Association of Basal Serum Androgen Concentration with Follicles Number on the Day of Triggering Final Oocyte Maturation in Low Responders According to the Bologna Criteria: A Prospective Cohort Study
Abstract
1. Introduction
2. Results
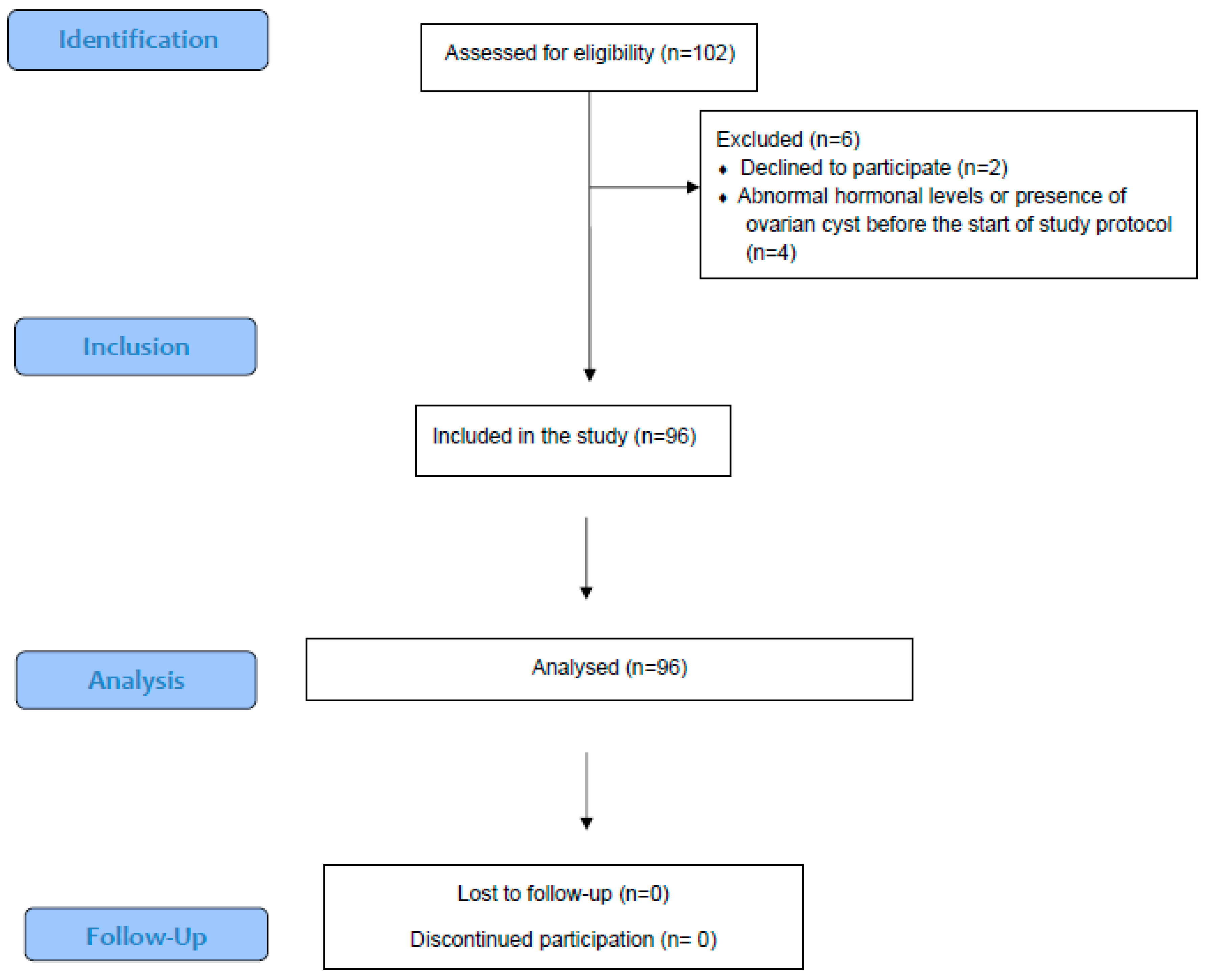
2.1. Patient Population and Cycle Characteristics
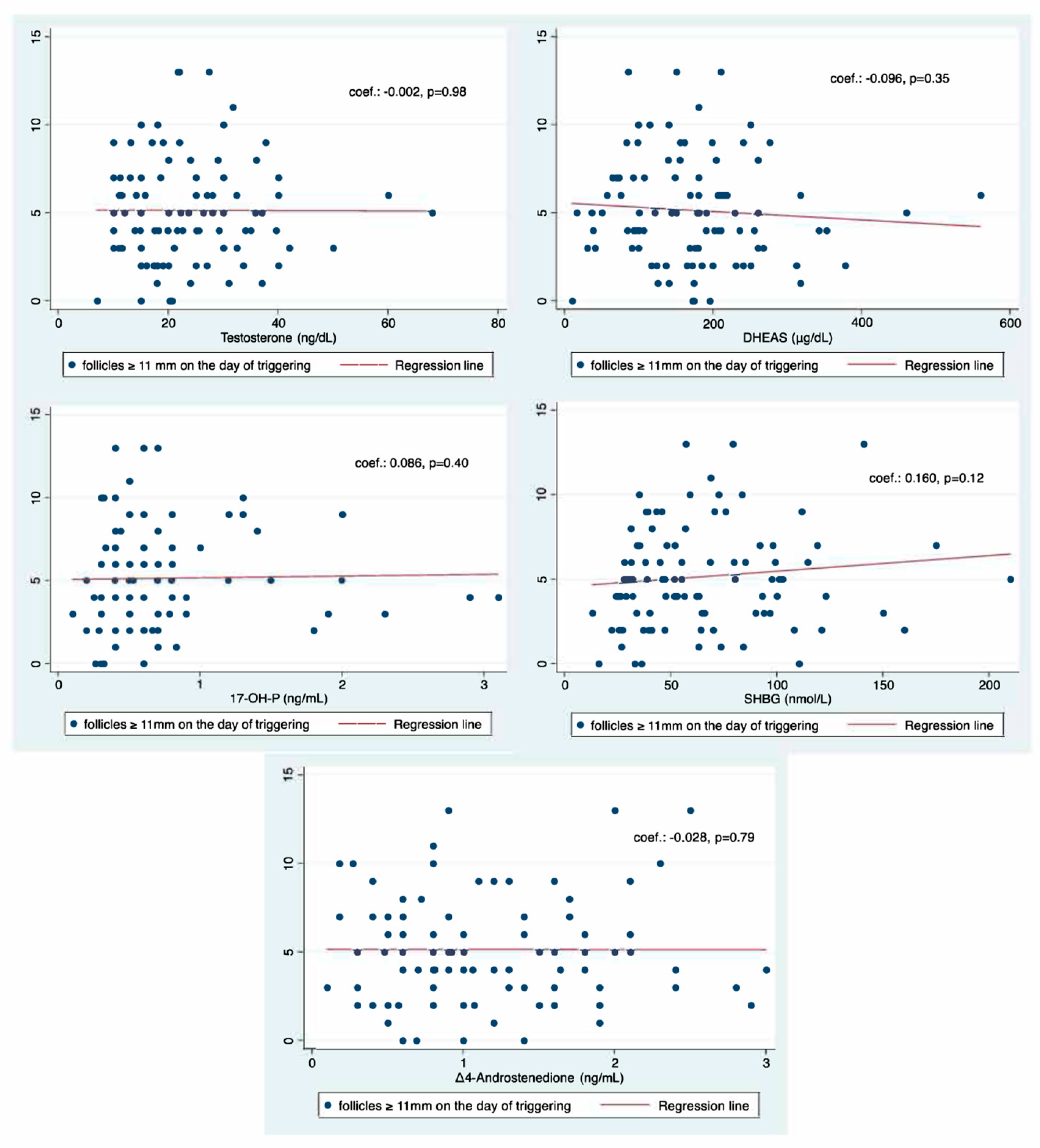
2.2. Primary Outcome Measure
2.3. Secondary Outcome Measures
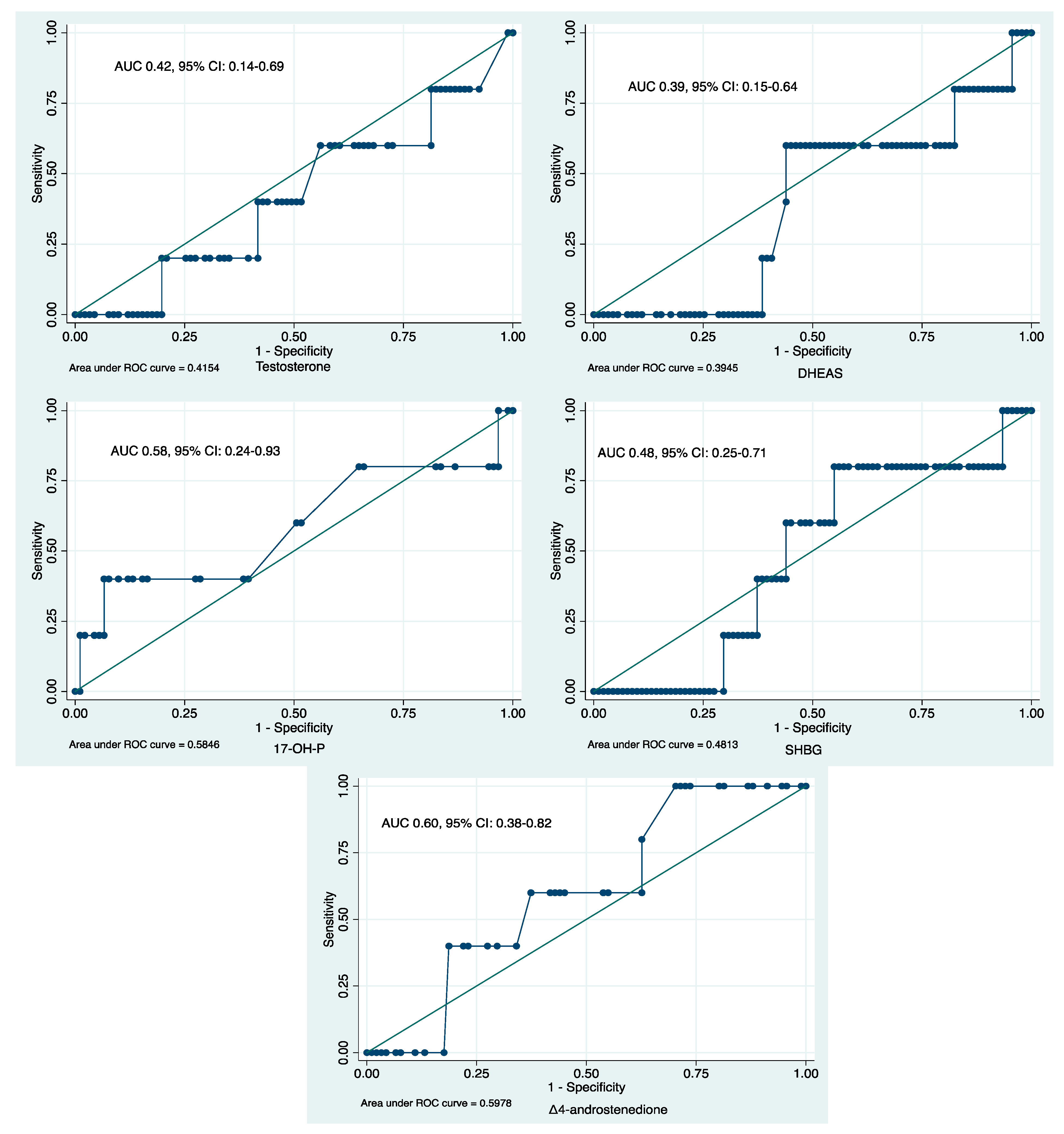
2.4. ROC Analyses
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Hormonal Measurements and Ovarian Stimulation
4.3. ART Procedure
4.4. Outcome Measures
4.5. Statistical Analysis
4.6. Sample Size
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venetis, C.A.; Tilia, L.; Panlilio, E.; Kan, A. Is more better? A higher oocyte yield is independently associated with more day-3 euploid embryos after ICSI. Hum. Reprod. 2019, 34, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.J.; Zhang, N.; Kolibianakis, E.M.; Costello, M.F.; Keller, E.; Chambers, G.M.; Venetis, C.A. Is there an optimal number of oocytes retrieved at which live birth rates or cumulative live birth rates per aspiration are maximized after ART? A systematic review. Reprod. Biomed. Online 2021, 42, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.K.; Rittenberg, V.; Raine-Fenning, N.; Bhattacharya, S.; Zamora, J.; Coomarasamy, A. Association between the number of eggs and live birth in IVF treatment: An analysis of 400,135 treatment cycles. Hum. Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Venetis, C.A.; Kolibianakis, E.M.; Tarlatzi, T.B.; Tarlatzis, B.C. Evidence-based management of poor ovarian response. Ann. N. Y. Acad. Sci. 2010, 1205, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, D.; Kolibianakis, E.M.; Venetis, C.A.; Papanikolaou, E.G.; Bontis, J.; Tarlatzis, B.C. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Fertil. Steril. 2009, 91, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Hardy, K. Androgen Action in the Ovary. Front Endocrinol. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Montoya-Botero, P.; Polyzos, N.P. Androgens and diminished ovarian reserve: The long road from basic science to clinical implementation. A comprehensive and systematic review with meta-analysis. Am. J. Obs. Gynecol. 2022, 227, 401–413.e18. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Rodriguez Paris, V.; Aflatounian, A.; Handelsman, D.J. Androgens and ovarian function: Translation from basic discovery research to clinical impact. J. Endocrinol. 2019, 242, R23–R50. [Google Scholar] [CrossRef]
- Vendola, K.; Zhou, J.; Wang, J.; Bondy, C.A. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum. Reprod. 1999, 14, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Bonser, J.; Walker, J.; Purohit, A.; Reed, M.J.; Potter, B.V.; Willis, D.S.; Franks, S.; Mason, H.D. Human granulosa cells are a site of sulphatase activity and are able to utilize dehydroepiandrosterone sulphate as a precursor for oestradiol production. J. Endocrinol. 2000, 167, 465–471. [Google Scholar] [CrossRef]
- Astapova, O.; Minor, B.M.N.; Hammes, S.R. Physiological and Pathological Androgen Actions in the Ovary. Endocrinology 2019, 160, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.L.; Bell, R.; Donath, S.; Montalto, J.G.; Davis, S.R. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005, 90, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Q.; Li, Y.; Wang, W.; Yang, D. Low level of basal testosterone: A significant risk factor for poor oocyte yield after ovulation induction. Reprod. Fertil. Dev. 2016, 28, 286–292. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Y.; Long, L.; Luo, C.; Mai, Q. Basal serum testosterone levels correlate with ovarian reserve and ovarian response in cycling women undergoing in vitro fertilization. Gynecol. Endocrinol. 2016, 32, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Sequeira, K.; Tapia-Pizarro, A.; Munoz, A.; Salinas, A.; Cespedes, P.; Escalona, J.; Godoy, A. Androgens Profile in Blood Serum and Follicular Fluid of Women With Poor Ovarian Response During Controlled Ovarian Stimulation Reveals Differences Amongst POSEIDON Stratification Groups: A Pilot Study. Front. Endocrinol. 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Q.; Li, Y.; Huang, J.; Wang, W.; Huang, L.; Zhao, X.; Yang, D. Predictive value of androgens and multivariate model for poor ovarian response. Reprod. Biomed. Online 2014, 28, 723–732. [Google Scholar] [CrossRef][Green Version]
- Meldrum, D.R.; de Ziegler, D. Introduction: Risk and safety management in infertility and assisted reproductive technology. Fertil. Steril. 2013, 100, 1497–1498. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Kushnir, V.A.; Shohat-Tal, A.; Lazzaroni, E.; Lee, H.J.; Barad, D.H. Is androgen production in association with immune system activation potential evidence for existence of a functional adrenal/ovarian autoimmune system in women? Reprod. Biol. Endocrinol. 2013, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Weil, S.; Vendola, K.; Zhou, J.; Bondy, C.A. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J. Clin. Endocrinol. Metab. 1999, 84, 2951–2956. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, Z.; Sun, M.; Geng, L.; Che, L.; Chen, Z.J. Association of basal serum testosterone levels with ovarian response and in vitro fertilization outcome. Reprod. Biol. Endocrinol. 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Shen, H.; Li, Y.; Zhang, C.; Wang, C.; Chen, X.; Liang, R.; Wei, L. Low testosterone levels in women with diminished ovarian reserve impair embryo implantation rate: A retrospective case-control study. J. Assist. Reprod. Genet. 2014, 31, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Abide Yayla, C.; Ozkaya, E.; Kayatas Eser, S.; Sanverdi, I.; Devranoglu, B.; Kutlu, T. Association of basal serum androgen levels with ovarian response and ICSI cycle outcome. Ir. J. Med. Sci. 2018, 187, 409–415. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef]
- Papathanasiou, A. Implementing the ESHRE ‘poor responder’ criteria in research studies: Methodological implications. Hum. Reprod. 2014, 29, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Venetis, C.A. The Bologna criteria for poor ovarian response: The good, the bad and the way forward. Hum. Reprod. 2014, 29, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Prizant, H.; Light, A.; Biswas, A.; Hayes, E.; Lee, H.J.; Barad, D.; Gleicher, N.; Hammes, S.R. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Qi, X.; Yun, C.; Qiao, J.; Pang, Y. Effects of Androgen Excess-Related Metabolic Disturbances on Granulosa Cell Function and Follicular Development. Front. Endocrinol. 2022, 13, 815968. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, C.G.; Bernuci, M.P.; Silva-de-Sa, M.F.; Rosa, E.S.A.C. The role of androgen hormones in early follicular development. ISRN Obs. Gynecol. 2014, 2014, 818010. [Google Scholar] [CrossRef] [PubMed]
- Katsika, E.T.; Bosdou, J.K.; Goulis, D.G.; Grimbizis, G.F.; Kolibianakis, E.M. Higher live birth rate following transdermal testosterone pretreatment in poor responders: A systematic review and meta-analysis. Reprod. Biomed. Online 2023, 46, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Bosdou, J.K.; Venetis, C.A.; Kolibianakis, E.M.; Toulis, K.A.; Goulis, D.G.; Zepiridis, L.; Tarlatzis, B.C. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 127–145. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; Le Clef, N.; et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI. Hum. Reprod. Open 2020, 2020, hoaa009. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Guenther, W.C. Desk Calculation of Probabilities for the Distribution of the Sample Correlation Coefficient. Am. Stat. 1977, 31, 45–48. [Google Scholar] [CrossRef]

| Patient Characteristics (n = 96) | Median | 95% CI |
|---|---|---|
| Age (years) | 42.0 | 41.5–42.9 |
| BMI (kg/m2) | 24.1 | 22.5–25.7 |
| AFC | 6.0 | 5.0–7.0 |
| AMH (ng/mL) | 0.8 | 0.6–1.0 |
| Serum androgen concentration | ||
| Testosterone (ng/dL) | 20.4 | 18.3–24.0 |
| DHEAS (μg/dL) | 170.1 | 148.0–180.0 |
| 17-OH-P (ng/mL) | 0.6 | 0.5–0.6 |
| Δ4-A (ng/mL) | 1.0 | 0.9–1.2 |
| SHBG (nmol/L) | 55.7 | 47.0–64.6 |
| Ovarian stimulation characteristics | ||
| Duration of stimulation (days) | 10.0 | 9.0–10.0 |
| Total dose of FSH (IU) | 3000 | 2700–3000 |
| Day of triggering final oocyte maturation | ||
| Number of follicles ≥ 11 mm | 5.0 | 4.0–5.1 |
| Number of follicles ≥ 17 mm | 2.0 | 2.0–3.0 |
| Estradiol (pg/mL) | 1154 | 921–1285 |
| IVF outcome | ||
| COCs retrieved | 3.0 | 2.0–4.0 |
| MII oocytes | 3.0 | 2.0–3.0 |
| 2pn oocytes | 2.0 | 2.0–2.0 |
| % (n) | 95% CI | |
| IVF ICSI | 20.8 (16) 79.2 (61) | 13.0–31.5 68.5–87.0 |
| Patients reaching ET | 52.1 (50) | 42.0–62.0 |
| Positive hCG test | 9.4 (9) | 4.9–17.2 |
| Ongoing pregnancy | 5.2 (5) | 2.1–12.1 |
| Live birth | 5.2 (5) | 2.1–12.1 |
| Androgen and SHBG | Coef. | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Testosterone | +0.003 | 0.60 | −0.007 to +0.013 |
| DHEAS | −0.001 | 0.30 | −0.002 to +0.001 |
| 17-OH-P | +0.015 | 0.89 | −0.197 to +0.227 |
| Δ4-A | −0.017 | 0.91 | −0.300 to +0.267 |
| SHBG | +0.002 | 0.24 | −0.001 to +0.005 |
| COCs | MII | 2pn | |
|---|---|---|---|
| Coefficient p-Value | |||
| Testosterone | −0.118 0.30 | −0.176 0.12 | −0.129 0.26 |
| DHEAS | −0.041 0.72 | −0.039 0.73 | +0.060 0.60 |
| 17-OH-P | −0.008 0.95 | −0.039 0.73 | −0.103 0.37 |
| Δ4-A | −0.036 0.75 | −0.070 0.54 | −0.045 0.69 |
| SHBG | +0.143 0.21 | +0.091 0.42 | +0.010 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosdou, J.K.; Venetis, C.A.; Anagnostis, P.; Savvaidou, D.; Chatzimeletiou, K.; Zepiridis, L.; Goulis, D.G.; Grimbizis, G.; Kolibianakis, E.M. Association of Basal Serum Androgen Concentration with Follicles Number on the Day of Triggering Final Oocyte Maturation in Low Responders According to the Bologna Criteria: A Prospective Cohort Study. Int. J. Mol. Sci. 2025, 26, 1656. https://doi.org/10.3390/ijms26041656
Bosdou JK, Venetis CA, Anagnostis P, Savvaidou D, Chatzimeletiou K, Zepiridis L, Goulis DG, Grimbizis G, Kolibianakis EM. Association of Basal Serum Androgen Concentration with Follicles Number on the Day of Triggering Final Oocyte Maturation in Low Responders According to the Bologna Criteria: A Prospective Cohort Study. International Journal of Molecular Sciences. 2025; 26(4):1656. https://doi.org/10.3390/ijms26041656
Chicago/Turabian StyleBosdou, Julia K., Christos A. Venetis, Panagiotis Anagnostis, Despoina Savvaidou, Katerina Chatzimeletiou, Leonidas Zepiridis, Dimitrios G. Goulis, Grigoris Grimbizis, and Efstratios M. Kolibianakis. 2025. "Association of Basal Serum Androgen Concentration with Follicles Number on the Day of Triggering Final Oocyte Maturation in Low Responders According to the Bologna Criteria: A Prospective Cohort Study" International Journal of Molecular Sciences 26, no. 4: 1656. https://doi.org/10.3390/ijms26041656
APA StyleBosdou, J. K., Venetis, C. A., Anagnostis, P., Savvaidou, D., Chatzimeletiou, K., Zepiridis, L., Goulis, D. G., Grimbizis, G., & Kolibianakis, E. M. (2025). Association of Basal Serum Androgen Concentration with Follicles Number on the Day of Triggering Final Oocyte Maturation in Low Responders According to the Bologna Criteria: A Prospective Cohort Study. International Journal of Molecular Sciences, 26(4), 1656. https://doi.org/10.3390/ijms26041656







