Extrapolation of PBBs Environmental Transformation Mechanisms and Toxicity Risks of Byproducts
Abstract
1. Introduction
2. Results and Discussion
2.1. Extrapolation of PBBs Environmental Transformation Pathways and the Summary of Their Transformation Products
2.1.1. Extrapolation of PBBs Photolysis Pathways
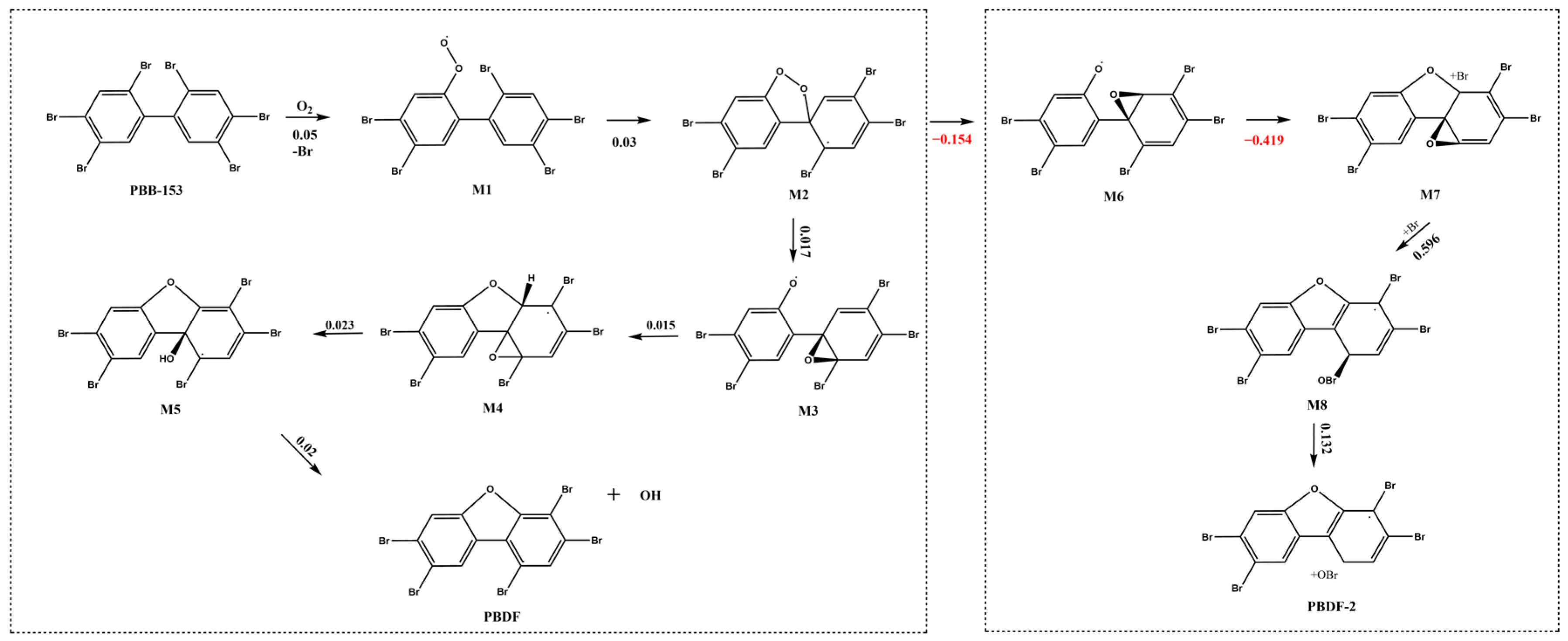
2.1.2. Extrapolation of Oxidative Conversion Pathways for the PBBs Combustion
2.1.3. Analysis of PBBs Microbial Reductive Debromination Conversion Pathways
2.1.4. Analysis of the PBBs Biometabolic Transformation Pathway
2.1.5. Summary of Transformation Products of PBBs and Their Substitutes
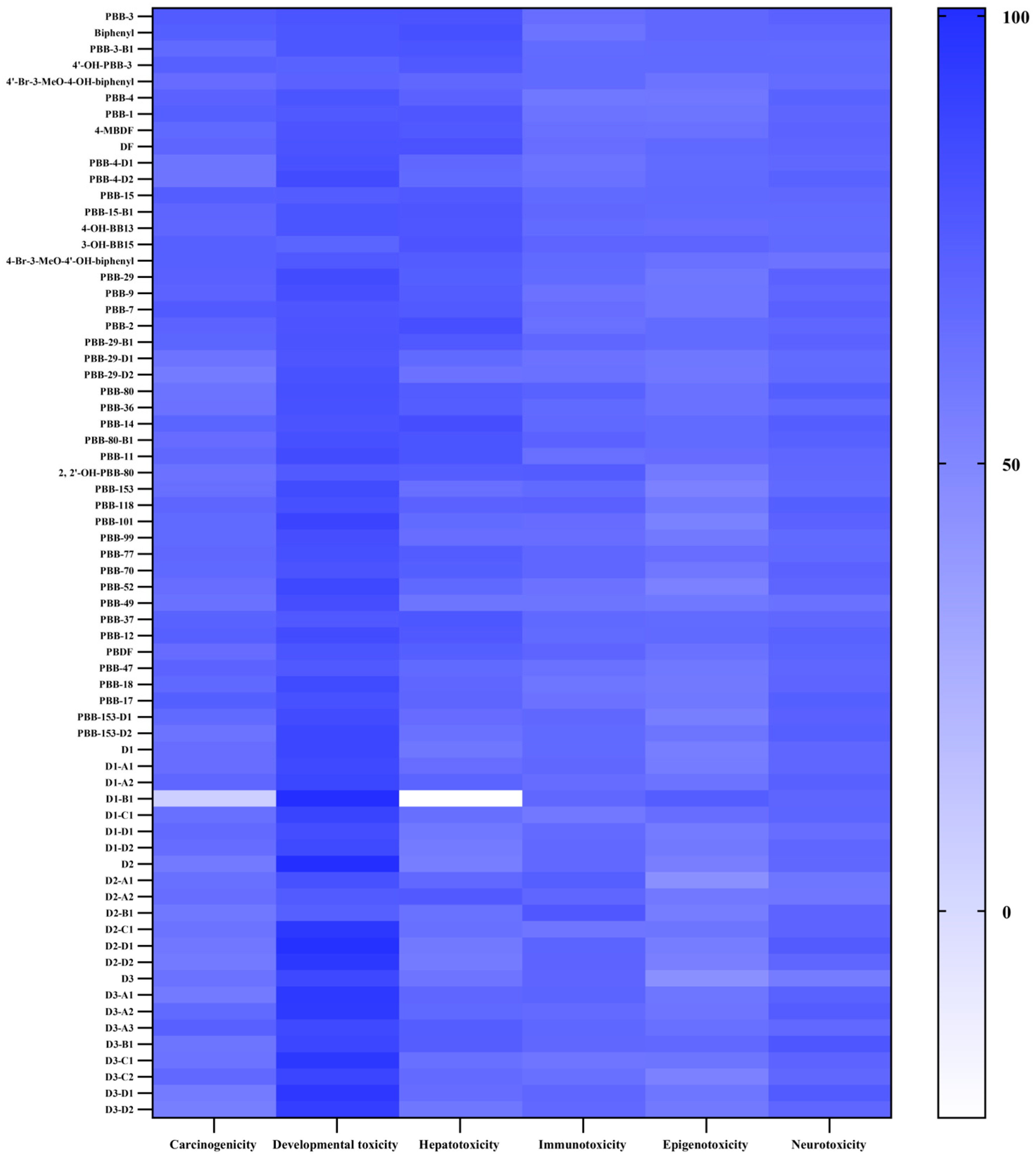
2.2. Biotoxicity Evaluation of PBBs and Their Substitutes’ Transformation Products Based on Toxicokinetics
2.2.1. Evaluation of Mutagenicity and Rodent Carcinogenicity of PBBs and Their Substitutes’ Transformation Products
2.2.2. Biotoxicity Evaluation of PBBs and Their Substitutes’ Transformation Products
2.2.3. Evaluation of Biological Tissue Toxicity and Potential Developmental Toxicity of PBBs and Their Substitutes’ Transformation Products
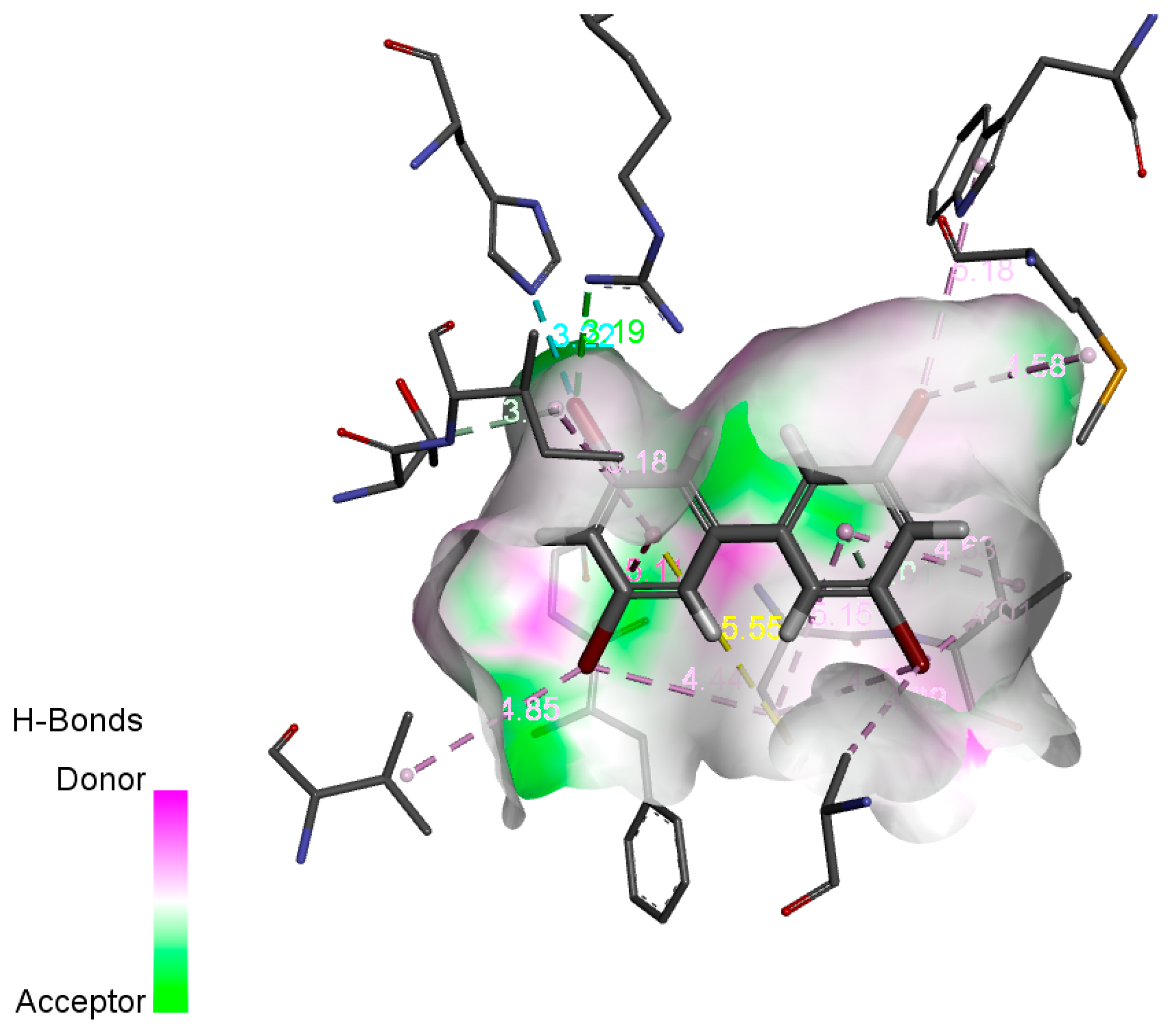
2.3. Potential Human Toxicity Risk Assessment of PBBs and Their Substitutes Based on 3D-QSAR Models
2.3.1. Construction and Evaluation of 3D-QSAR Models of Human Toxicity of PBBs and Their Substitutes
2.3.2. Potential Carcinogenic Risks of PBBs and Their Substitutes’ Transformation Products
2.3.3. Potential Developmental Toxicity Risks of PBBs and Their Substitutes’ Transformation Products
2.3.4. Potential Hepatotoxicity Risks of PBBs and Their Substitutes’ Transformation Products
2.3.5. Potential Epigenotoxicity Risks of PBBs and Their Substitutes’ Transformation Products
2.3.6. Potential Human Neurotoxic Risks of PBBs and Their Substitutes’ Transformation Products
2.3.7. Potential Immunotoxicity Risks of PBBs and Their Substitutes’ Transformation Products
3. Materials and Methods
3.1. Calculation of Energy Barriers for PBBs Transformation Pathways—Gaussian Calculation
3.2. Animal Toxicity Evaluation of PBBs and Their Transformation Products—Toxicokinetic
3.3. Prediction of PBBs and Their Transformation Products’ Human Toxicities—Molecular Docking and 3D-QSAR Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBBs | Polybrominated biphenyls |
| Br | Bromine |
| PBDF | Polybrominated dibenzofuran |
| BP | Biphenyl |
| TOPKAT | Toxicokinetics |
References
- Robert, B.H.; Metrecia, L.T.; Alicia, K.S.; Sarah, C.; Karen, C.; Melanie, P.; Hillary, B.; Dana, B.B.; Elizabeth, M.M.; Michele, M. Elimination of PBB-153; findings from a cohort of Michigan adults. Environ. Res. 2023, 220, 115146. [Google Scholar]
- Greeson, K.W.; Fowler, K.L.; Estave, P.M.; Thompson, S.K.; Wagner, C.; Edenfield, R.C.; Symosko, K.M.; Steves, A.N.; Marder, E.M.; Terrell, M.L.; et al. Detrimental effects of flame retardant, PBB153, exposure on sperm and future generations. Sci. Rep. 2020, 10, 8567. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Darrow, L.A.; Barr, D.B.; Howards, P.P.; Lyles, R.H.; Terrell, M.L.; Smith, A.K.; Conneely, K.N.; Marder, M.E.; Marcus, M. Serum polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) and thyroid function among Michigan adults several decades after the 1973–1974 PBB contamination of livestock feed. Environ. Health Perspect. 2017, 125, 097020. [Google Scholar] [CrossRef]
- Bahn, A.K.; Mills, J.L.; Snyder, P.J.; Gann, P.H.; Houten, L.; Bialik, O.; Hollmann, L.; Utiger, R.D. Hypothyroidism in workers exposed to polybrominated biphenyls. N. Engl. J. Med. 1980, 302, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.B.; Terrell, M.L.; Mardovich, S.; Somers, E.C.; Pearson, M.; Barton, H.; Tomlinson, M.S.; Marder, M.E.; Barr, D.B.; Marcus, M. Polybrominated Biphenyls (PBBs) and Prevalence of Autoimmune Disorders among Members of the Michigan PBB Registry. Environ. Res. 2023, 239, 117312. [Google Scholar] [CrossRef] [PubMed]
- Terrell, M.L.; Hartnett, K.P.; Lim, H.; Wirth, J.; Marcus, M. Maternal exposure to brominated flame retardants and infant Apgar scores. Chemosphere 2015, 118, 178–186. [Google Scholar] [CrossRef]
- Blanck, H.M.; Marcus, M.; Tolbert, P.E.; Rubin, C.; Henderson, A.K.; Hertzberg, V.S.; Zhang, R.H.; Cameron, L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 2000, 11, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Terrell, M.L.; Rosenblatt, K.A.; Wirth, J.; Cameron, L.L.; Marcus, M. Breast cancer among women in Michigan following exposure to brominated flame retardants. Occup. Environ. Med. 2016, 73, 564–567. [Google Scholar] [CrossRef]
- Small, C.M.; Murray, D.; Terrell, M.L.; Marcus, M. Reproductive outcomes among women exposed to a brominated flame retardant in utero. Arch. Environ. Occup. Health 2011, 66, 201–208. [Google Scholar] [CrossRef]
- Roboz, J.; Greaves, J.; Bekesi, J.G. Polybrominated biphenyls in model and environmentally contaminated human blood: Protein binding and immunotoxicological studies. Environ. Health Perspect. 1985, 60, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Small, C.M.; DeCaro, J.J.; Terrell, M.L.; Dominguez, C.; Cameron, L.L.; Wirth, J.; Marcus, M. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ. Health Perspect. 2009, 117, 1175–1179. [Google Scholar] [CrossRef]
- Zhang, S.F. Molecular Mechanisms of Thyroid Disrupting Effects of Typical Tetrabromobiphenyl (BB80) and Its Hydroxylation Products. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2020. [Google Scholar]
- Davis, E.F.; Klosterhaus, S.L.; Stapleton, H.M. Measurement of flame retardants and triclosan in municipal sewage sludge and biosolids. Environ. Int. 2012, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z. Formation of polybrominated dibenzofurans from polybrominated biphenyls. Chemosphere 2015, 119, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, T.; Feng, S.Y.; Chen, X.W.; Dang, D.; Huang, K.B.; Tao, X.Q.; Yin, H.; Dang, Z.; Lu, G.N. Experimental and theoretical investigations on debromination pathways of polybrominated biphenyls (PBBs) under ultraviolet light. Chemosphere 2018, 212, 1–7. [Google Scholar] [CrossRef]
- Wang, R.; Tang, T.; Huang, K.B.; Zou, M.Y.; Tao, X.Q.; Yin, H.; Lin, Z.; Dang, Z.; Lu, F.N. Debromination of polybrominated biphenyls (PBBs) by zero valent metals and iron-based bimetallic particles: Mechanisms, pathways and predicting descriptor. Chem. Eng. J. 2018, 351, 773–781. [Google Scholar] [CrossRef]
- Morris, P.J.; Quensen, J.F.; Tiedje, J.M.; Boyd, S.A. Reductive debromination of the commercial polybrominated biphenyl mixture Firemaster BP6 by anaerobic microorganisms from sediments. Appl. Environ. Microbiol. 1992, 58, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- von der Recke, R.; Vetter, W. Photolytic Transformation of Polybrominated Biphenyls Leading to the Structures of Unknown Hexa- to Nonabromo-Congeners. J. Chromatogr. A 2007, 1167, 184–194. [Google Scholar] [CrossRef]
- Letcher, R.J.; Gebbink, W.A.; Sonne, C.; Born, E.W.; McKinney, M.A.; Dietz, R. Bioaccumulation and Biotransformation of Brominated and Chlorinated Contaminants and Their Metabolites in Ringed Seals (Pusa hispida) and Polar Bears (Ursus maritimus) from East Greenland. Environ. Int. 2009, 35, 1118–1124. [Google Scholar] [CrossRef]
- Tang, C.; Jin, J.; Peng, X. Research advances in computational toxicological assessment and environmental behavior modeling of pollutants. Environ. Monitor. Forewarning 2016, 8, 1–15. [Google Scholar]
- Zhao, L.; Zhou, M.Y.; Zhao, Y.Y.; Yang, J.W.; Pu, Q.K.; Yang, H.; Wu, Y.; Lyu, C.; Li, Y. Potential toxicity risk assessment and priority control strategy for PAHs metabolism and transformation behaviors in the environment. Int. J. Environ. Res. Public Health 2022, 19, 10972. [Google Scholar] [CrossRef]
- Yamada, S.; Naito, Y.; Funakawa, M.; Nakai, S.; Hosomi, M. Photodegradation fates of cis-chlordane, trans-chlordane, and heptachlor in ethanol. Chemosphere 2008, 70, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Man, M.M.; Sun, Q.X.; Liu, Q.Z.; Wu, K.C.; Yang, D.F. Density functional theory study of marine polybrominated diphenyl ethers in anaerobic degradation. J. Ocean Univ. China 2023, 22, 1353–1360. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Elissawy, A.M.; Youssef, F.S.; Kicsak, M.; Kurtan, T.; Singab, A.N.B.; Kalscheuer, R. Austalide derivative from marine-derived Aspergillus sp. and evaluation of its cytotoxic and ADME/TOPKAT properties. RSC Adv. 2023, 13, 16480–16487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Liu, H.W.; Liu, M.H.; Liu, Y.C.; Wang, J.Q.; Wang, Y.; Wang, X.; Xiang, Z.; Liu, W. Network toxicology prediction and molecular docking-based strategy to explore the potential toxicity mechanism of Metformin chlorination byproducts in drinking water. Comb. Chem. High Throughput Screen. 2024, 27, 101–117. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, S.; Yu, M.; Wang, Q.; Yan, F. Two QSAR models for predicting the toxicity of chemicals towards Tetrahymena pyriformis based on topological-norm descriptors and spatial-norm descriptors. SAR QSAR Environ. Res. 2023, 34, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Sun, G.H.; Zhao, L.J.; Zhang, N.; Zhang, R.G.; Peng, Y.Z. Quantitative structure-Activity relationship (QSAR) studies on the toxic effects of nitroaromatic compounds (NACs): A systematic review. Int. J. Mol. Sci. 2021, 22, 8557. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.R.; Li, L.; Sun, S.F.; Jiang, X.X.; Wang, M.; Zheng, M.H. Sources, analytical methods and environmental characteristics of polybrominated biphenyls. Prog. Chem. 2014, 26, 1434–14444. [Google Scholar]
- Hakk, H.; Letcher, R.J. Metabolism in the toxicokinetics and fate of brominated flame retardants—A review. Environ. Int. 2003, 29, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wen, X.; Peng, M.G.; Yu, Y.J. Characteristics and risk assessment of polybrominated biphenyls from electronic waste dismantling places. China Environ. Sci. 2017, 37, 4781–4789. [Google Scholar]
- Ojha, A.K.; Snehasis, B. Different Proton Transfer Channels for the Transformation of Zwitterionic Alanine–(H2O) N=2-4 to Nonzwitterionic Alanine–(H2O) N=2-4: A Density Functional Theory Study. J. Mol. Model. 2014, 20, 2124. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Long, Y.X.; Zhu, J.; Cheng, S.; Fu, J.; Liu, L. An Experimental and Density Functional Theory Investigation of the Influence of HZSM-5 on Pyrolysis Oils Produced from Vanillin, a Model Lignin Compound. J. Energy Inst. 2024, 116, 101761. [Google Scholar] [CrossRef]
- Zhang, W.H.; Gu, W.W.; Sun, R.H.; Zhou, M.Y.; Han, Z.Z.; Li, Y. An adjusted 3D-QSAR model for the combined activity of fluoroquinolones photodegradation and microbial degradation assisted by dynamic simulation and its application in molecular modification. Ecotoxicol. Environ. Saf. 2021, 212, 111973. [Google Scholar] [CrossRef]
- Wei, H.; Zou, Y.; Li, A.; Christensen, E.R.; Rockne, K.J. Photolytic debromination pathway of polybrominated diphenyl ethers in hexane by sunlight. Environ. Pollut. 2013, 174, 194–200. [Google Scholar] [CrossRef]
- Amran, N.A.; Bello, U.; Hazwan Ruslan, M.S. The Role of Antioxidants in Improving Biodiesel’s Oxidative Stability, Poor Cold Flow Properties, and the Effects of the Duo on Engine Performance: A Review. Heliyon 2022, 8, e09846. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A Review on Lignin Pyrolysis: Pyrolytic Behavior, Mechanism, and Relevant Upgrading for Improving Process Efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.C.; Wang, Z.Y.; Zeng, X.L. Study on the Thermodynamic Properties and Stability of a Series of Polybrominated Dibenzo-furans by Density Functional Theory. Acta Chim. Sin. 2006, 19, 1961–1968. [Google Scholar]
- Hiroki, O.; Ando, K. The Role of Intermolecular Hydrogen Bond on Dielectric Properties in Hydrogen-Bonded Material 5-Bromo-9-Hydroxyphenalenone: Theoretical Investigation. Phys. Chem. Chem. Phys. 2011, 13, 10719. [Google Scholar]
- Yu, A.Y.; Kürşat, E.; Yang, R.; Hu, Q.M. A Comprehensive Theoretical Investigation of the Molecular Properties of Methyl Bromide (CH3Br). Z. Naturforsch. A 2015, 70, 1025–1030. [Google Scholar] [CrossRef]
- Hu, J.W.; Zhuang, Y.; Luo, J.; Wei, X.H.; Huang, X.F. A Theoretical Study on Reductive Debromination of Polybrominated Diphenyl Ethers. Int. J. Mol. Sci. 2012, 13, 9332–9342. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, S.C.; Macdonald, R.W.; Wright, C.A.; Burd, B.; Shaw, D.P.; van Roodselaar, A. Joined by Geochemistry, Divided by History: PCBs and PBDEs in Strait of Georgia Sediments. Mar. Environ. Res. 2008, 66, S112–S120. [Google Scholar] [CrossRef]
- Norman, R.O.C. Principles of Organic Synthesis, 3rd ed.; Routledge: London, UK, 1993. [Google Scholar]
- Deraet, X.; Desmedt, E.; Lommel, R.V.; Speybroeck, V.V.; Proft, F.D. The Electrophilic Aromatic Bromination of Benzenes: Mechanistic and Regioselective Insights from Density Functional Theory. Phys. Chem. Chem. Phys. 2023, 25, 28581–28594. [Google Scholar] [CrossRef]
- Song, H.Y.; Zhao, H.; Yan, X.; Shi, X.F.; Ma, J. Adsorption Characteristics of Marine Contaminant Polychlorinated Biphenyls Based on Surface-Enhanced Raman Spectroscopy. Spec. Spectr. Anal. 2022, 42, 704. [Google Scholar]
- Han, W.L.; Liu, Y.; Feng, K.W. Spatiotemporal Differentiation and Degradation Analysis of Polybrominated Diphenyl Ethers in Sediments of Shanmei Reservoir and Its Inflowing River, Quanzhou, China. Environ. Sci. 2020, 41, 4525–4538. [Google Scholar]
- Zhao, S.Y.; Rogers, M.J.; Ding, C.; He, J.Z. Reductive Debromination of Polybrominated Diphenyl Ethers—Microbes, Processes and Dehalogenases. Front. Microbiol. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Kohli, J.; Crawford, A. Fire Master BP 6: Fractionation, metabolic and enzyme induction studies. Environ. Health Perspect. 1978, 23, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Cui, S.X.; Pan, L.M.; Dong, W.H.; Ma, M.; Liu, W.P.; Zhuang, S.L. The molecular mechanism of the antagonistic activity of hydroxylated polybrominated biphenyl (OH-BB80) toward thyroid receptor β. Sci. Total Environ. 2019, 697, 134040. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Wang, Y.; Chen, J.W.; Wang, Z.Y.; Wang, X.B. How PBDEs are transformed into dihydroxylated and dioxin metabolites catalyzed by the active center of cytochrome P450s: A DFT study. Environ. Sci. Technol. 2016, 50, 8155–8163. [Google Scholar] [CrossRef]
- Markus, H.; Wolfgang, M.; Helmut, G.A. The influence of halogen substituents at the ligand framework of (α-diimine)nickel(II) catalyst precursors on their behavior in ethylene oligomerization and polymerization. J. Mol. Catal. A Chem. 2003, 197, 1–13. [Google Scholar]
- Wang, H.; Jiang, M.; Li, S.; Chung-Yun, H.; Jin, C.; Sun, F.; Li, Z. Design of Cinnamaldehyde Amino Acid Schiff Base Compounds Based on the Quantitative Structure–Activity Relationship. R. Soc. Open Sci. 2017, 4, 170516. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, M.; Wencel-Delord, J. Diaryl Hypervalent Bromines and Chlorines: Synthesis, Structures and Reactivities. Chem. Sci. 2024, 15, 1557–1569. [Google Scholar] [CrossRef]
- Patel, C.; Bassin, J.P.; Scott, M.; Flye, J.; Hunter, A.P.; Martin, L.; Goyal, M. Synthesis and Antimicrobial Activity of 1,2-Benzothiazine Derivatives. Molecules 2016, 21, 861. [Google Scholar] [CrossRef]
- Yang, L.Z.; Liu, M. 3D-QSAR model of polybrominated biphenyls tri-effect modified by standard deviation standardization method and its application in environmental friendly molecular modification. Chem. J. Chin. Univ. 2019, 40, 2471–2479. [Google Scholar]
- Yang, L.Z.; Liu, M. Construction of a 3D-QSAR Model with Dual Spectral Effects and Its Application in Molecular Modification of Environmentally Friendly PBBs. Spectrosc. Spect. Anal. 2021, 41, 430–434. [Google Scholar]
- Yang, L.Z.; Li, M.H.; Liu, M. Establishment of a 3D-QSAR model based on the combined activity of bioconcentration, long-range transport, and highest infrared signal intensity and molecular design of environmentally friendly PBB substitutes. Polymers 2021, 13, 356. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhou, Y.X.; Zhu, G.H.; Li, M.F.; Gong, H.P.; Liu, J.S. Determination of 20 polybrominated biphenyls in soil and sediment by isotope dilution gas chromatography-high resolution mass spectrometry. Environ. Chem. 2018, 37, 1994–2001. [Google Scholar]
- Chang, C.J.; Terrell, M.L.; Marcus, M.; Marder, M.E.; Panuwet, P.; Ryan, P.B.; Pearson, M.; Barton, H.; Barr, D.B. Serum Concentrations of Polybrominated Biphenyls (PBBs), Polychlorinated Biphenyls (PCBs) and Polybrominated Diphenyl Ethers (PBDEs) in the Michigan PBB Registry 40 Years after the PBB Contamination Incident. Environ. Int. 2020, 137, 105526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Qiu, X.; Li, R.; Liu, S.; Li, K.; Wang, F.; Zhu, P.; Li, G.; Zhu, T. Exposure to Typical Persistent Organic Pollutants from an Electronic Waste Recycling Site in Northern China. Chemosphere 2013, 91, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, Z.; Zhou, H.; Zhao, Q. Burdens of PBBs, PBDEs, and PCBs in Tissues of the Cancer Patients in the E-Waste Disassembly Sites in Zhejiang, China. Sci. Total Environ. 2009, 407, 4831–4837. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Dhoone, M.S.; Mello, F.V.; Miranda, J.A.T.; Fogaca, F.H.S.; Alonso, M.B.; Torres, J.P.M.; Fernandes, J.O. Survey on Endocrine-Disrupting Chemicals in Seafood: Occurrence and Distribution. Environ. Res. 2022, 210, 112886. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.M.; Marcus, M.; Hertzberg, V.; Tolbert, P.E.; Rubin, C.; Henderson, A.K.; Zhang, R.H. Determinants of Polybrominated Biphenyl Serum Decay among Women in the Michigan PBB Cohort. Environ. Health Perspect. 2000, 108, 147. [Google Scholar] [CrossRef] [PubMed]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The Impact of Endocrine-Disrupting Chemical Exposure in the Mammalian Hypothalamic-Pituitary Axis. Mol. Cell. Endocrinology 2020, 518, 110997. [Google Scholar] [CrossRef]
- Wu, J.P.; Mo, L.; Zhi, H.; Peng, Y.; Tao, L.; Ren, Z.H.; Luo, X.J.; Mai, B.X. Hepatic Ethoxyresorufin-O-Deethylase Induction in the Common Kingfisher from an Electronic Waste Recycling Site. Environ. Toxicol. Chem. 2015, 35, 1594–1599. [Google Scholar] [CrossRef]
- Zhang, W.H.; Sun, R.H.; Zhao, X.H.; Li, Y. Environmental conversion path inference of new designed fluoroquinolones and their potential environmental risk. Arch. Environ. Contam. Toxicol. 2020, 78, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Wicks, B.J.; Randall, D.J. The effect of feeding and fasting on ammonia toxicity in juvenile rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2002, 59, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Li, K.; Yan, D.L. Toxicity Assessment of 4 Azo Dyes in Zebrafish Embryos. Int. J. Toxicol. 2020, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Deziel, N.C.; Alfonso-Garrido, J.; Warren, J.L.; Huang, H.; Sjodin, A.; Zhang, Y. Exposure to polybrominated diphenyl ethers and a polybrominated biphenyl and risk of thyroid cancer in women: Single and multi-pollutant approaches. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, G.L.; Han, X.; Zhu, C.; Kilfoy, B.A.; Zhu, Y.; Boyle, P.; Zheng, T.Z. Do polybrominated diphenyl ethers (PBDEs) increase the risk of thyroid cancer? Biosci. Hypotheses 2008, 1, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Lilis, R.; Selikoff, I.J. Unanticipated prevalence of symptoms among dairy farmers in Michigan and Wisconsin. Environ. Health Perspect. 1978, 23, 217–226. [Google Scholar] [CrossRef]
- Fan, Y.; Boivin, G.P.; Knudsen, E.S.; Nebert, D.W.; Xia, Y.; Puga, A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010, 70, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Cousins, I.; Mackay, D. Comparison of two methods for obtaining degradation half-lives. Chemosphere 2004, 56, 531–535. [Google Scholar] [CrossRef]
- Gu, W.W.; Chen, Y.; Li, Y. Attenuation of the Atmospheric Migration Ability of Polychlorinated Naphthalenes (PCN-2) Based on Three-dimensional QSAR Models with Full Factor Experimental Design. Bull. Environ. Contam. Toxicol. 2017, 99, 276–280. [Google Scholar] [CrossRef]
- Navarro-Acosta, D.; Ludis, C.J.; León-Sotomayor, W.; Vivas-Reyes, R.; Néstor, C. Integrative Computational Approach for Hyperuricemia Treatment: 3D QSAR, Molecular Docking and Dynamics of Flavonoid Analogues as Xanthine Oxidase Inhibitors. J. Mol. Struct. 2024, 1322, 140395. [Google Scholar] [CrossRef]
- Khaldan, A.; Bouamrane, S.; Ouabane, M.; El-mernissi, R.; Alaqarbeh, M.; Alnajjare, R.; Gürer, E.S.; Kaya, S.; Maghat, H.; Bouachrine, M.; et al. Design, 3D-QSAR, Molecular Docking, MD Simulations, ADME/Tox Properties and DFT Study of Benzimidazole Derivatives as Promising α-Glucosidase Inhibitors. J. Mol. Struct. 2025, 1328, 141351. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, S.; Wang, F.; Jiang, R.; Yin, X.; Wang, S.; Jin, R.; Guo, H.; Tang, Y.; Wang, Y. 3D-QSAR, Scaffold Hopping, Virtual Screening, and Molecular Dynamics Simulations of Pyridin-2-One as MIDH1 Inhibitors. Int. J. Mol. Sci. 2024, 25, 7434. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Liu, H.; Wang, B.; Wang, M.; Zhang, E.; Li, J.; Ding, L. A Molecular Simulation Study of Sulfonamide HBV Core Protein Allosteric Modulators. ChemistrySelect 2024, 9, e202403645. [Google Scholar] [CrossRef]
- Tong, J.B.; Xiao, X.C.; Luo, D.; Xu, H.Y.; Xing, Y.C.; Gao, P.; Liu, Y. Discovery of Novel BRD4-BD2 Inhibitors via in Silico Approaches: QSAR Techniques, Molecular Docking, and Molecular Dynamics Simulations. Mol. Divers. 2023, 28, 671–692. [Google Scholar] [CrossRef]
- El fadili, M.; Er-rajy, M.; Imtara, H.; Kara, M.; Zarougui, S.; Altwaijry, N.; Al kamaly, O.; Al Sfouk, A.; Elhallaoui, M. 3D-QSAR, ADME-Tox in Silico Prediction and Molecular Docking Studies for Modeling the Analgesic Activity against Neuropathic Pain of Novel NR2B-Selective NMDA Receptor Antagonists. Processes 2022, 10, 1462. [Google Scholar] [CrossRef]
- Li, X.A.; Hou, Y.L.; Li, Q.; Gu, W.W.; Li, Y. Molecular design of high-efficacy and high drug safety Fluoroquinolones suitable for a variety of aerobic biodegradation bacteria. J. Environ. Manag. 2021, 299, 113628. [Google Scholar] [CrossRef]
- Li, A.P.; Zhang, W.N.; Zhang, L.C.; Liu, Y.T.; Li, K.; Du, G.H.; Qin, X.M. Elucidating the time-dependent changes in the urinary metabolome under doxorubicin-induced nephrotoxicity. Toxicol. Lett. 2020, 319, 204–212. [Google Scholar] [CrossRef]
- Caro, D.; Florez, M.; Gaitan, R.; Martinez, E.; Baldiris, R.; Vivas-Reyes, R. Study of relationship chemical structure antimalarial activity of quinoidal compounds obtained by synthesis. Afinidad 2017, 74, 185–193. [Google Scholar]
- Luo, L.J.; Xiao, Z.Y.; Zhou, X.Y.; Yang, L.H.; Luo, S.S.; Zhao, C.Y.; Luan, T.G. Quantum chemical calculation to elucidate the biodegradation pathway of methylphenanthrene by green microalgae. Water Res. 2020, 173, 115598. [Google Scholar] [CrossRef]
- Gu, W.W.; Li, Q.; Li, Y. Environment-friendly PCN substitutes design and environmental behavior simulation based on a multi-activity 3D-QSAR model and molecular dynamics. J. Hazard. Mater. 2020, 393, 122339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Chu, Z.H.; Li, Y. Molecular Design of Lower Photodegradation Fluoroquinolone Antibiotics and Their Photolysis Paths Inference. Chem. J. Chin. Univ. 2019, 39, 2707–2718. [Google Scholar]

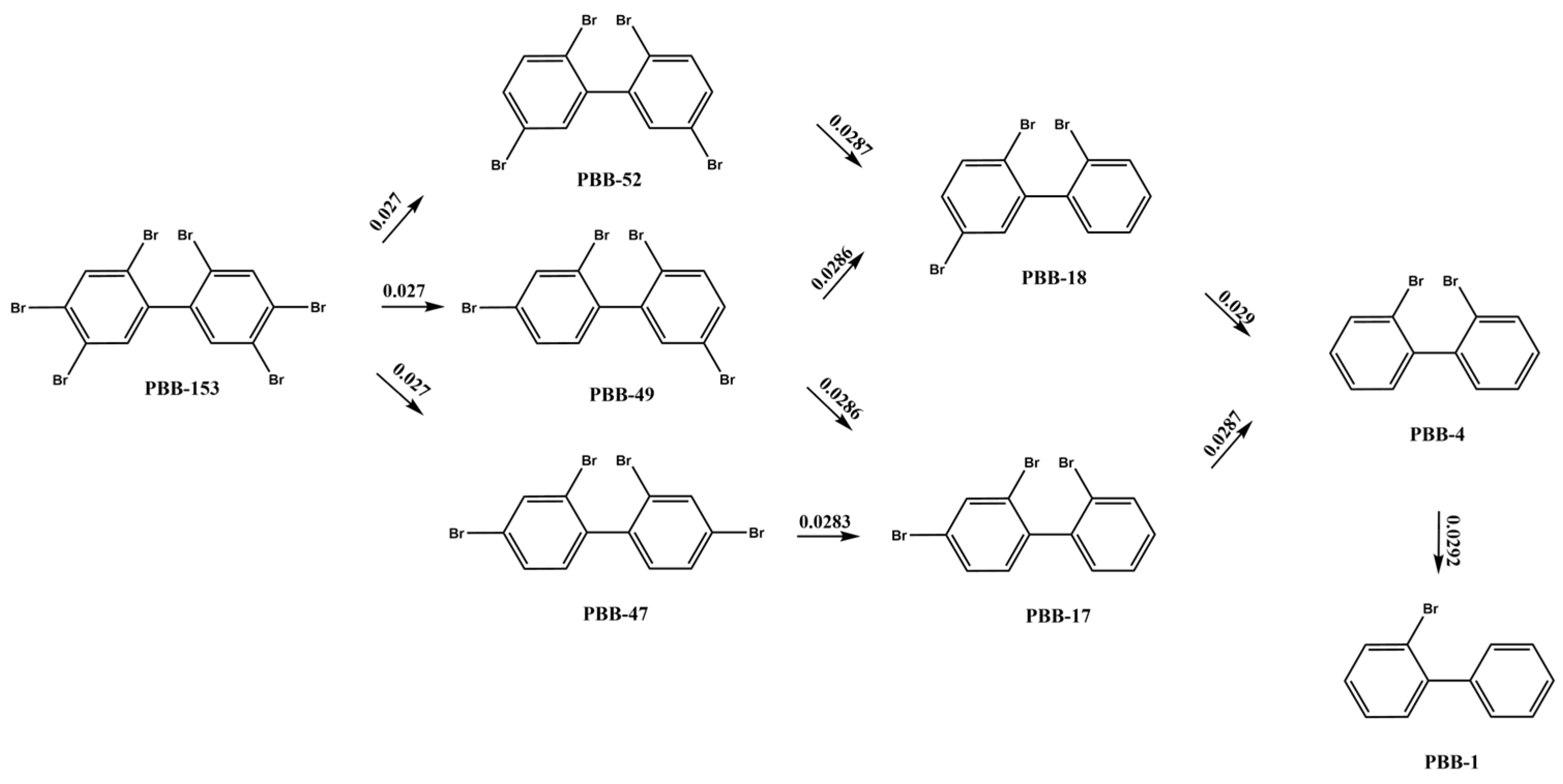

| Number of Debromination | Debromination Sites | Energy Barrier/a.u. |
|---|---|---|
| 1 | 2 | 0.001 |
| 4 | 0.027 | |
| 5 | 0.027 | |
| 2 | 2, 4 | 0.164 |
| 2, 5 | 0.163 | |
| 2, 2′ | 0.011 | |
| 2, 4′ | 0.164 | |
| 2, 5′ | 0.164 | |
| 3 | 2, 2′, 3 | 0.163 |
| 2, 2′, 4 | 0.164 | |
| 4 | 2, 2′, 3, 3 | 0.162 |
| 2, 2′, 3, 4 | 0.164 | |
| 2, 2′, 3, 4′ | 0.002 | |
| 5 | 2, 2′, 3, 4′, 3 | 0.161 |
| 2, 2′, 3, 4′, 4 | 0.164 | |
| 6 | 2, 2′, 3, 4′, 3, 4 | 0.003 |
| PBBs | Transformation Products | Transformation Pathways |
|---|---|---|
| PBB-3 | Biphenyl | Photodegradation |
| PBB-4 | PBB-1, Biphenyl | |
| PBB-15 | PBB-3, Biphenyl | |
| PBB-29 | PBB-9, PBB-7, PBB-3, PBB-2, PBB-1, biphenyl | |
| PBB-80 | PBB-36, PBB-14, PBB-2, Biphenyl | |
| PBB-153 | PBB-118, PBB-101, PBB-99, PBB-77, PBB-70, PBB-52, PBB-49, PBB-37, PBB-12, PBB-3, biphenyl | |
| D1 | D1-A1, D1-A2 | |
| D2 | D2-A1, D2-A2 | |
| D3 | D3-A1, D3-A2, D3-A3 | |
| PBB-3 | PBB-3-B1 | Combustion oxidation |
| PBB-4 | 4-MBDF, DF | |
| PBB-15 | PBB-15-B1 | |
| PBB-29 | PBB-29-B1 | |
| PBB-80 | PBB-80-B1 | |
| PBB-153 | PBDF | |
| D1 | D1-B1 | |
| D2 | D2-B1 | |
| D3 | D3-B1 | |
| PBB-3 | Biphenyl | Microbial reduction |
| PBB-4 | PBB-1, Biphenyl | |
| PBB-15 | PBB-3, Biphenyl | |
| PBB-29 | PBB-7, PBB-9, PBB-1, Biphenyl | |
| PBB-80 | PBB-36, PBB-14, PBB-11, PBB-2, Biphenyl | |
| PBB-153 | PBB-52, PBB-49, PBB-47, PBB-18, PBB-17, PBB-4, PBB-1 | |
| D1 | D1-C1 | |
| D2 | D2-C1 | |
| D3 | D3-C1, D3-C2 | |
| PBB-3 | 4′-OH-PBB-3, 4′-Br-3-MeO-4-OH-biphenyl, | Biological metabolism |
| PBB-4 | PBB-4-D1, PBB-4-D2 | |
| PBB-15 | 4-OH-PBB-13, 3-OH-PBB-15, 4-Br-3-MeO-4′-OH-biphenyl | |
| PBB-29 | PBB-29-D1, PBB-29-D2 | |
| PBB-80 | 2, 2′-OH-PBB-80 | |
| PBB-153 | PBB-153-D1, PBB-153-D2 | |
| D1 | D1-D1, D1-D2 | |
| D2 | D2-D1, D2-D2 | |
| D3 | D3-D1, D3-D2 |
| 3D-QSAR Models | q2 | n | R2 | SEE | F | SEP | r2pred |
|---|---|---|---|---|---|---|---|
| Carcinogenicity model | 0.827 | 10 | 0.994 | 0.532 | 111.695 | 3.279 | 0.853 |
| Developmental toxicity model | 0.818 | 5 | 0.966 | 0.879 | 68.755 | 6.225 | 0.856 |
| Hepatotoxicity model | 0.836 | 5 | 0.99 | 0.94 | 227.902 | 4.427 | 0.784 |
| Epigenotoxicity model | 0.895 | 7 | 0.991 | 0.598 | 158.151 | 4.318 | 0.633 |
| Neurotoxicity model | 0.807 | 8 | 0.995 | 0.494 | 220.73 | 0.868 | 0.915 |
| Immunotoxicity model | 0.825 | 8 | 0.993 | 0.403 | 156.833 | 2.276 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Liu, Q.; Cui, W.; Tao, L.; Chi, Y.; Yang, L. Extrapolation of PBBs Environmental Transformation Mechanisms and Toxicity Risks of Byproducts. Int. J. Mol. Sci. 2025, 26, 1753. https://doi.org/10.3390/ijms26041753
Xu B, Liu Q, Cui W, Tao L, Chi Y, Yang L. Extrapolation of PBBs Environmental Transformation Mechanisms and Toxicity Risks of Byproducts. International Journal of Molecular Sciences. 2025; 26(4):1753. https://doi.org/10.3390/ijms26041753
Chicago/Turabian StyleXu, Bohan, Qian Liu, Weihan Cui, Li Tao, Yuanquan Chi, and Luze Yang. 2025. "Extrapolation of PBBs Environmental Transformation Mechanisms and Toxicity Risks of Byproducts" International Journal of Molecular Sciences 26, no. 4: 1753. https://doi.org/10.3390/ijms26041753
APA StyleXu, B., Liu, Q., Cui, W., Tao, L., Chi, Y., & Yang, L. (2025). Extrapolation of PBBs Environmental Transformation Mechanisms and Toxicity Risks of Byproducts. International Journal of Molecular Sciences, 26(4), 1753. https://doi.org/10.3390/ijms26041753





