ADRB2 Polymorphisms (rs1042713 and rs1042714) and Blood Pressure Response to the Cold Pressor Test in Combat Athletes and Non-Athletes
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
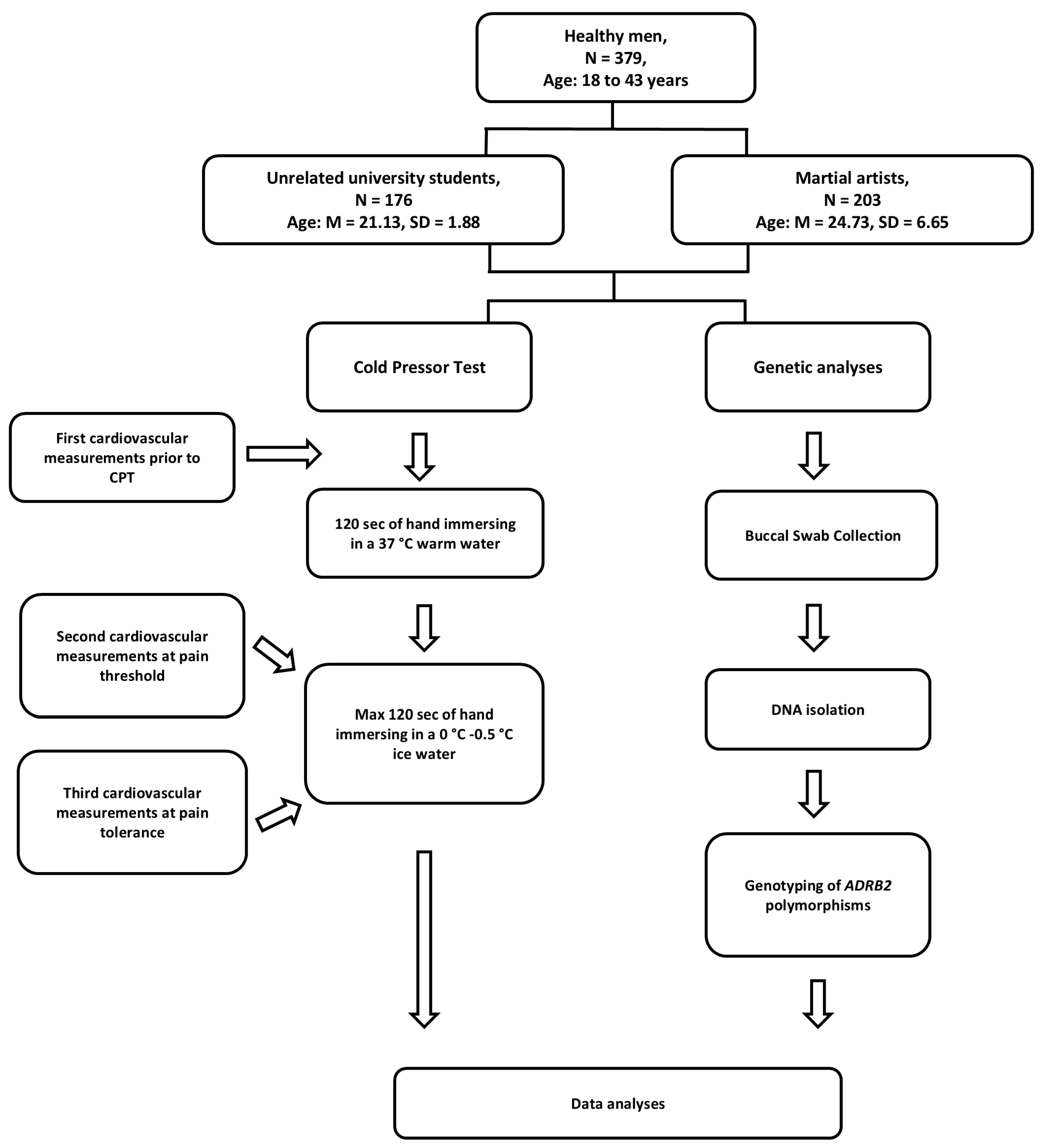
4.2. Participants
4.3. Procedure and Measurements
4.4. Genotyping
4.5. Statistical Analyses
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matuskova, L.; Czippelova, B.; Turianikova, Z.; Svec, D.; Kolkova, Z.; Lasabova, Z.; Javorka, M. Beta-adrenergic receptors gene polymorphisms are associated with cardiac contractility and blood pressure variability. Physiol. Res. 2021, 70 (Suppl. S3), S327–S337. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.; Rossi, F.; Giacchè, M.; Perani, C.; Rivadossi, F.; Muiesan, M.L.; Salvetti, M.; Beschi, M.; Rizzoni, D.; Agabiti-Rosei, E. Beta(2)-adrenergic receptor gene polymorphism, age, and cardiovascular phenotypes. Hypertension 2023, 41, 361–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Littlejohn, M.D.; Taylor, R.T.; Allison, L.; Miller. A.L. Kennedy, M.A. Determination of b2-Adrenergic Receptor (ADRB2) Haplotypes by a Multiplexed Polymerase Chain Reaction Assay. Hum. Mutat. 2002, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Ahles, A.; Engelhardt, S. Polymorphic variants of adrenoceptors: Pharmacology, physiology, and role in disease. Pharmacol. Rev. 2014, 66, 598–637. [Google Scholar] [CrossRef]
- van Duijvenboden, S.; Ramírez, J.; Young, W.J.; Olczak, K.J.; Ahmed, F.; Alhammadi, M.J.; International Consortium of Blood Pressure; Bell, C.G.; Morris, A.P.; Munroe, P.B. Integration of genetic fine-mapping and multi-omics data reveals candidate effector genes for hypertension. Am. J. Hum. Genet. 2023, 110, 1718–1734. [Google Scholar] [CrossRef]
- Pouwels, S.; Van Genderen, M.; Kreeftenberg, H.G.; Ribeiro, R.; Parmar, C.; Topal, B.; Celik, A.; Ugale, S. Utility of the cold pressor test to predict future cardiovascular events. Expert Rev. Cardiovasc. Ther. 2019, 4, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Keller-Ross, M.L.; Cunningham, H.A.; Carter, J.R. Impact of age and sex on neural cardiovascular responsiveness to cold pressor test in humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Leźnicka, K.; Pawlak, M.; Maciejewska-Skrendo, A.; Buczny, J.; Wojtkowska, A.; Pawlus, G.; Mahoy-Mokrzyńska, A.; Jażdzeska, A. Is Physical Activity an Effective Factor for Modulating Pressure Pain Threshold and Pain Tolerance after Cardiovascular Incidents? Int. J. Environ. Res. Public Health 2022, 19, 11276. [Google Scholar] [CrossRef] [PubMed]
- Leźnicka, K.; Starkowska, A.; Tomczak, M.; Cięszczyk, P.; Białecka, M.; Ligocka, M.; Żmijewski, P.; Pawlak, M. Temperament as a modulating factor of pain sensitivity in combat sport athletes. Physiol. Behav. 2017, 180, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, G.; Boes, C.J.; Low, P.A.; Coon, E.A. The expanding role of the cold pressor test: A brief history. Clin. Auton. Res. 2021, 31, 153–155. [Google Scholar] [CrossRef]
- Drost, L.; Finke, J.B.; Bachmann, P.; Schächinger, H. Cold pressor stress effects on cardiac repolarization. Stress 2024, 27, 2352626. [Google Scholar] [CrossRef] [PubMed]
- Ege, F.; Aslanyavrusu, M.; Uzunok, B.; Özdemir, O. Effects of the Cold Pressor Test on Popliteal Vein Diameter, Flow Velocity, and Blood Flow in the Lower Limb in 60 Healthy Individuals. Med. Sci. Monit. 2024, 30, e944560. [Google Scholar] [CrossRef]
- Bachmann, P.; Zhang, X.; Larra, M.F.; Rebeck, D.; Schönbein, K.; Koch, K.P. Validation of an automated bilateral feet cold pressor test. Int. J. Psychophysiol. 2017, 124, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.O.; Ajayi, R.E. Effect of sympathetic autonomic stress from the cold pressor test on left ventricular function in young healthy adults. Physiol. Rep. 2019, 7, e13985. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Gómez, J.; Blanco, M.; Rodriguez, I. The cold pressor test: Pharmacological and therapeutic aspects. Am. J. Ther. 1997, 4, 34–38. [Google Scholar] [CrossRef]
- Allen, A.P.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014, 38, 94–124. [Google Scholar] [CrossRef]
- Leźnicka, K.; Pawlak, M.; Białecka, M.; Safranow, K.; Cięszczyk, P. Pain perception and cardiovascular system response among athletes playing contact sports. Res. Sports Med. 2017, 25, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Dworkin, S.F.; Haug, J.; Gehrig, J. Human pain resposivity in a tonic pain model: Physiological determinants. Pain 1989, 37, 143–160. [Google Scholar] [CrossRef]
- Kolifarhood, G.; Daneshpour, M.; Hadaegh, F.; Sabour, S.; Saadati, H.M.; Haghdoust, A.; Akbarzadeh, M.; Sedaghati-Khayat, B.; Khosravi, N. Heritability of blood pressure traits in diverse populations: A systematic review and meta-analysis. J. Hum. Hypertens. 2019, 33, 775–785. [Google Scholar] [CrossRef]
- Reynes, E.; Loran, J. Effect of traditional judo training on aggressiveness among young boys. Percept. Mot. Ski. 2002, 94, 21–25. [Google Scholar] [CrossRef]
- Sohrabi, F.; Atashak, S.; Aliloo, M.M. Psychological profile of athletes in contact and noncontact sports. Middle-East J. Sci. Res. 2011, 9, 638–644. [Google Scholar]
- Masuo, K.; Katsuya, T.; Fu, Y.; Rakugi, H.; Ogihara, T.; Tuck, M.L. Beta2- and beta3-adrenergic receptor polymorphisms are related to the onset of weight gain and blood pressure elevation over 5 years. Circulation 2005, 111, 3429–3434. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Turner, S.T.; Joyner, M.J.; Eisenach, J.H.; Johnson, B.D. The Arg16Gly polymorphism of the β2-adrenergic receptor and the natriuretic response to rapid saline infusion in humans. J. Psychiol. 2006, 574, 947–954. [Google Scholar] [CrossRef]
- Kelsey, R.M.; Alpert, B.S.; Dahmer, M.K.; Krushkal, J.; Quasney, M.W. Beta-adrenergic receptor gene polymorphisms and cardiovascular reactivity to stress in Black adolescents and young adults. Psychophysiology 2010, 47, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.H.; McGuire, A.M.; Schwingler, R.M.; Turner, S.T.; Joyner, M.J. The Arg16/Gly beta2-adrenergic receptor polymorphism is associated with altered cardiovascular responses to isometric exercise. Physiol. Genom. 2004, 16, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Atala, M.M.; Goulart, A.; Guerra, G.M.; Mostarda, C.; Rodrigues, B.; Mello, P.R.; Casarine, D.E.; Irigoyen, M.C.; Pereira, A.C.; Consolim-Kolombo, F.M. Arg16Gly and Gln27Glu β2 adrenergic polymorphisms influence cardiac autonomic modulation and baroreflex sensitivity in healthy young Brazilians. Am. J. Transl. Res. 2015, 7, 153–161. [Google Scholar]
- Busjahn, A.; Li, G.H.; Faulhaber, H.D.; Rosenthal, M.; Becker, A.; Jeschke, E.; Schuster, H.; Timmermann, B.; Hoehe, P.; Luft, F.C. β-2 Adrenergic Receptor Gene Variations, Blood Pressure, and Heart Size in Normal Twins. Hypertension 2000, 35, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Turki, J.; Innis, M.; Liggett, S.B. Amino-terminal polymorphisms of the human b2-adrenergic receptor impart distinct agonistpromoted regulatory properties. Biochemistry 1994, 33, 9414–9419. [Google Scholar] [CrossRef]
- Green, S.A.; Turki, J.; Bejarano, P.; Hall, I.P.; Liggett, S.B. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 1995, 13, 25–33. [Google Scholar] [CrossRef]
- Koryakina, Y.; Jones, S.M.; Cornett, L.E.; Seely, K.; Brents, L.; Prather, P.L.; Kofman, A.; Kurten, R.C. Effects of the β-agonist, isoprenaline, on the down-regulation, functional responsiveness and trafficking of β2-adrenergic receptors with N-terminal polymorphisms. Cell Biol. Int. 2012, 36, 1171–1183. [Google Scholar] [CrossRef]
- Gratze, G.; Fortin, J.; Labugger, R.; Binder, A.; Kotanko, P.; Timmermann, B.; Luft, F.C.; Hoehe, M.R.; Skrabal, F. Beta-2 adrenergic receptor variants affect resting blood pressure and agonist-induced vasodilation in young adult Caucasians. Hypertension 1999, 33, 1425–1430. [Google Scholar] [CrossRef]
- Dishy, V.; Sofowora, G.G.; Xie, H.G.; Kim, R.B.; Byrne, D.W.; Stein, C.M.; Wood, A.J. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N. Engl. J. Med. 2001, 345, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Liggett, S.B. Molecular and genetic basis of beta2-adrenergic receptor function. J. Allergy Clin. Immunol. 1999, 104, 42–46. [Google Scholar] [CrossRef]
- Keogh, E.; Bond, F.W.; Hanmer, R.; Tilston, J. Comparing acceptance- and control-based coping instructions on the cold-pressor pain experiences of healthy men and women. Eur. J. Pain 2005, 9, 591–598. [Google Scholar] [CrossRef]
- Jamovi Version 2.5 Computer Software. Available online: https://www.jamovi.org (accessed on 15 June 2024).
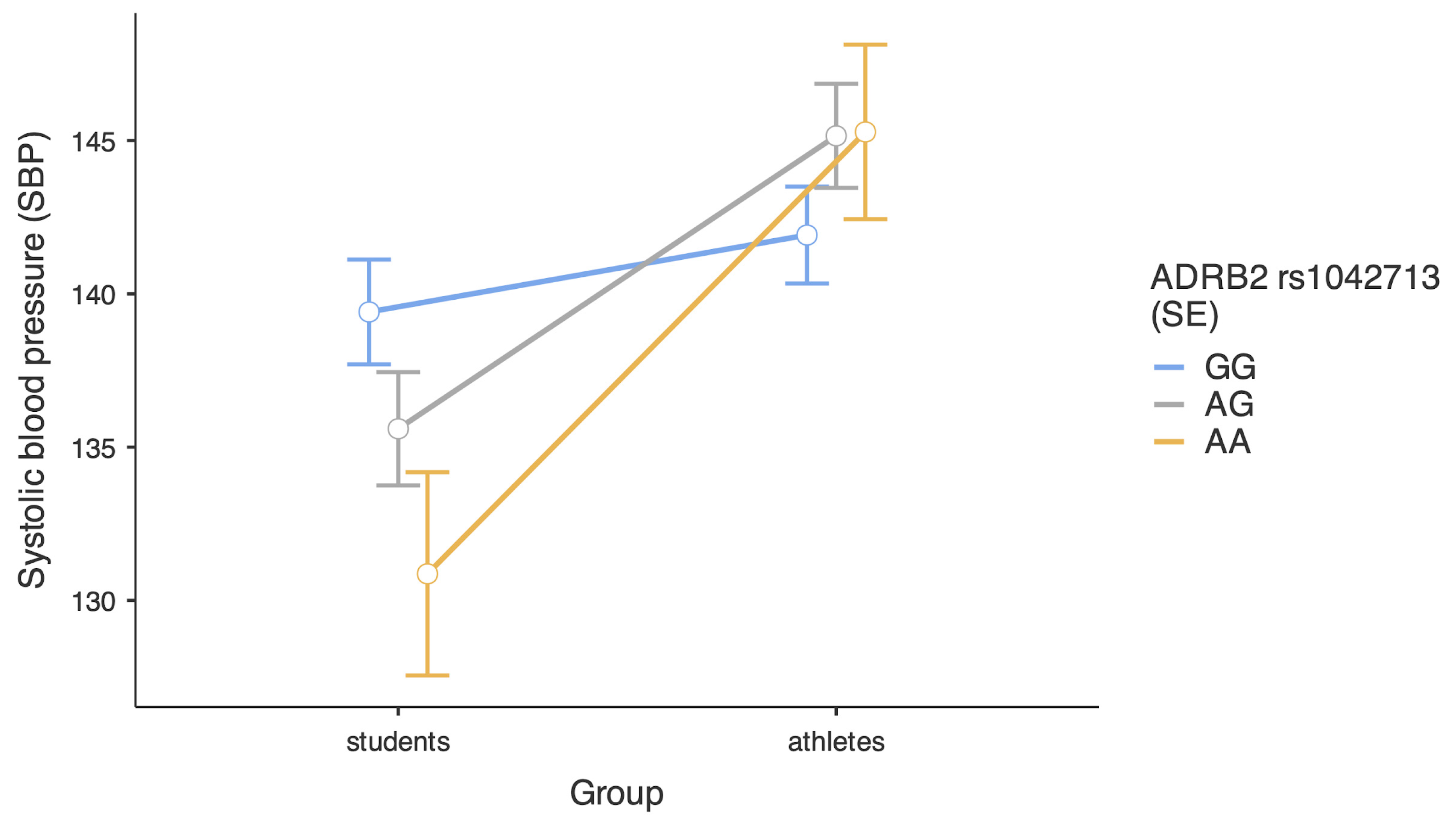
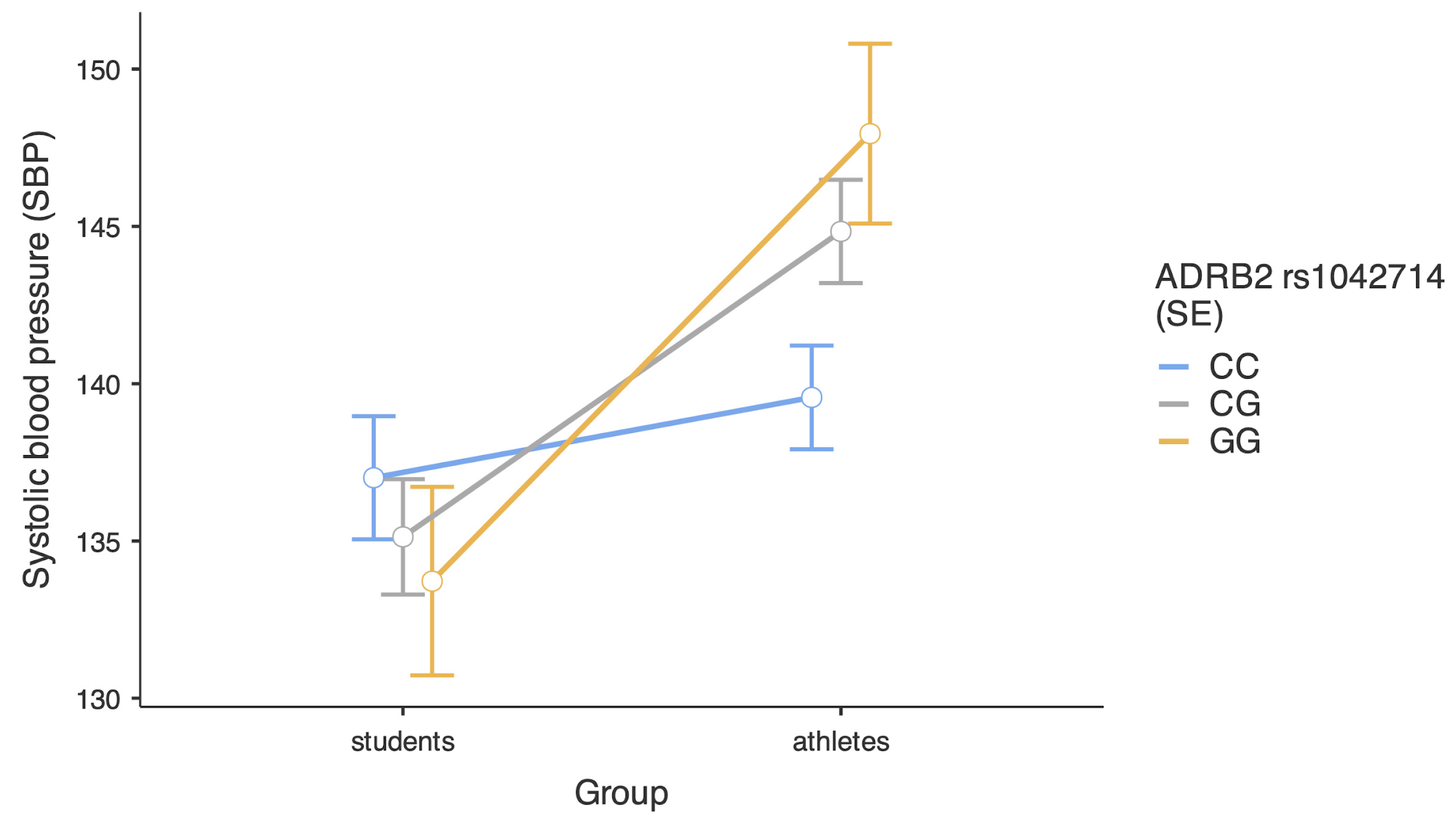
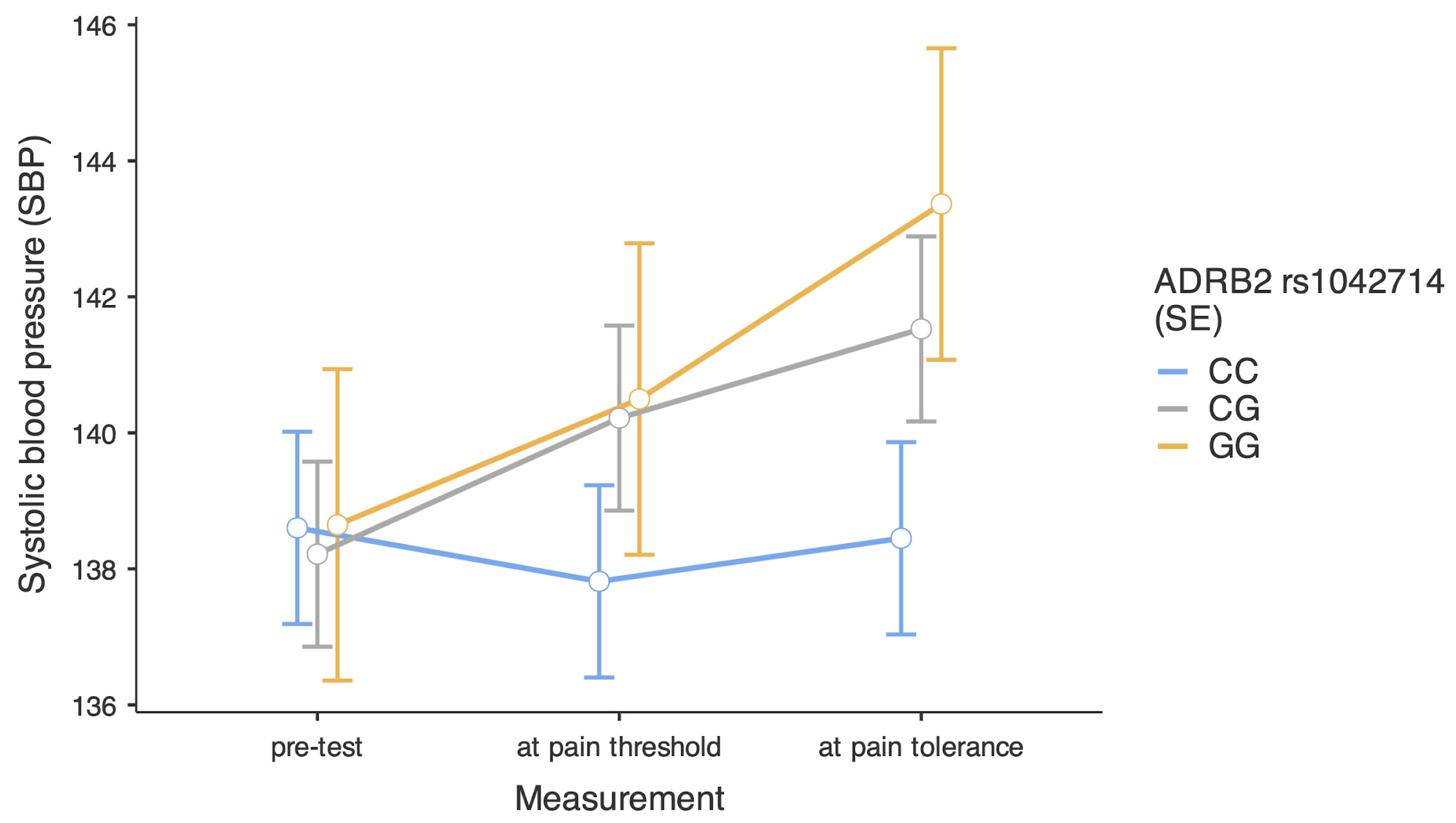
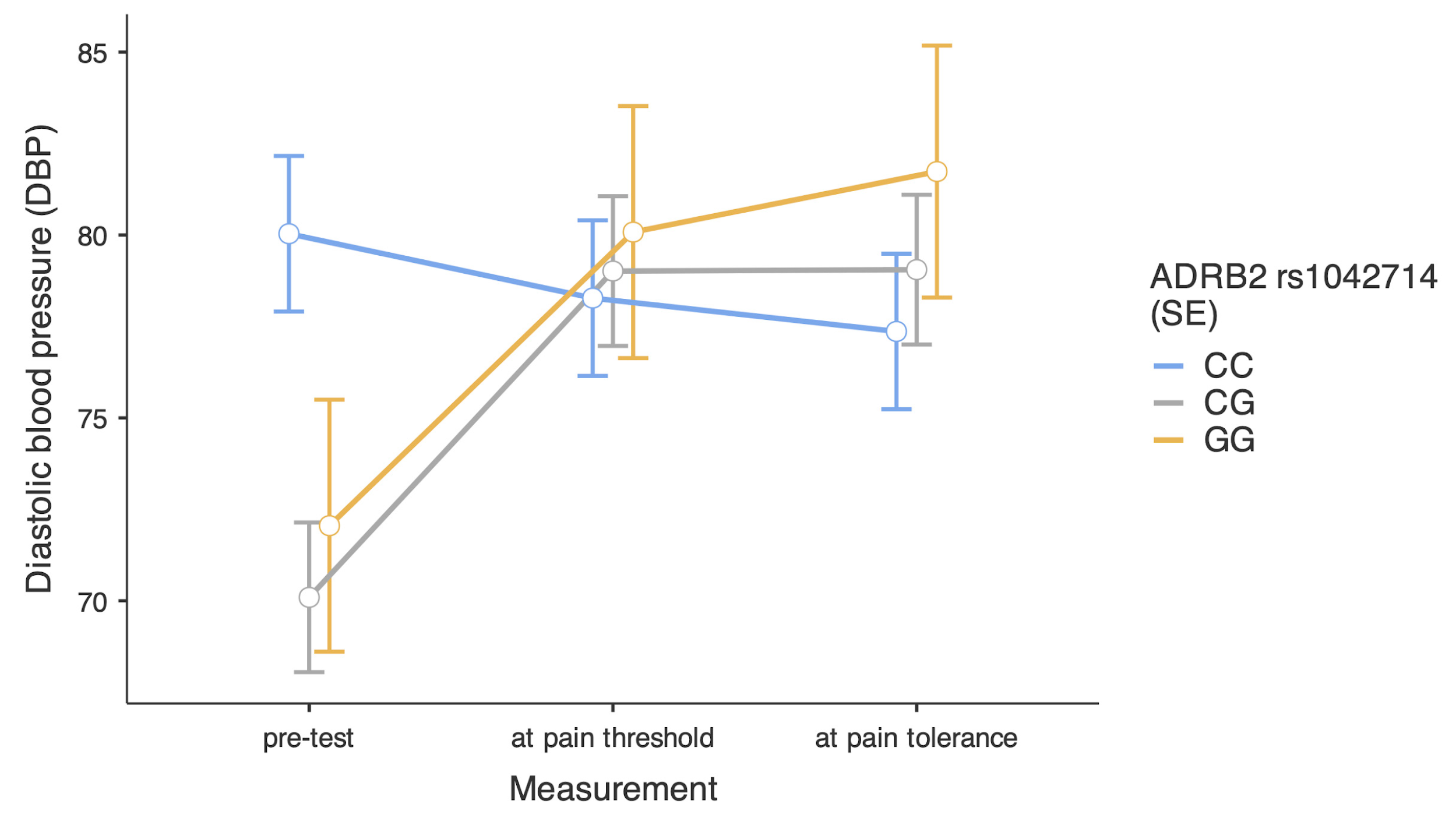
| Variables | Combat Athletes | Comparison Group | U Statistics | p | Effect Size | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | Cohens’s d | |||
| Age (years) | 24.73 | 6.65 | 21.13 | 1.88 | 13,884.0 | 0.001 | 0.21 |
| Height (m) | 1.79 | 0.07 | 1.82 | 0.08 | 13,713.5 | 0.001 | 0.22 |
| Body Mass (kg) | 78.30 | 12.96 | 77.86 | 9.66 | 17,342.5 | 0.816 | 0.01 |
| BMI (kg/m2) | 24.47 | 3.28 | 23.55 | 2.16 | 14,808.0 | 0.008 | 0.16 |
| CPT 1 | 25.12 | 27.41 | 26.62 | 23.96 | 16,612.5 | 0.231 | 0.07 |
| CPT 2 | 98.89 | 31.63 | 86.22 | 34.81 | 13,995.0 | 0.001 | 0.22 |
| Names | Effect | b | SE | Lower | Upper | df | t | p |
|---|---|---|---|---|---|---|---|---|
| (Intercept) | (Intercept) | 139.70 | 0.87 | 137.99 | 141.42 | 369 | 159.94 | 0.001 |
| Group | athletes–students | 8.83 | 1.75 | 5.40 | 12.26 | 369 | 5.05 | 0.001 |
| Measurement | at pain threshold–pre-test | 1.02 | 0.71 | −0.38 | 2.42 | 738 | 1.43 | 0.153 |
| at pain tolerance–pre-test | 2.63 | 0.71 | 1.22 | 4.03 | 738 | 3.68 | 0.001 | |
| rs1042713 | AG–GG | −0.29 | 1.75 | −3.72 | 3.15 | 369 | −0.16 | 0.870 |
| AA–GG | −2.59 | 2.71 | −7.91 | 2.73 | 369 | −0.96 | 0.340 | |
| rs1042714 | CG–CC | 1.70 | 1.90 | −2.03 | 5.43 | 369 | 0.89 | 0.372 |
| GG–CC | 2.55 | 2.65 | −2.66 | 7.75 | 369 | 0.96 | 0.338 | |
| Group × Measurement | (athletes–students) × (at pain threshold–pre-test) | 1.71 | 1.43 | −1.09 | 4.51 | 738 | 1.20 | 0.232 |
| (athletes–students) × (at pain tolerance–pre-test) | 2.00 | 1.43 | −0.81 | 4.80 | 738 | 1.40 | 0.163 | |
| Group × rs1042713 | (athletes–students)×(AG–GG) | 7.05 | 3.50 | 0.17 | 13.92 | 369 | 2.01 | 0.045 |
| (athletes–students)×(AA–GG) | 11.91 | 5.42 | 1.27 | 22.54 | 369 | 2.20 | 0.029 | |
| Measurement × rs1042713 | (at pain threshold–pre-test) × (AG–GG) | 0.19 | 1.43 | −2.62 | 3.00 | 738 | 0.14 | 0.892 |
| (at pain tolerance–pre-test) × (AG–GG) | 1.14 | 1.43 | −1.67 | 3.95 | 738 | 0.80 | 0.427 | |
| (at pain threshold–pre-test) × (AA–GG) | 0.20 | 2.22 | −4.15 | 4.55 | 738 | 0.09 | 0.927 | |
| (at pain tolerance–pre-test) × (AA–GG) | 0.92 | 2.22 | −3.43 | 5.26 | 738 | 0.41 | 0.680 | |
| Group × rs1042714 | (athletes–students) × (CG–CC) | 7.15 | 3.80 | −0.30 | 14.61 | 369 | 1.88 | 0.061 |
| (athletes–students) × (GG–CC) | 11.67 | 5.31 | 1.26 | 22.08 | 369 | 2.20 | 0.029 | |
| Measurement × rs1042714 | (at pain threshold–pre-test) × (CG–CC) | 2.79 | 1.55 | −0.26 | 5.84 | 738 | 1.80 | 0.073 |
| (at pain tolerance–pre-test) × (CG–CC) | 3.47 | 1.55 | 0.42 | 6.51 | 738 | 2.23 | 0.026 | |
| (at pain threshold–pre-test) × (GG–CC) | 2.64 | 2.17 | −1.62 | 6.90 | 738 | 1.22 | 0.225 | |
| (at pain tolerance–pre-test) × (GG–CC) | 4.87 | 2.17 | 0.61 | 9.13 | 738 | 2.25 | 0.025 | |
| Group × Measurement × rs1042713 | (athletes–students) × (at pain threshold–pre-test) × (AG–GG) | 1.84 | 2.86 | −3.78 | 7.46 | 738 | 0.64 | 0.520 |
| (athletes–students) × (at pain tolerance–pre-test) × (AG–GG) | −0.12 | 2.86 | −5.74 | 5.50 | 738 | −0.04 | 0.968 | |
| (athletes–students) × (at pain threshold–pre-test) × (AA–GG) | 2.90 | 4.43 | −5.80 | 11.59 | 738 | 0.65 | 0.514 | |
| (athletes–students) × (at pain tolerance–pre-test) × (AA–GG) | 0.41 | 4.43 | −8.29 | 9.10 | 738 | 0.09 | 0.927 | |
| Group × Measurement × rs1042714 | (athletes–students) × (at pain threshold–pre-test) × (CG–CC) | 2.12 | 3.11 | −3.97 | 8.22 | 738 | 0.68 | 0.495 |
| (athletes–students) × (at pain tolerance–pre-test) × (CG–CC) | −2.36 | 3.11 | −8.45 | 3.74 | 738 | −0.76 | 0.448 | |
| (athletes–students) × (at pain threshold–pre-test) × (GG–CC) | 1.90 | 4.34 | −6.62 | 10.41 | 738 | 0.44 | 0.662 | |
| (athletes–students) × (at pain tolerance–pre-test) × (GG–CC) | −4.40 | 4.34 | −12.91 | 4.12 | 738 | −1.01 | 0.311 |
| Names | Effect | b | SE | Lower | Upper | df | t | p |
|---|---|---|---|---|---|---|---|---|
| (Intercept) | (Intercept) | 77.52 | 0.98 | 75.60 | 79.44 | 369 | 79.30 | 0.001 |
| Group | athletes–students | 4.17 | 1.96 | 0.33 | 8.00 | 369 | 2.13 | 0.034 |
| Measurement | at pain threshold–pre-test | 5.06 | 1.86 | 1.41 | 8.72 | 738 | 2.72 | 0.007 |
| at pain tolerance–pre-test | 5.32 | 1.86 | 1.67 | 8.98 | 738 | 2.86 | 0.004 | |
| rs1042713 | AG–GG | 0.61 | 1.96 | −3.24 | 4.45 | 369 | 0.31 | 0.758 |
| AA–GG | −3.81 | 3.03 | −9.76 | 2.14 | 369 | −1.26 | 0.210 | |
| rs1042714 | CG–CC | −2.50 | 2.13 | −6.68 | 1.67 | 369 | −1.18 | 0.240 |
| GG–CC | −0.60 | 2.97 | −6.43 | 5.23 | 369 | −0.20 | 0.839 | |
| Group × Measurement | (athletes–students) × (at pain threshold–pre-test) | −1.71 | 3.73 | −9.02 | 5.60 | 738 | −0.46 | 0.646 |
| (athletes–students) × (at pain tolerance–pre-test) | −0.30 | 3.73 | −7.61 | 7.01 | 738 | −0.08 | 0.935 | |
| Group × rs1042713 | (athletes–students) × (AG–GG) | 4.58 | 3.92 | −3.11 | 12.27 | 369 | 1.17 | 0.244 |
| (athletes–students) × (AA–GG) | 1.97 | 6.07 | −9.93 | 13.88 | 369 | 0.33 | 0.745 | |
| Measurement × rs1042713 | (at pain threshold–pre-test) × (AG–GG) | −1.69 | 3.73 | −9.02 | 5.63 | 738 | −0.45 | 0.650 |
| (at pain tolerance–pre-test) × (AG–GG) | −0.71 | 3.73 | −8.04 | 6.62 | 738 | −0.19 | 0.850 | |
| (at pain threshold–pre-test) × (AA–GG) | 8.46 | 5.78 | −2.88 | 19.80 | 738 | 1.46 | 0.144 | |
| (at pain tolerance–pre-test) × (AA–GG) | 10.42 | 5.78 | −0.92 | 21.75 | 738 | 1.80 | 0.072 | |
| Group × rs1042714 | (athletes–students) × (CG–CC) | 0.56 | 4.25 | −7.78 | 8.91 | 369 | 0.13 | 0.895 |
| (athletes–students) × (GG–CC) | 1.54 | 5.94 | −10.12 | 13.20 | 369 | 0.26 | 0.796 | |
| Measurement × rs1042714 | (at pain threshold–pre-test) × (CG–CC) | 10.68 | 4.05 | 2.73 | 18.63 | 738 | 2.64 | 0.009 |
| (at pain tolerance–pre-test) × (CG–CC) | 11.63 | 4.05 | 3.68 | 19.58 | 738 | 2.87 | 0.004 | |
| (at pain threshold–pre-test) × (GG–CC) | 9.79 | 5.66 | −1.32 | 20.89 | 738 | 1.73 | 0.084 | |
| (at pain tolerance–pre-test) × (GG–CC) | 12.35 | 5.66 | 1.25 | 23.46 | 738 | 2.18 | 0.029 | |
| Group × Measurement × rs1042713 | (athletes–students) × (at pain threshold–pre-test) × (AG–GG) | −3.35 | 7.47 | −18.01 | 11.30 | 738 | −0.45 | 0.654 |
| (athletes–students) × (at pain tolerance–pre-test) × (AG–GG) | 0.77 | 7.47 | −13.88 | 15.43 | 738 | 0.10 | 0.917 | |
| (athletes–students) × (at pain threshold–pre-test) × (AA–GG) | 9.03 | 11.56 | −13.65 | 31.71 | 738 | 0.78 | 0.435 | |
| (athletes–students) × (at pain tolerance–pre-test) × (AA–GG) | 9.24 | 11.56 | −13.44 | 31.92 | 738 | 0.80 | 0.424 | |
| Group × Measurement × rs1042714 | (athletes–students) × (at pain threshold–pre-test) × (CG–CC) | 7.08 | 8.10 | −8.82 | 22.97 | 738 | 0.87 | 0.383 |
| (athletes–students) × (at pain tolerance–pre-test) × (CG–CC) | 5.43 | 8.10 | −10.46 | 21.33 | 738 | 0.67 | 0.503 | |
| (athletes–students) × (at pain threshold–pre-test) × (GG–CC) | 5.35 | 11.32 | −16.85 | 27.56 | 738 | 0.47 | 0.636 | |
| (athletes–students) × (at pain tolerance–pre-test) × (GG–CC) | 5.20 | 11.32 | −17.00 | 27.41 | 738 | 0.46 | 0.646 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawczuk, M.; Gąsiorowska, A.; Maciejewska-Skrendo, A.; Chudecka, M.; Kotarska, K.; Proia, P.; Marszałek, J.; Małkowska, P.; Leźnicka, K. ADRB2 Polymorphisms (rs1042713 and rs1042714) and Blood Pressure Response to the Cold Pressor Test in Combat Athletes and Non-Athletes. Int. J. Mol. Sci. 2025, 26, 1765. https://doi.org/10.3390/ijms26041765
Sawczuk M, Gąsiorowska A, Maciejewska-Skrendo A, Chudecka M, Kotarska K, Proia P, Marszałek J, Małkowska P, Leźnicka K. ADRB2 Polymorphisms (rs1042713 and rs1042714) and Blood Pressure Response to the Cold Pressor Test in Combat Athletes and Non-Athletes. International Journal of Molecular Sciences. 2025; 26(4):1765. https://doi.org/10.3390/ijms26041765
Chicago/Turabian StyleSawczuk, Marek, Agata Gąsiorowska, Agnieszka Maciejewska-Skrendo, Monika Chudecka, Katarzyna Kotarska, Patrizia Proia, Jolanta Marszałek, Paulina Małkowska, and Katarzyna Leźnicka. 2025. "ADRB2 Polymorphisms (rs1042713 and rs1042714) and Blood Pressure Response to the Cold Pressor Test in Combat Athletes and Non-Athletes" International Journal of Molecular Sciences 26, no. 4: 1765. https://doi.org/10.3390/ijms26041765
APA StyleSawczuk, M., Gąsiorowska, A., Maciejewska-Skrendo, A., Chudecka, M., Kotarska, K., Proia, P., Marszałek, J., Małkowska, P., & Leźnicka, K. (2025). ADRB2 Polymorphisms (rs1042713 and rs1042714) and Blood Pressure Response to the Cold Pressor Test in Combat Athletes and Non-Athletes. International Journal of Molecular Sciences, 26(4), 1765. https://doi.org/10.3390/ijms26041765







