treatment drug | ||||
| 1 | Ogsiveo | nirogacestat | 27 November 2023 | To treat adults with progressing desmoid tumors who require systemic treatment |
| 2 | Truqap | capivasertib | 16 November 2023 | To treat breast cancer that meets certain disease criteria |
| 3 | Augtyro | repotrectinib | 15 November 2023 | To treat ROS1-positive non-small-cell lung cancer |
| 4 | Fruzaqla | fruquintinib | 8 November 2023 | To treat refractory, metastatic colorectal cancer |
| 5 | Loqtorzi | toripalimab-tpzi | 27 October 2023 | To treat recurrent or metastatic nasopharyngeal carcinoma when used together with or following other therapies |
| 6 | Elrexfio | elranatamab-bcmm | 14 August 2023 | To treat adults with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy |
| 7 | Talvey | talquetamab-tgvs | 9 August 2023 | To treat adults with relapsed or refractory multiple myeloma who have received at least four prior therapies |
| 8 | Vanflyta | quizartinib | 20 July 2023 | To use as part of a treatment regimen for newly diagnosed acute myeloid leukemia that meets certain criteria |
| 9 | Columvi | glofitamab-gxbm | 15 June 2023 | To treat diffuse large B-cell lymphoma, not otherwise specified, or large B-cell lymphoma arising from follicular lymphoma after two or more lines of systemic therapy |
| 10 | Epkinly | epcoritamab-bysp | 19 May 2023 | To treat relapsed or refractory diffuse large B-cell lymphoma (not otherwise specified) and high-grade B-cell lymphoma after two or more lines of systemic therapy |
| 11 | Zynyz | retifanlimab-dlwr | 22 March 2023 | To treat metastatic or recurrent locally advanced Merkel cell carcinoma |
| 12 | Orserdu | elacestrant | 27 January 2023 | To treat estrogen receptor-positive, human epidermal growth factor receptor 2-negative, ESR1-mutated, advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy |
| 13 | Jaypirca | pirtobrutinib | 27 January 2023 | To treat relapsed or refractory mantle cell lymphoma in adults who have had at least two lines of systemic therapy, including a BTK inhibitor |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Aphexda | motixafortide | 8 September 2023 | To use with filgrastim (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma |
| 2 | Posluma | flotufolastat F 18 | 25 May 2023 | To use with positron emission tomography imaging in certain patients with prostate cancer |
| 2022 | ||||
| Cancer treatment drug | ||||
| 1 | Lunsumio | mosunetuzumab-axgb | 22 December 2022 | To treat adults with relapsed or refractory follicular lymphoma, a type of non-Hodgkin lymphoma |
| 2 | Krazati | adagrasib | 12 December 2022 | To treat KRAS G12C-mutated locally advanced or metastatic non-small-cell lung cancer in adults who have received at least one prior systemic therapy |
| 3 | Rezlidhia | olutasidenib | 1 December 2022 | To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation |
| 4 | Elahere | mirvetuximab soravtansine-gynx | 14 November 2022 | To treat patients with recurrent ovarian cancer that is resistant to platinum therapy |
| 5 | Tecvayli | teclistamab-cqyv | 25 October 2022 | To treat relapsed or refractory multiple myeloma among adults who have received at least four specific lines of therapy |
| 6 | Imjudo | tremelimumab | 21 October 2022 | To treat unresectable hepatocellular carcinoma |
| 7 | Lytgobi | futibatinib | 30 September 2022 | To treat intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements |
| 8 | Pluvicto | lutetium (177Lu) vipivotide tetraxetan | 23 March 2022 | To treat prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer following other therapies |
| 9 | Opdualag | nivolumab and relatlimab-rmbw | 18 March 2022 | To treat unresectable or metastatic melanoma |
| 10 | Vonjo | pacritinib | 28 February 2022 | To treat intermediate or high-risk primary or secondary myelofibrosis in adults with low platelets |
| 11 | Kimmtrak | tebentafusp-tebn | 25 January 2022 | To treat unresectable or metastatic uveal melanoma |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Elucirem | gadopiclenol | 21 September 2022 | To detect and visualize lesions, together with MRI, with abnormal vascularity in the central nervous system and the body |
| 2 | Rolvedon | eflapegrastim | 9 September 2022 | To decrease the incidence of infection in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia |
| 2021 | ||||
| Cancer treatment drug | ||||
| 1 | Besremi | ropeginterferon alfa-2b-njft | 12 November 2021 | To treat polycythemia vera, a blood disease that causes the overproduction of red blood cells |
| 2 | Scemblix | asciminib | 29 October 2021 | To treat Philadelphia chromosome-positive chronic myeloid leukemia with disease that meets certain criteria |
| 3 | Tivdak | tisotumab vedotin-tftv | 20 September 2021 | To treat recurrent or metastatic cervical cancer with disease progression on or after chemotherapy |
| 4 | Exkivity | mobocertinib | 15 September 2021 | To treat locally advanced or metastatic non-small-cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations |
| 5 | Rylaze | asparaginase erwinia chrysanthemi (recombinant)-rywn | 30 June 2021 | To treat acute lymphoblastic leukemia and lymphoblastic lymphoma in patients who are allergic to E. coli-derived asparaginase products, as a component of a chemotherapy regimen |
| 6 | Truseltiq | infigratinib | 28 May 2021 | To treat cholangiocarcinoma whose disease meets certain criteria |
| 7 | Lumakras | sotorasib | 28 May 2021 | To treat types of non-small-cell lung cancer |
| 8 | Rybrevant | amivantamab-vmjw | 21 May 2021 | To treat a subset of non-small-cell lung cancer |
| 9 | Zynlonta | loncastuximab tesirine-lpyl | 23 April 2021 | To treat certain types of relapsed or refractory large B-cell lymphoma |
| 10 | Jemperli | dostarlimab-gxly | 22 April 2021 | To treat endometrial cancer |
| 11 | Fotivda | tivozanib | 10 March 2021 | To treat renal cell carcinoma |
| 12 | Pepaxto | melphalan flufenamide | 26 February 2021 | To treat relapsed or refractory multiple myeloma |
| 13 | Ukoniq | umbralisib | 5 February 2021 | To treat marginal zone lymphoma and follicular lymphoma |
| 14 | Tepmetko | tepotinib | 3 February 2021 | To treat non-small-cell lung cancer |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Cytalux | pafolacianine | 29 November 2021 | To help identify ovarian cancer lesions |
| 2 | Rylaze | asparaginase erwinia chrysanthemi (recombinant)-rywn | 30 June 2021 | To treat acute lymphoblastic leukemia and lymphoblastic lymphoma in patients who are allergic to E. coli-derived asparaginase products, as a component of a chemotherapy regimen |
| 3 | Pylarify | piflufolastat F 18 | 26 May 2021 | To identify prostate-specific membrane antigen-positive lesions in prostate cancer |
| 2020 | ||||
| Cancer treatment drug | ||||
| 1 | Orgovyx | relugolix | 18 December 2020 | To treat advanced prostate cancer |
| 2 | Margenza | margetuximab (anti-HER2 mAb | 16 December 2020 | To treat HER2+ breast cancer |
| 3 | Danyelza | naxitamab-gqgk | 25 November 2020 | To treat high-risk refractory or relapsed neuroblastoma |
| 4 | Gavreto | pralsetinib | 4 September 2020 | To treat non-small lung cancer |
| 5 | Blenrep | belantamab mafodotin-blmf | 5 August 2020 | To treat multiple myeloma |
| 6 | Monjuvi | tafasitamab-cxix | 31 July 2020 | To treat relapsed or refractory diffuse large B-cell lymphoma |
| 7 | Inqovi | decitabine and cedazuridine | 7 July 2020 | To treat adult patients with myelodysplastic syndromes |
| 8 | Zepzelca | lurbinectedin | 15 June 2020 | To treat metastatic small-cell lung cancer |
| 9 | Qinlock | ripretinib | 15 May 2020 | To treat advanced gastrointestinal-stromal tumors |
| 10 | Retevmo | selpercatinib | 8 May 2020 | To treat lung and thyroid cancers |
| 11 | Tabrecta | capmatinib | 6 May 2020 | To treat patients with non small-cell lung cancer |
| 12 | Trodelvy | sacituzumab govitecan-hziy | 22 April 2020 | To treat adult patients with metastatic triple-negative breast cancer who received at least two prior therapies for metastatic disease |
| 13 | Pemazyre | pemigatinib | 17 April 2020 | To treat certain patients with cholangiocarcinoma, a rare form of cancer that forms in bile ducts |
| 14 | Tukysa | tucatinib | 17 April 2020 | To treat advanced unresectable or metastatic HER2-positive breast cancer |
| 15 | Koselugo | selumetinib | 10 April 2020 | To treat neurofibromatosis type 1, a genetic disorder of the nervous system causing tumors to grow on nerves |
| 16 | Sarclisa | isatuximab | 2 March 2020 | To treat multiple myloma |
| 17 | Tazverik | tazemetostat | 23 January 2020 | To treat epithelioid sarcoma |
| 18 | Ayvakit | avapritinib | 9 January 2020 | To treat adults with unresectable or metastatic gastrointestinal stromal tumor (GIST) |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Gallium 68 PSMA-11 | Gallium 68 PSMA-11 | 1 December 2020 | For detection and localization of prostate cancer |
| 2 | Detectnet | copper Cu 64 dotatate injection | 3 September 2020 | To help detect certain types of neuroendocrine tumors |
| 3 | Cerianna | fluoroestradiol F18 | 20 May 2020 | Diagnostic imaging agent for certain patients with breast cancer |
| 2019 | ||||
| Cancer treatment drug | ||||
| 1 | Enhertu | fam-trastuzumab deruxtecan-nxki | 20 December 2019 | To treat metastatic breast cancer |
| 2 | Padcev | enfortumab vedotin-ejfv | 18 December 2019 | To treat refractory bladder cancer |
| 3 | Brukinsa | zanubrutinib | 14 November 2019 | To treat certain patients with mantle cell lymphoma, a form of blood cancer |
| 4 | Inrebic | fedratinib | 16 August 2019 | To treat adult patients with intermediate-2 or high-risk primary or secondary myelofibrosis |
| 5 | Rozlytrek | entrectinib | 15 August 2019 | To treat adult patients with metastatic non-small-cell lung cancer (NSCLC) whose tumors are ROS1-positiveTo treat adult and pediatric patients 12 years of age and older with solid tumors |
| 6 | Turalio | pexidartinib | 2 August 2019 | To treat adult patients with symptomatic tenosynovial giant cell tumor |
| 7 | Nubeqa | darolutamide | 30 July 2019 | To treat adult patients with non-metastatic castration resistant prostate cancer |
| 8 | Xpovio | selinexor | 3 July 2019 | To treat adult patients with relapsed or refractory multiple myeloma (RRMM) |
| 9 | Polivy | polatuzumab vedotin-piiq | 10 June 2019 | To treat adult patients with relapsed or refractory diffuse large B-cell lymphoma |
| 10 | Piqray | alpelisib | 24 May 2019 | To treat breast cancer |
| 11 | Balversa | erdafitinib | 12 April 2019 | To treat adult patients with locally advanced or metastatic bladder cancer |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Ga-68-DOTATOC | Ga-68-DOTATOC | 21 August 2019 | For use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) |
| 2018 | ||||
| Cancer treatment drug | ||||
| 1 | Elzonris | tagraxofusp-erzs | 21 December 2018 | To treat blastic plasmacytoid dendritic cell neoplasm (BPDCN) |
| 2 | Asparlas | calaspargase pegol-mknl | 20 December 2018 | To treat acute lymphoblastic leukemia (ALL) in pediatric and young adult patients age 1 month to 21 years |
| 3 | Xospata | gilteritinib | 28 November 2018 | To treat patients who have relapsed or refractory acute myeloid leukemia (AML) |
| 4 | Vitrakvi | larotrectinib | 26 November 2018 | To treat patients whose cancers have a specific genetic feature (biomarker) |
| 5 | Daurismo | glasdegib | 21 November 2018 | To treat newly-diagnosed acute myeloid leukemia (AML) in adult patients |
| 6 | Lorbrena | lorlatinib | 2 November 2018 | To treat patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small-cell lung cancer |
| 7 | Talzenna | talazoparib | 16 October 2018 | To treat locally advanced or metastatic breast cancer patients with a germline BRCA mutation. |
| 8 | Libtayo | cemiplimab-rwlc | 28 September 2018 | To treat cutaneous squamous cell carcinoma (CSCC) |
| 9 | Vizimpro | dacomitinib | 27 September 2018 | To treat metastatic non-small-cell lung cancer |
| 10 | Copiktra | duvelisib | 24 September 2018 | To treat relapsed or refractory chronic lymphocytic leukemia, small lymphocytic lymphoma and follicular lymphoma |
| 11 | Lumoxiti | moxetumomab pasudotox-tdfk | 13 September 2018 | To treat hairy cell leukemia |
| 12 | Poteligeo | mogamulizumab-kpkc | 8 August 2018 | To treat two rare types of non-Hodgkin lymphoma |
| 13 | Tibsovo | ivosidenib | 20 July 2018 | To treat patients with relapsed or refractory acute myeloid leukemia |
| 14 | Braftovi | encorafenib | 27 June 2018 | To treat unresectable or metastatic melanoma |
| 15 | Mektovi | binimetinib | 27 June 2018 | To treat unresectable or metastatic melanoma |
| 16 | Erleada | apalutamide | 14 February 2018 | To treat a certain type of prostate cancer using novel clinical trial endpoint |
| 17 | Lutathera | lutetium Lu 177 dotatate | 26 January 2018 | To treat a type of cancer that affects the pancreas or gastrointestinal tract called gastroenteropancreatic neuroendocrine tumors (GEP-NETs) |
| Cancer treatment aid (diagnostics, therapy mitigation) | ||||
| 1 | Akynzeo | fosnetupitant and palonosetron | 19 April 2018 | To prevent acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy |
References
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Loeb, K.R.; Loeb, L.A. Significance of Multiple Mutations in Cancer. Carcinogenesis 2000, 21, 379–385. [Google Scholar] [CrossRef]
- Yokota, J. Tumor Progression and Metastasis. Carcinogenesis 2000, 21, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Zefferino, R.; Piccoli, C.; Di Gioia, S.; Capitanio, N.; Conese, M. Gap Junction Intercellular Communication in the Carcinogenesis Hallmarks: Is This a Phenomenon or Epiphenomenon? Cells 2019, 8, 896. [Google Scholar] [CrossRef]
- Ashkenazi, R.; Gentry, S.N.; Jackson, T.L. Pathways to Tumorigenesis—Modeling Mutation Acquisition in Stem Cells and Their Progeny. Neoplasia 2008, 10, 1170–1182, IN1–IN6. [Google Scholar] [CrossRef] [PubMed]
- Luo, W. Nasopharyngeal Carcinoma Ecology Theory: Cancer as Multidimensional Spatiotemporal “Unity of Ecology and Evolution” Pathological Ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Chiang, S.P.H.; Cabrera, R.M.; Segall, J.E. Tumor Cell Intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Liotta, L.A. Adhere, Degrade, and Move: The Three-Step Model of Invasion. Cancer Res. 2016, 76, 3115–3117. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Courtneidge, S.A. The “ins” and “Outs” of Podosomes and Invadopodia: Characteristics, Formation and Function. Nat. Rev. Mol. Cell Biol. 2011, 12, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.T.; Condeelis, J. Digging a Little Deeper: The Stages of Invadopodium Formation and Maturation. Eur. J. Cell Biol. 2014, 93, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.T.; Wang, Y.; Bravo-Cordero, J.J.; Sharma, V.P.; Miskolci, V.; Hodgson, L.; Condeelis, J. Talin Regulates Moesin–NHE-1 Recruitment to Invadopodia and Promotes Mammary Tumor Metastasis. J. Cell Biol. 2014, 205, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.K.; Courtneidge, S.A. Invadosomes Are Coming: New Insights into Function and Disease Relevance. FEBS J. 2018, 285, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Yan, J.; Schwartz, M.A. Talin as a Mechanosensitive Signaling Hub. J. Cell Biol. 2018, 217, 3776–3784. [Google Scholar] [CrossRef]
- Smith, S.J.; McCann, R.O. A C-Terminal Dimerization Motif Is Required for Focal Adhesion Targeting of Talin1 and the Interaction of the Talin1 I/LWEQ Module with F-Actin. Biochemistry 2007, 46, 10886–10898. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Minn, A.J.; Kang, Y.; Siegel, P.M.; Serganova, I.; Cordón-Cardo, C.; Olshen, A.B.; Gerald, W.L.; Massagué, J. Identifying Site-Specific Metastasis Genes and Functions. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA; Volume 70, pp. 149–158.
- Saxena, M.; Christofori, G. Rebuilding Cancer Metastasis in the Mouse. Mol. Oncol. 2013, 7, 283–296. [Google Scholar] [CrossRef]
- Andasari, V.; Chaplain, M.A.J. Intracellular Modelling of Cell-Matrix Adhesion during Cancer Cell Invasion. Math. Model. Nat. Phenom. 2012, 7, 29–48. [Google Scholar] [CrossRef][Green Version]
- Devreotes, P.; Horwitz, A.R. Signaling Networks That Regulate Cell Migration. Cold Spring Harb. Perspect. Biol. 2015, 7, a005959. [Google Scholar] [CrossRef]
- Xiao, Y.; Riahi, R.; Torab, P.; Zhang, D.D.; Wong, P.K. Collective Cell Migration in 3D Epithelial Wound Healing. ACS Nano 2019, 13, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Hons, M.; Kopf, A.; Hauschild, R.; Leithner, A.; Gaertner, F.; Abe, J.; Renkawitz, J.; Stein, J.V.; Sixt, M. Chemokines and Integrins Independently Tune Actin Flow and Substrate Friction during Intranodal Migration of T Cells. Nat. Immunol. 2018, 19, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Mayor, R. Collective Cell Migration in Development. J. Cell Biol. 2016, 212, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuzé, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and Low Adhesion Induce Fast Amoeboid Migration of Slow Mesenchymal Cells. Cell 2015, 160, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.G.; Vignjevic, D.M. Modes of Cancer Cell Invasion and the Role of the Microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell Migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar]
- Yin, M.; Ma, W.; An, L. Cortactin in Cancer Cell Migration and Invasion. Oncotarget 2017, 8, 88232–88243. [Google Scholar] [CrossRef]
- Nammalwar, R.C.; Heil, A.; Gerke, V. Ezrin Interacts with the Scaffold Protein IQGAP1 and Affects Its Cortical Localization. Biochim Biophys Acta Mol. Cell Res. 2014, 1853, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. Actin and Microtubules in Cell Motility: Which One Is in Control? Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef]
- Moshfegh, Y.; Bravo-Cordero, J.J.; Miskolci, V.; Condeelis, J.; Hodgson, L. A Trio–Rac1–Pak1 Signalling Axis Drives Invadopodia Disassembly. Nat. Cell Biol. 2014, 16, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Machacek, M.; Hodgson, L.; Welch, C.; Elliott, H.; Pertz, O.; Nalbant, P.; Abell, A.; Johnson, G.L.; Hahn, K.M.; Danuser, G. Coordination of Rho GTPase Activities during Cell Protrusion. Nature 2009, 461, 99–103. [Google Scholar] [CrossRef]
- Jacob, A.; Prekeris, R. The Regulation of MMP Targeting to Invadopodia during Cancer Metastasis. Front. Cell Dev. Biol. 2015, 3, 4. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as Biomechanical Sensors of the Microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Gough, R.E.; Goult, B.T. The Tale of Two Talins—Two Isoforms to Fine-Tune Integrin Signalling. FEBS Lett. 2018, 592, 2108–2125. [Google Scholar] [CrossRef]
- Klapholz, B.; Brown, N.H. Talin—The Master of Integrin Adhesions. J. Cell Sci. 2017, 130, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Nicolas, A. Physically Based Principles of Cell Adhesion Mechanosensitivity in Tissues. Rep. Prog. Phys. 2012, 75, 116601. [Google Scholar] [CrossRef]
- Monkley, S.J.; Pritchard, C.A.; Critchley, D.R. Analysis of the Mammalian Talin2 Gene TLN2. Biochem. Biophys. Res. Commun. 2001, 286, 880–885. [Google Scholar] [CrossRef]
- Molony, L.; McCaslin, D.; Abernethy, J.; Paschal, B.; Burridge, K. Properties of Talin from Chicken Gizzard Smooth Muscle. J. Biol. Chem. 1987, 262, 7790–7795. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Connell, L. A New Protein of Adhesion Plaques and Ruffling Membranes. J. Cell Biol. 1983, 97, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, A.; Duggan, K.; Buck, C.; Beckerle, M.C.; Burridge, K. Interaction of Plasma Membrane Fibronectin Receptor with Talin—A Transmembrane Linkage. Nature 1986, 320, 531–533. [Google Scholar] [CrossRef]
- Muguruma, M.; Matsumura, S.; Fukazawa, T. Direct Interactions between Talin and Actin. Biochem. Biophys. Res. Commun. 1990, 171, 1217–1223. [Google Scholar] [CrossRef]
- Gingras, A.R.; Ziegler, W.H.; Frank, R.; Barsukov, I.L.; Roberts, G.C.K.; Critchley, D.R.; Emsley, J. Mapping and Consensus Sequence Identification for Multiple Vinculin Binding Sites within the Talin Rod. J. Biol. Chem. 2005, 280, 37217–37224. [Google Scholar] [CrossRef]
- Chen, H.C.; Appeddu, P.A.; Parsons, J.T.; Hildebrand, J.D.; Schaller, M.D.; Guan, J.L. Interaction of Focal Adhesion Kinase with Cytoskeletal Protein Talin. J. Biol. Chem. 1995, 270, 16995–16999. [Google Scholar] [CrossRef] [PubMed]
- Zacharchenko, T.; Qian, X.; Goult, B.T.; Jethwa, D.; Almeida, T.B.; Ballestrem, C.; Critchley, D.R.; Lowy, D.R.; Barsukov, I.L. LD Motif Recognition by Talin: Structure of the Talin-DLC1 Complex. Structure 2016, 24, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Chishti, A.H.; Kim, A.C.; Marfatia, S.M.; Lutchman, M.; Hanspal, M.; Jindal, H.; Liu, S.-C.; Low, P.S.; Rouleau, G.A.; Mohandas, N.; et al. The FERM Domain: A Unique Module Involved in the Linkage of Cytoplasmic Proteins to the Membrane. Trends Biochem. Sci. 1998, 23, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Bouaouina, M.; Elliott, P.R.; Bate, N.; Patel, B.; Gingras, A.R.; Grossmann, J.G.; Roberts, G.C.K.; Calderwood, D.A.; Critchley, D.R.; et al. Structure of a Double Ubiquitin-like Domain in the Talin Head: A Role in Integrin Activation. EMBO J. 2010, 29, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Zacharchenko, T.; Bate, N.; Tsang, R.; Hey, F.; Gingras, A.R.; Elliott, P.R.; Roberts, G.C.K.; Ballestrem, C.; Critchley, D.R.; et al. RIAM and Vinculin Binding to Talin Are Mutually Exclusive and Regulate Adhesion Assembly and Turnover. J. Biol. Chem. 2013, 288, 8238–8249. [Google Scholar] [CrossRef]
- Bate, N.; Gingras, A.R.; Bachir, A.; Horwitz, R.; Ye, F.; Patel, B.; Goult, B.T.; Critchley, D.R. Talin Contains A C-Terminal Calpain2 Cleavage Site Important In Focal Adhesion Dynamics. PLoS ONE 2012, 7, e34461. [Google Scholar] [CrossRef]
- Goult, B.T.; Bouaouina, M.; Harburger, D.S.; Bate, N.; Patel, B.; Anthis, N.J.; Campbell, I.D.; Calderwood, D.A.; Barsukov, I.L.; Roberts, G.C.; et al. The Structure of the N-Terminus of Kindlin-1: A Domain Important for AIIbβ3 Integrin Activation. J. Mol. Biol. 2009, 394, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.R.; Goult, B.T.; Kopp, P.M.; Bate, N.; Grossmann, J.G.; Roberts, G.C.K.; Critchley, D.R.; Barsukov, I.L. The Structure of the Talin Head Reveals a Novel Extended Conformation of the FERM Domain. Structure 2010, 18, 1289–1299. [Google Scholar] [CrossRef]
- Bouaouina, M.; Lad, Y.; Calderwood, D.A. The N-Terminal Domains of Talin Cooperate with the Phosphotyrosine Binding-like Domain to Activate Β1 and Β3 Integrins *. J. Biol. Chem. 2008, 283, 6118–6125. [Google Scholar] [CrossRef]
- Sun, Z.; Costell, M.; Fässler, R. Integrin Activation by Talin, Kindlin and Mechanical Forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, L.; Rees, D.J.; Ohanian, V.; Bolton, S.J.; Gilmore, A.P.; Patel, B.; Priddle, H.; Trevithick, J.E.; Hynes, R.O.; Critchley, D.R. Talin Contains Three Actin-Binding Sites Each of Which Is Adjacent to a Vinculin-Binding Site. J. Cell Sci. 1996, 109 Pt 1, 2715–2726. [Google Scholar] [CrossRef]
- Gingras, A.R.; Ziegler, W.H.; Bobkov, A.A.; Joyce, M.G.; Fasci, D.; Himmel, M.; Rothemund, S.; Ritter, A.; Grossmann, J.G.; Patel, B.; et al. Structural Determinants of Integrin Binding to the Talin Rod. J. Biol. Chem. 2009, 284, 8866–8876. [Google Scholar] [CrossRef]
- Saxena, M.; Changede, R.; Hone, J.; Wolfenson, H.; Sheetz, M.P. Force-Induced Calpain Cleavage of Talin Is Critical for Growth, Adhesion Development, and Rigidity Sensing. Nano Lett. 2017, 17, 7242–7251. [Google Scholar] [CrossRef]
- Franco, S.J.; Rodgers, M.A.; Perrin, B.J.; Han, J.; Bennin, D.A.; Critchley, D.R.; Huttenlocher, A. Calpain-Mediated Proteolysis of Talin Regulates Adhesion Dynamics. Nat. Cell Biol. 2004, 6, 977–983. [Google Scholar] [CrossRef]
- Huang, C.; Rajfur, Z.; Yousefi, N.; Chen, Z.; Jacobson, K.; Ginsberg, M.H. Talin Phosphorylation by Cdk5 Regulates Smurf1-Mediated Talin Head Ubiquitylation and Cell Migration. Nat. Cell Biol. 2009, 11, 624–630. [Google Scholar] [CrossRef]
- Sakamoto, S.; McCann, R.O.; Dhir, R.; Kyprianou, N. Talin1 Promotes Tumor Invasion and Metastasis via Focal Adhesion Signaling and Anoikis Resistance. Cancer Res. 2010, 70, 1885–1895. [Google Scholar] [CrossRef]
- Jin, J.-K.; Tien, P.-C.; Cheng, C.-J.; Song, J.H.; Huang, C.; Lin, S.-H.; Gallick, G.E. Talin1 Phosphorylation Activates Β1 Integrins: A Novel Mechanism to Promote Prostate Cancer Bone Metastasis. Oncogene 2015, 34, 1811–1821. [Google Scholar] [CrossRef]
- McCann, R.O.; Craig, S.W. Functional Genomic Analysis Reveals the Utility of the I/LWEQ Module as a Predictor of Protein: Actin Interaction. Biochem. Biophys. Res. Commun. 1999, 266, 135–140. [Google Scholar] [CrossRef]
- Qi, L.; Jafari, N.; Li, X.; Chen, Z.; Li, L.; Hytönen, V.P.; Goult, B.T.; Zhan, C.-G.; Huang, C. Talin2-Mediated Traction Force Drives Matrix Degradation and Cell Invasion. J. Cell Sci. 2016, 129, 3661–3674. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Zhu, Y.; Qi, L.; Azizi, L.; Hytönen, V.P.; Zhan, C.-G.; Huang, C. The Molecular Basis of Talin2’s High Affinity toward Β1-Integrin. Sci. Rep. 2017, 7, 41989. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, G.; Cai, Y.; Monkley, S.J.; Critchley, D.R.; Sheetz, M.P. Talin Depletion Reveals Independence of Initial Cell Spreading from Integrin Activation and Traction. Nat. Cell Biol. 2008, 10, 1062–1068. [Google Scholar] [CrossRef]
- Praekelt, U.; Kopp, P.M.; Rehm, K.; Linder, S.; Bate, N.; Patel, B.; Debrand, E.; Manso, A.M.; Ross, R.S.; Conti, F.; et al. New Isoform-Specific Monoclonal Antibodies Reveal Different Sub-Cellular Localisations for Talin1 and Talin2. Eur. J. Cell Biol. 2012, 91, 180–191. [Google Scholar] [CrossRef]
- Senetar, M.A.; Moncman, C.L.; McCann, R.O. Talin2 Is Induced during Striated Muscle Differentiation and Is Targeted to Stable Adhesion Complexes in Mature Muscle. Cell Motil. Cytoskelet. 2007, 64, 157–173. [Google Scholar] [CrossRef]
- Protein Expression Overview of TLN1. The Human Protein Atlas. Available online: https://v22.proteinatlas.org/ENSG00000137076-TLN1 (accessed on 23 May 2023).
- Protein Expression Overview of TLN2. The Human Protein Atlas. Available online: https://v22.proteinatlas.org/ENSG00000171914-TLN2 (accessed on 23 May 2023).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 23 May 2023).
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical Regulation of a Molecular Clutch Defines Force Transmission and Transduction in Response to Matrix Rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Qi, L.; Rychahou, P.; Jafari, N.; Huang, C. The Role of Talin2 in Breast Cancer Tumorigenesis and Metastasis. Oncotarget 2017, 8, 106876–106887. [Google Scholar] [CrossRef][Green Version]
- Baster, Z.; Li, L.; Rajfur, Z.; Huang, C. Talin2 Mediates Secretion and Trafficking of Matrix Metallopeptidase 9 during Invadopodium Formation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118693. [Google Scholar] [CrossRef]
- Senetar, M.A.; Foster, S.J.; McCann, R.O. Intrasteric Inhibition Mediates the Interaction of the I/LWEQ Module Proteins Talin1, Talin2, Hip1, and Hip12 with Actin. Biochemistry 2004, 43, 15418–15428. [Google Scholar] [CrossRef]
- Azizi, L.; Cowell, A.R.; Mykuliak, V.V.; Goult, B.T.; Turkki, P.; Hytönen, V.P. Cancer Associated Talin Point Mutations Disorganise Cell Adhesion and Migration. Sci. Rep. 2021, 11, 347. [Google Scholar] [CrossRef]
- Azizi, L.; Varela, L.; Turkki, P.; Mykuliak, V.V.; Korpela, S.; Ihalainen, T.O.; Church, J.; Hytönen, V.P.; Goult, B.T. Talin Variant P229S Compromises Integrin Activation and Associates with Multifaceted Clinical Symptoms. Hum. Mol. Genet. 2022, 31, 4159–4172. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Ahn, S.J.; Huang, B.; Kumar, A.; Schwartz, M.A. Actin Flow-Dependent and-Independent Force Transmission through Integrins. Proc. Natl. Acad. Sci. USA 2020, 117, 32413–32422. [Google Scholar] [CrossRef]
- Gingras, A.R.; Bate, N.; Goult, B.T.; Hazelwood, L.; Canestrelli, I.; Grossmann, J.G.; Liu, H.; Putz, N.S.M.; Roberts, G.C.K.; Volkmann, N.; et al. The Structure of the C-Terminal Actin-Binding Domain of Talin. EMBO J. 2008, 27, 458–469. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin Ligands at a Glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Xia, T.; Takagi, J.; Coller, B.S.; Wang, J.H.; Springer, T.A. Structural Basis for Allostery in Integrins and Binding to Fibrinogen-Mimetic Therapeutics. Nature 2004, 432, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.R.; Owens, T.W.; Naylor, M.J. Structural and Mechanical Functions of Integrins. Biophys. Rev. 2014, 6, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Petre, B.M.; Walz, T.; Springer, T.A. Global Conformational Earrangements in Integrin Extracellular Domains in Outside-in and inside-out Signaling. Cell 2002, 110, 599–611. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Iacob, R.E.; Engen, J.R.; Springer, T.A. General Structural Features That Regulate Integrin Affinity Revealed by Atypical AVβ8. Nat. Commun. 2019, 10, 5481. [Google Scholar] [CrossRef]
- Kong, F.; García, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Demonstration of Catch Bonds between an Integrin and Its Ligand. J. Cell Biol. 2009, 185, 1275–1284. [Google Scholar] [CrossRef]
- Li, J.; Springer, T.A. Integrin Extension Enables Ultrasensitive Regulation by Cytoskeletal Force. Proc. Natl. Acad. Sci. USA 2017, 114, 4685–4690. [Google Scholar] [CrossRef]
- Byron, A.; Humphries, J.D.; Askari, J.A.; Craig, S.E.; Mould, A.P.; Humphries, M.J. Anti-Integrin Monoclonal Antibodies. J. Cell Sci. 2009, 122, 4009–4011. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of Integrins in Cell Invasion and Migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Haeger, A.; Alexander, S.; Vullings, M.; Kaiser, F.M.P.; Veelken, C.; Flucke, U.; Koehl, G.E.; Hirschberg, M.; Flentje, M.; Hoffman, R.M.; et al. Collective Cancer Invasion Forms an Integrin-Dependent Radioresistant Niche. J. Exp. Med. 2020, 217, e20181184. [Google Scholar] [CrossRef]
- Evans, R.; Patzak, I.; Svensson, L.; De Filippo, K.; Jones, K.; McDowall, A.; Hogg, N. Integrins in Immunity. J. Cell Sci. 2009, 122, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Streuli, C.H. Signalling Pathways Linking Integrins with Cell Cycle Progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Assoian, R.K. Integrins and Cell Proliferation: Regulation of Cyclin-Dependent Kinases via Cytoplasmic Signaling Pathways. J. Cell Sci. 2001, 114, 2553–2560. [Google Scholar] [CrossRef]
- Brown, K.E.; Yamada, K.M. The Role of Integrins during Vertebrate Development. Semin. Dev. Biol. 1995, 6, 69–77. [Google Scholar] [CrossRef]
- Bouvard, D.; Brakebusch, C.; Gustafsson, E.; Aszódi, A.; Bengtsson, T.; Berna, A.; Fässler, R. Functional Consequences of Integrin Gene Mutations in Mice. Circ. Res. 2001, 89, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Saher, G.; Fässler, R.; Bornemann, A.; Echtermeyer, F.; von der Mark, H.; Miosge, N.; Pösch, E.; von der Mark, K. Absence of Integrin A7 Causes a Novel Form of Muscular Dystrophy. Nat. Genet. 1997, 17, 318–323. [Google Scholar] [CrossRef]
- Romaine, A.; Sørensen, I.W.; Zeltz, C.; Lu, N.; Erusappan, P.M.; Melleby, A.O.; Zhang, L.; Bendiksen, B.; Robinson, E.L.; Aronsen, J.M.; et al. Overexpression of Integrin A11 Induces Cardiac Fibrosis in Mice. Acta Physiologica 2018, 222, e12932. [Google Scholar] [CrossRef] [PubMed]
- Wickström, S.A.; Radovanac, K.; Fässler, R. Genetic Analyses of Integrin Signaling. Cold Spring Harb. Perspect. Biol. 2011, 3, a005116. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Hayashido, Y.; Kitano, H.; Sakaue, T.; Fujii, T.; Suematsu, M.; Sakurai, S.; Okamoto, T. Overexpression of Integrin Av Facilitates Proliferation and Invasion of Oral Squamous Cell Carcinoma Cells via Mek/Erk Signaling Pathway That Is Activated by Interaction of Integrin Avβ8 with Type I Collagen. Int. J. Oncol. 2014, 45, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Q.; Popova, S.N.; Brown, E.R.S.; Barsyte-Lovejoy, D.; Navab, R.; Shih, W.; Li, M.; Lu, M.; Jurisica, I.; Penn, L.Z.; et al. Integrin A11 Regulates IGF2 Expression in Fibroblasts to Enhance Tumorigenicity of Human Non-Small-Cell Lung Cancer Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11754–11759. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Alushin, G.M.; Waterman, C.M. Mechanosensation: A Catch Bond That Only Hooks One Way. Curr. Biol. 2017, 27, R1158–R1160. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Hinczewski, M.; Thirumalai, D. Plasticity of Hydrogen Bond Networks Regulates Mechanochemistry of Cell Adhesion Complexes. Proc. Natl. Acad. Sci. USA 2014, 111, 9048–9053. [Google Scholar] [CrossRef]
- Benito-Jardón, M.; Klapproth, S.; Gimeno-Lluch, I.; Petzold, T.; Bharadwaj, M.; Müller, D.J.; Zuchtriegel, G.; Reichel, C.A.; Costell, M. The Fibronectin Synergy Site Re-Enforces Cell Adhesion and Mediates a Crosstalk between Integrin Classes. elife 2017, 6, e22264. [Google Scholar] [CrossRef] [PubMed]
- Anthis, N.J.; Wegener, K.L.; Ye, F.; Kim, C.; Goult, B.T.; Lowe, E.D.; Vakonakis, I.; Bate, N.; Critchley, D.R.; Ginsberg, M.H.; et al. The Structure of an Integrin/Talin Complex Reveals the Basis of inside-out Signal Transduction. EMBO J. 2009, 28, 3623–3632. [Google Scholar] [CrossRef]
- Lai, X.; Li, Q.; Wu, F.; Lin, J.; Chen, J.; Zheng, H.; Guo, L. Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons From Somatic Cell Reprogramming. Front. Cell Dev. Biol. 2020, 8, 760. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Tan, X.; Choi, S.; Wise, T.; Anderson, R.A. PIPKIγ and Talin Couple Phosphoinositide and Adhesion Signaling to Control the Epithelial to Mesenchymal Transition. Oncogene 2017, 36, 899–911. [Google Scholar] [CrossRef]
- Bolós, V.; Gasent, J.M.; López-Tarruella, S.; Grande, E. The Dual Kinase Complex FAK-Src as a Promising Therapeutic Target in Cancer. OncoTargets Ther. 2010, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Goyette, M.-A.; Chapelle, J.; Boufaied, N.; Al Rahbani, J.; Schonewolff, M.; Danek, E.I.; Muller, W.J.; Labbé, D.P.; Côté, J.-F.; et al. CdGAP Is a Talin-Binding Protein and a Target of TGF-β Signaling That Promotes HER2-Positive Breast Cancer Growth and Metastasis. Cell Rep. 2023, 42, 112936. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pursell, B.; Lu, S.; Chang, T.-K.; Mercurio, A.M. Regulation of Β4-Integrin Expression by Epigenetic Modifications in the Mammary Gland and during the Epithelial-to-Mesenchymal Transition. J. Cell Sci. 2009, 122, 2473–2480. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the Cytoskeleton, and Cancer Cell Invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Petersen, O.W.; Ronnov-Jessen, L.; Weaver, V.M.; Bissell, M.J. Differentiation and Cancer in the Mammary Gland: Shedding Light on an Old Dichotomy. Adv. Cancer Res. 1998, 75, 135. [Google Scholar] [CrossRef]
- Desiniotis, A.; Kyprianou, N. Significance of Talin in Cancer Progression and Metastasis. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2011; pp. 117–147. [Google Scholar]
- Harryman, W.L.; Marr, K.D.; Nagle, R.B.; Cress, A.E. Integrins and Epithelial-Mesenchymal Cooperation in the Tumor Microenvironment of Muscle-Invasive Lethal Cancers. Front. Cell Dev. Biol. 2022, 10, 837585. [Google Scholar] [CrossRef] [PubMed]
- Bécam, I.E.; Tanentzapf, G.; Lepesant, J.-A.; Brown, N.H.; Huynh, J.-R. Integrin-Independent Repression of Cadherin Transcription by Talin during Axis Formation in Drosophila. Nat. Cell Biol. 2005, 7, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.P.; Eddy, R.; Entenberg, D.; Kai, M.; Gertler, F.B.; Condeelis, J. Tks5 and SHIP2 Regulate Invadopodium Maturation, but Not Initiation, in Breast Carcinoma Cells. Curr. Biol. 2013, 23, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor Cell Invadopodia: Invasive Protrusions That Orchestrate Metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef]
- Beaty, B.T.; Sharma, V.P.; Bravo-Cordero, J.J.; Simpson, M.A.; Eddy, R.J.; Koleske, A.J.; Condeelis, J. Β1 Integrin Regulates Arg to Promote Invadopodial Maturation and Matrix Degradation. Mol. Biol. Cell 2013, 24, 1661–1675. [Google Scholar] [CrossRef]
- Schoumacher, M.; Goldman, R.D.; Louvard, D.; Vignjevic, D.M. Actin, Microtubules, and Vimentin Intermediate Filaments Cooperate for Elongation of Invadopodia. J. Cell Biol. 2010, 189, 541–556. [Google Scholar] [CrossRef]
- Bravo-Cordero, J.J.; Magalhaes, M.A.O.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of Cofilin in Cell Locomotion and Invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Sleeboom, J.J.F.; Eslami Amirabadi, H.; Nair, P.; Sahlgren, C.M.; den Toonder, J.M.J. Metastasis in Context: Modeling the Tumor Microenvironment with Cancer-on-a-Chip Approaches. Dis. Model. Mech. 2018, 11, dmm.033100. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Doyle, A.D.; Shinsato, Y.; Wang, S.; Bodendorfer, M.A.; Zheng, M.; Yamada, K.M. Basement Membrane Regulates Fibronectin Organization Using Sliding Focal Adhesions Driven by a Contractile Winch. Dev. Cell 2020, 52, 631–646.e4. [Google Scholar] [CrossRef]
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. Β1 Integrin, ILK and MTOR Regulate Collagen Synthesis in Mechanically Loaded Tendon Cells. Sci. Rep. 2020, 10, 12644. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.S.; Sun, X.; Baird, M.A.; Hourwitz, M.J.; Seo, B.R.; Pasapera, A.M.; Mehta, S.B.; Losert, W.; Fischbach, C.; Fourkas, J.T.; et al. Contractility, Focal Adhesion Orientation, and Stress Fiber Orientation Drive Cancer Cell Polarity and Migration along Wavy ECM Substrates. Proc. Natl. Acad. Sci. USA 2021, 118, e2021135118. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Artym, V.V.; Green, J.A.; Yamada, K.M. The Matrix Reorganized: Extracellular Matrix Remodeling and Integrin Signaling. Curr. Opin. Cell Biol. 2006, 18, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.A.; Heck, T.; Smeets, B.; Ramon, H.; Parameswaran, H.; Van Oosterwyck, H. Intercellular Adhesion Stiffness Moderates Cell Decoupling as a Function of Substrate Stiffness. Biophys. J. 2020, 119, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Vachon, P.H. Integrin Signaling, Cell Survival, and Anoikis: Distinctions, Differences, and Differentiation. J. Signal Transduct. 2011, 2011, 738137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Kuo, J.-C.; Wei, M.-T.; Chen, Y.-C.; Yang, M.-H.; Chiou, A. Early Stage Mechanical Remodeling of Collagen Surrounding Head and Neck Squamous Cell Carcinoma Spheroids Correlates Strongly with Their Invasion Capability. Acta Biomater. 2019, 84, 280–292. [Google Scholar] [CrossRef]
- Fata, J.E.; Werb, Z.; Bissell, M.J. Regulation of Mammary Gland Branching Morphogenesis by the Extracellular Matrix and Its Remodeling Enzymes. Breast Cancer Res. 2003, 6, 1. [Google Scholar] [CrossRef]
- Seo, D.W.; Li, H.; Guedez, L.; Wingfield, P.T.; Diaz, T.; Salloum, R.; Wei, B.Y.; Stetler-Stevenson, W.G. TIMP-2 Mediated Inhibition of Angiogenesis: An MMP-Independent Mechanism. Cell 2003, 114, 171–180. [Google Scholar] [CrossRef]
- Barbazán, J.; Alonso-Alconada, L.; Muinelo-Romay, L.; Vieito, M.; Abalo, A.; Alonso-Nocelo, M.; Candamio, S.; Gallardo, E.; Fernández, B.; Abdulkader, I.; et al. Molecular Characterization of Circulating Tumor Cells in Human Metastatic Colorectal Cancer. PLoS ONE 2012, 7, e40476. [Google Scholar] [CrossRef] [PubMed]
- Kai, F.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Alanko, J.; Mai, A.; Jacquemet, G.; Schauer, K.; Kaukonen, R.; Saari, M.; Goud, B.; Ivaska, J. Integrin Endosomal Signalling Suppresses Anoikis. Nat. Cell Biol. 2015, 17, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Baster, Z.; Li, L.; Kukkurainen, S.; Chen, J.; Pentikäinen, O.; Győrffy, B.; Hytönen, V.P.; Zhu, H.; Rajfur, Z.; Huang, C. Cyanidin-3-glucoside Binds to Talin and Modulates Colon Cancer Cell Adhesions and 3D Growth. FASEB J. 2020, 34, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Irby, R.; Mao, W.; Coppola, D.; Jove, R.; Gamero, A.; Cuthbertson, D.; Fujita, D.J.; Yeatman, T.J. Overexpression of Normal C-Src in Poorly Metastatic Human Colon Cancer Cells Enhances Primary Tumor Growth but Not Metastatic Potential. Cell Growth Differ. 1997, 8, 1287–1295. [Google Scholar] [PubMed]
- Staley, C.A.; Parikh, N.U.; Gallick, G.E. Decreased Tumorigenicity of a Human Colon Adenocarcinoma Cell Line by an Antisense Expression Vector Specific for C-Src. Cell Growth Differ. 1997, 8, 269–274. [Google Scholar]
- Rezaie, Y.; Fattahi, F.; Mashinchi, B.; Kamyab Hesari, K.; Montazeri, S.; Kalantari, E.; Madjd, Z.; Saeednejad Zanjani, L. High Expression of Talin-1 Is Associated with Tumor Progression and Recurrence in Melanoma Skin Cancer Patients. BMC Cancer 2023, 23, 302. [Google Scholar] [CrossRef]
- Wang, X.; Baster, Z.; Azizi, L.; Li, L.; Rajfur, Z.; Hytönen, V.P.; Huang, C. Talin2 Binds to Non-Muscle Myosin IIa and Regulates Cell Attachment and Fibronectin Secretion. Sci. Rep. 2024, 14, 20175. [Google Scholar] [CrossRef]
- Kovács, S.A.; Fekete, J.T.; Győrffy, B. Predictive Biomarkers of Immunotherapy Response with Pharmacological Applications in Solid Tumors. Acta Pharmacol. Sin. 2023, 44, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Posta, M.; Győrffy, B. Analysis of a Large Cohort of Pancreatic Cancer Transcriptomic Profiles to Reveal the Strongest Prognostic Factors. Clin. Transl. Sci. 2023, 16, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Transcriptome-level Discovery of Survival-associated Biomarkers and Therapy Targets in Non-small-cell Lung Cancer. Br. J. Pharmacol. 2024, 181, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Discovery and Ranking of the Most Robust Prognostic Biomarkers in Serous Ovarian Cancer. Geroscience 2023, 45, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Survival Analysis across the Entire Transcriptome Identifies Biomarkers with the Highest Prognostic Power in Breast Cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chu, S.; Chiou, H.; Kuo, W.; Chiang, C.-L.; Hsieh, Y.-S. Mulberry Anthocyanins, Cyanidin 3-Rutinoside and Cyanidin 3-Glucoside, Exhibited an Inhibitory Effect on the Migration and Invasion of a Human Lung Cancer Cell Line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Cruz da Silva, E.; Dontenwill, M.; Choulier, L.; Lehmann, M. Role of Integrins in Resistance to Therapies Targeting Growth Factor Receptors in Cancer. Cancers 2019, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network Global Burden of Disease Study 2019 (GBD 2019) Reference Life Table; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2021. [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Tosolini, M.; Charoentong, P.; Kirilovsky, A.; Bindea, G.; Berger, A.; Camus, M.; Gillard, M.; Bruneval, P.; Fridman, W.; et al. Biomolecular Network Reconstruction Identifies T-Cell Homing Factors Associated with Survival in Colorectal Cancer. Gastroenterology 2010, 138, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Yoong, K.F.; McNab, G.; Hübscher, S.G.; Adams, D.H. Vascular Adhesion Protein-1 and ICAM-1 Support the Adhesion of Tumor-Infiltrating Lymphocytes to Tumor Endothelium in Human Hepatocellular Carcinoma. J. Immunol. 1998, 160, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; McCabe, K.E.; Bartakova, A.; Delaney, J.; Pizzo, D.P.; Newbury, R.O.; Varner, J.A.; Schlaepfer, D.D.; Stupack, D.G. Integrin A4 Enhances Metastasis and May Be Associated with Poor Prognosis in MYCNlow Neuroblastoma. PLoS ONE 2015, 10, e0120815. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-B.; Chen, G.-Y.; Xia, J.-G.; Zang, X.-W.; Yang, H.-Y.; Yang, L. Association of VCAM-1 Overexpression with Oncogenesis, Tumor Angiogenesis and Metastasis of Gastric Carcinoma. World J. Gastroenterol. 2003, 9, 1409–1414. [Google Scholar] [CrossRef]
- Moore, K.M.; Thomas, G.J.; Duffy, S.W.; Warwick, J.; Gabe, R.; Chou, P.; Ellis, I.O.; Green, A.R.; Haider, S.; Brouilette, K.; et al. Therapeutic Targeting of Integrin Avβ6 in Breast Cancer. JNCI J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Reader, C.S.; Vallath, S.; Steele, C.W.; Haider, S.; Brentnall, A.; Desai, A.; Moore, K.M.; Jamieson, N.B.; Chang, D.; Bailey, P.; et al. The Integrin Avβ6 Drives Pancreatic Cancer through Diverse Mechanisms and Represents an Effective Target for Therapy. J. Pathol. 2019, 249, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Busenhart, P.; Montalban-Arques, A.; Katkeviciute, E.; Morsy, Y.; Van Passen, C.; Hering, L.; Atrott, K.; Lang, S.; Garzon, J.F.G.; Naschberger, E.; et al. Inhibition of Integrin Avβ6 Sparks T-Cell Antitumor Response and Enhances Immune Checkpoint Blockade Therapy in Colorectal Cancer. J. Immunother. Cancer 2022, 10, e003465. [Google Scholar] [CrossRef] [PubMed]
- Bagati, A.; Kumar, S.; Jiang, P.; Pyrdol, J.; Zou, A.E.; Godicelj, A.; Mathewson, N.D.; Cartwright, A.N.R.; Cejas, P.; Brown, M.; et al. Integrin Avβ6–TGFβ–SOX4 Pathway Drives Immune Evasion in Triple-Negative Breast Cancer. Cancer Cell 2021, 39, 54–67.e9. [Google Scholar] [CrossRef] [PubMed]
- Lainé, A.; Labiad, O.; Hernandez-Vargas, H.; This, S.; Sanlaville, A.; Léon, S.; Dalle, S.; Sheppard, D.; Travis, M.A.; Paidassi, H.; et al. Regulatory T Cells Promote Cancer Immune-Escape through Integrin Avβ8-Mediated TGF-β Activation. Nat. Commun. 2021, 12, 6228. [Google Scholar] [CrossRef]
- Vannini, A.; Leoni, V.; Barboni, C.; Sanapo, M.; Zaghini, A.; Malatesta, P.; Campadelli-Fiume, G.; Gianni, T. Avβ3-Integrin Regulates PD-L1 Expression and Is Involved in Cancer Immune Evasion. Proc. Natl. Acad. Sci. USA 2019, 116, 20141–20150. [Google Scholar] [CrossRef]
- Yousefi, H.; Vatanmakanian, M.; Mahdiannasser, M.; Mashouri, L.; Alahari, N.V.; Monjezi, M.R.; Ilbeigi, S.; Alahari, S.K. Understanding the Role of Integrins in Breast Cancer Invasion, Metastasis, Angiogenesis, and Drug Resistance. Oncogene 2021, 40, 1043–1063. [Google Scholar] [CrossRef]
- Seguin, L.; Kato, S.; Franovic, A.; Camargo, M.F.; Lesperance, J.; Elliott, K.C.; Yebra, M.; Mielgo, A.; Lowy, A.M.; Husain, H.; et al. An Integrin Β3–KRAS–RalB Complex Drives Tumour Stemness and Resistance to EGFR Inhibition. Nat. Cell Biol. 2014, 16, 457–468. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, M.; Yang, L.; Tu, G.; Zhu, Q.; Chen, M.; Cheng, H.; Luo, H.; Fu, W.; Li, Z.; et al. Acquisition of Epithelial-Mesenchymal Transition Phenotype in the Tamoxifen-Resistant Breast Cancer Cell: A New Role for G Protein-Coupled Estrogen Receptor in Mediating Tamoxifen Resistance through Cancer-Associated Fibroblast-Derived Fibronectin and Β1-integrin signaling pathway in tumor cells. Breast Cancer Res. 2015, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.S.; Mavingire, N.; Khan, S.; Rowland, L.K.; Wooten, J.V.; Opoku-Agyeman, A.; Guevara, A.; Soto, U.; Cavalli, F.; Loaiza-Pérez, A.I.; et al. AhR Ligand Aminoflavone Suppresses A6-integrin–Src–Akt Signaling to Attenuate Tamoxifen Resistance in Breast Cancer Cells. J. Cell Physiol. 2019, 234, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, S.; Chen, J.; Xie, Z. The Interplay between Integrins and Immune Cells as a Regulator in Cancer Immunology. Int. J. Mol. Sci. 2023, 24, 6170. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/novel-drug-approvals-fda (accessed on 14 January 2025).
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of Deaths from Cancer Caused by Metastases? Cancer Med 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.A.; Kluetz, P.G.; Pazdur, R. Metastasis-Free Survival—A New End Point in Prostate Cancer Trials. N. Engl. J. Med. 2018, 378, 2458–2460. [Google Scholar] [CrossRef]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-Metastatic and Anti-Invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef]
- Raudenská, M.; Petrláková, K.; Juriňáková, T.; Leischner Fialová, J.; Fojtů, M.; Jakubek, M.; Rösel, D.; Brábek, J.; Masařík, M. Engine Shutdown: Migrastatic Strategies and Prevention of Metastases. Trends Cancer 2023, 9, 293–308. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging Therapeutic Opportunities for Integrin Inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov, National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 14 January 2025).
- Delbaldo, C.; Raymond, E.; Vera, K.; Hammershaimb, L.; Kaucic, K.; Lozahic, S.; Marty, M.; Faivre, S. Phase I and Pharmacokinetic Study of Etaracizumab (AbegrinTM), a Humanized Monoclonal Antibody against Avβ3 Integrin Receptor, in Patients with Advanced Solid Tumors. Investig. New Drugs 2008, 26, 35–43. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Eickhoff, J.; Lee, F.T.; King, D.M.; Alberti, D.; Thomas, J.P.; Friedl, A.; Kolesar, J.; Marnocha, R.; Volkman, J.; et al. Phase I Trial of a Monoclonal Antibody Specific for Avβ3 Integrin (MEDI-522) in Patients with Advanced Malignancies, Including an Assessment of Effect on Tumor Perfusion. Clin. Cancer Res. 2005, 11, 7851–7860. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Maschauer, S.; Prante, O. PET Radiopharmaceuticals for Imaging Integrin Expression: Tracers in Clinical Studies and Recent Developments. Biomed. Res. Int. 2014, 2014, 871609. [Google Scholar] [CrossRef]
- NCT06094530; Clinical Trial: 18F-FAPI-RGD PET/CT in Various Tumor Types. Sichuan Provincial People’s Hospital: Chengdu, China, 2023.
- NCT05976607; Clinical Trial: Clinical Study of 18F -FAPI-RGD in Renal Tumor. Sichuan Provincial People’s Hospital: Chengdu, China, 2023.
- NCT06228482; Clinical Trial: Molecularly Targeted Radionuclide Therapy Via the Integrin Alphavbeta6. University of California: Davis, CA, USA, 2024.
- Chen, J.-R.; Zhao, J.-T.; Xie, Z.-Z. Integrin-Mediated Cancer Progression as a Specific Target in Clinical Therapy. Biomed. Pharmacother. 2022, 155, 113745. [Google Scholar] [CrossRef]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Cayrol, F.; Revuelta, M.V.; Debernardi, M.; Paulazo, A.; Phillip, J.M.; Zamponi, N.; Sterle, H.; Díaz Flaqué, M.C.; Magro, C.; Marullo, R.; et al. Inhibition of Integrin AVβ3 Signaling Improves the Antineoplastic Effect of Bexarotene in Cutaneous T-Cell Lymphoma. Mol. Cancer Ther. 2022, 21, 1485–1496. [Google Scholar] [CrossRef]
- Shekari, N.; Javadian, M.; Ghasemi, M.; Baradaran, B.; Darabi, M.; Kazemi, T. Synergistic Beneficial Effect of Docosahexaenoic Acid (DHA) and Docetaxel on the Expression Level of Matrix Metalloproteinase-2 (MMP-2) and MicroRNA-106b in Gastric Cancer. J. Gastrointest. Cancer 2020, 51, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bhatt, M.; Chowdhury, D.; Chaudhuri, D.; Haldar, S. Talin-Drug Interaction Reveals a Key Molecular Determinant for Biphasic Mechanical Effect: Studied under Single-Molecule Resolution. bioRxiv 2022. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Yamada, K.M.; Collins, J.W.; Cruz Walma, D.A.; Doyle, A.D.; Morales, S.G.; Lu, J.; Matsumoto, K.; Nazari, S.S.; Sekiguchi, R.; Shinsato, Y.; et al. Extracellular Matrix Dynamics in Cell Migration, Invasion and Tissue Morphogenesis. Int. J. Exp. Pathol. 2019, 100, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.D.; Isenberg, B.C.; Chua, S.G.; Wong, J.Y. Extracellular Matrix Type Modulates Cell Migration on Mechanical Gradients. Exp. Cell Res. 2017, 359, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Latella, G.; Gaudio, E.; Sferra, R.; Vetuschi, A. The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall. Front. Med. 2021, 8, 610189. [Google Scholar] [CrossRef]
- Kozyrina, A.N.; Piskova, T.; Di Russo, J. Mechanobiology of Epithelia from the Perspective of Extracellular Matrix Heterogeneity. Front. Bioeng. Biotechnol. 2020, 8, 596599. [Google Scholar] [CrossRef]
- Schwarzbauer, J. Basement Membrane: Putting up the Barriers. Curr. Biol. 1999, 9, R242–R244. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin Basement Membrane: The Foundation of Epidermal Integrity—BM Functions and Diverse Roles of Bridging Molecules Nidogen and Perlecan. Biomed. Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef]
- Hohenester, E.; Yurchenco, P.D. Laminins in Basement Membrane Assembly. Cell Adh. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef]
- Jurj, A.; Ionescu, C.; Berindan-Neagoe, I.; Braicu, C. The Extracellular Matrix Alteration, Implication in Modulation of Drug Resistance Mechanism: Friends or Foes? J. Exp. Clin. Cancer Res. 2022, 41, 276. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; Matrisian, L.M. Matrix Metalloproteinases: They’re Not Just for Matrix Anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Newby, A.C. Matrix Metalloproteinases Regulate Migration, Proliferation, and Death of Vascular Smooth Muscle Cells by Degrading Matrix and Non-Matrix Substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in Translation: From Molecule to Brain Physiology, Pathology, and Therapy. J. Neurochem. 2016, 139, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, C.; Christodoulou, N.; Hamilton, R.S.; Nahaboo, W.; Boomgaard, D.S.; Amadei, G.; Migeotte, I.; Zernicka-Goetz, M. Basement Membrane Remodelling Regulates Mouse Embryogenesis. Nature 2020, 582, 253–258. [Google Scholar] [CrossRef]
- Stamenkovic, I. Matrix Metalloproteinases in Tumor Invasion and Metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef]
- Poincioux, R.; Lizárraga, F.; Chavrier, P. Matrix Invasion by Tumour Cells: A Focus on MT1-MMP Trafficking to Invadopodia. J. Cell Sci. 2009, 122, 3015–3024. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 Decreases Cellular Migration, and Angiogenesis in In Vitro Models of Retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Jing, J.; Lee, J.; Schedin, P.; Gilbert, S.M.; Peden, A.A.; Junutula, J.R.; Prekeris, R. Rab40b Regulates Trafficking of MMP2 and MMP9 during Invadopodia Formation and Invasion of Breast Cancer Cells. J. Cell Sci. 2013, 126, 4647–4658. [Google Scholar] [CrossRef]
- Toth, M.; Chvyrkova, I.; Bernardo, M.M.; Hernandez-Barrantes, S.; Fridman, R. Pro-MMP-9 Activation by the MT1-MMP/MMP-2 Axis and MMP-3: Role of TIMP-2 and Plasma Membranes. Biochem. Biophys. Res. Commun. 2003, 308, 386–395. [Google Scholar] [CrossRef]
- Li, Z.; Takino, T.; Endo, Y.; Sato, H. Activation of MMP-9 by Membrane Type-1 MMP/MMP-2 Axis Stimulates Tumor Metastasis. Cancer Sci. 2017, 108, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family with Structural and Functional Diversity. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Murphy, G. Tailoring TIMPs for Selective Metalloproteinase Inhibition. In The Cancer Degradome: Proteases and Cancer Biology; Springer: New York, NY, USA, 2008; pp. 787–810. ISBN 9780387690568. [Google Scholar]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile Extracellular Regulators in Cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Peeney, D.; Jensen, S.M.; Castro, N.P.; Kumar, S.; Noonan, S.; Handler, C.; Kuznetsov, A.; Shih, J.; Tran, A.D.; Salomon, D.S.; et al. TIMP-2 Suppresses Tumor Growth and Metastasis in Murine Model of Triple-Negative Breast Cancer. Carcinogenesis 2020, 41, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Fridman, R.; Toth, M.; Chvyrkova, I.; Meroueh, S.O.; Mobashery, S. Cell Surface Association of Matrix Metalloproteinase-9 (Gelatinase B). Cancer Metastasis Rev. 2003, 22, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hey, S.; Linder, S. Matrix Metalloproteinases at a Glance. J. Cell Sci. 2024, 137, jcs261898. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baster, Z.; Russell, L.; Rajfur, Z. A Review of Talin- and Integrin-Dependent Molecular Mechanisms in Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2025, 26, 1798. https://doi.org/10.3390/ijms26051798
Baster Z, Russell L, Rajfur Z. A Review of Talin- and Integrin-Dependent Molecular Mechanisms in Cancer Invasion and Metastasis. International Journal of Molecular Sciences. 2025; 26(5):1798. https://doi.org/10.3390/ijms26051798
Chicago/Turabian StyleBaster, Zbigniew, Lindsay Russell, and Zenon Rajfur. 2025. "A Review of Talin- and Integrin-Dependent Molecular Mechanisms in Cancer Invasion and Metastasis" International Journal of Molecular Sciences 26, no. 5: 1798. https://doi.org/10.3390/ijms26051798
APA StyleBaster, Z., Russell, L., & Rajfur, Z. (2025). A Review of Talin- and Integrin-Dependent Molecular Mechanisms in Cancer Invasion and Metastasis. International Journal of Molecular Sciences, 26(5), 1798. https://doi.org/10.3390/ijms26051798