Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue
Abstract
1. Introduction
2. Results
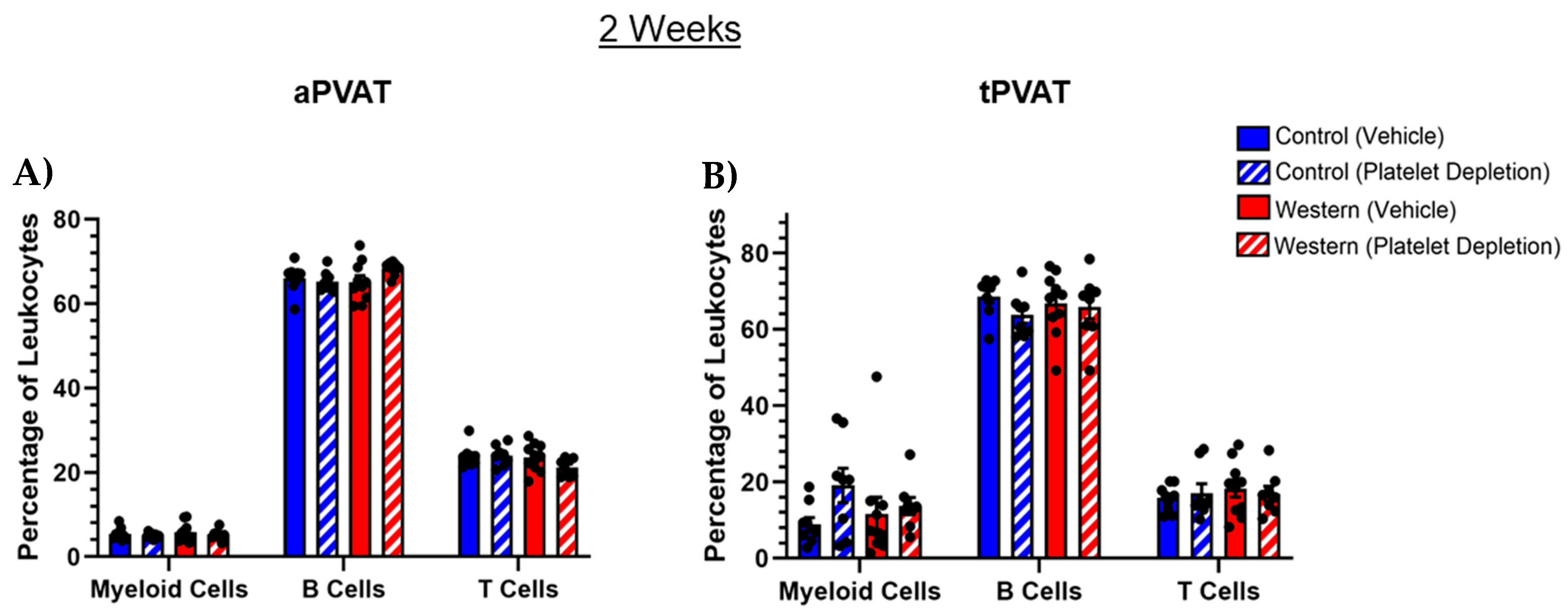
2.1. Leukocyte PVAT Composition with Acute Western Diet Feeding
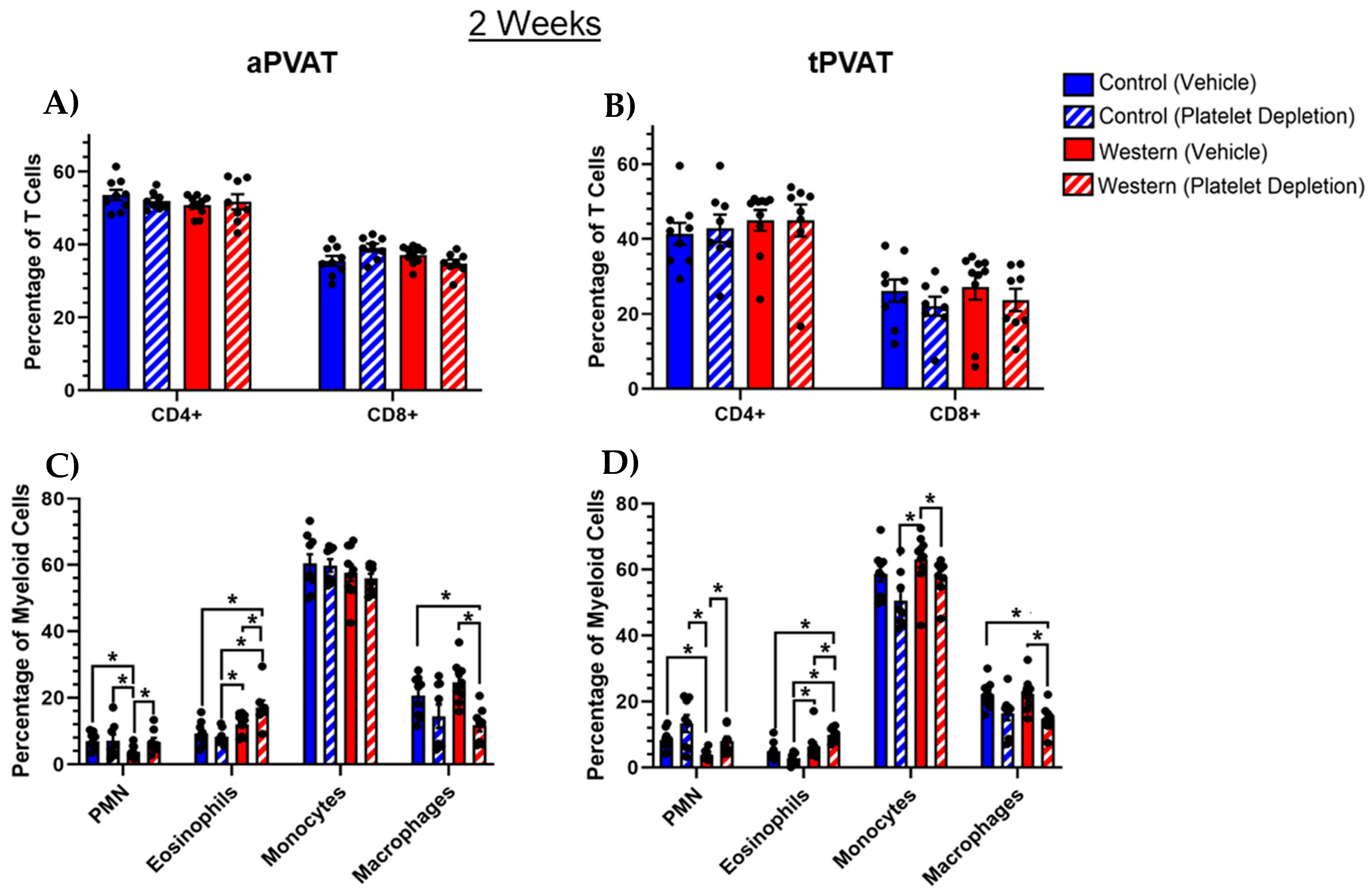
2.2. T and Myeloid Cell Subsets Within PVAT Following Acute Feeding
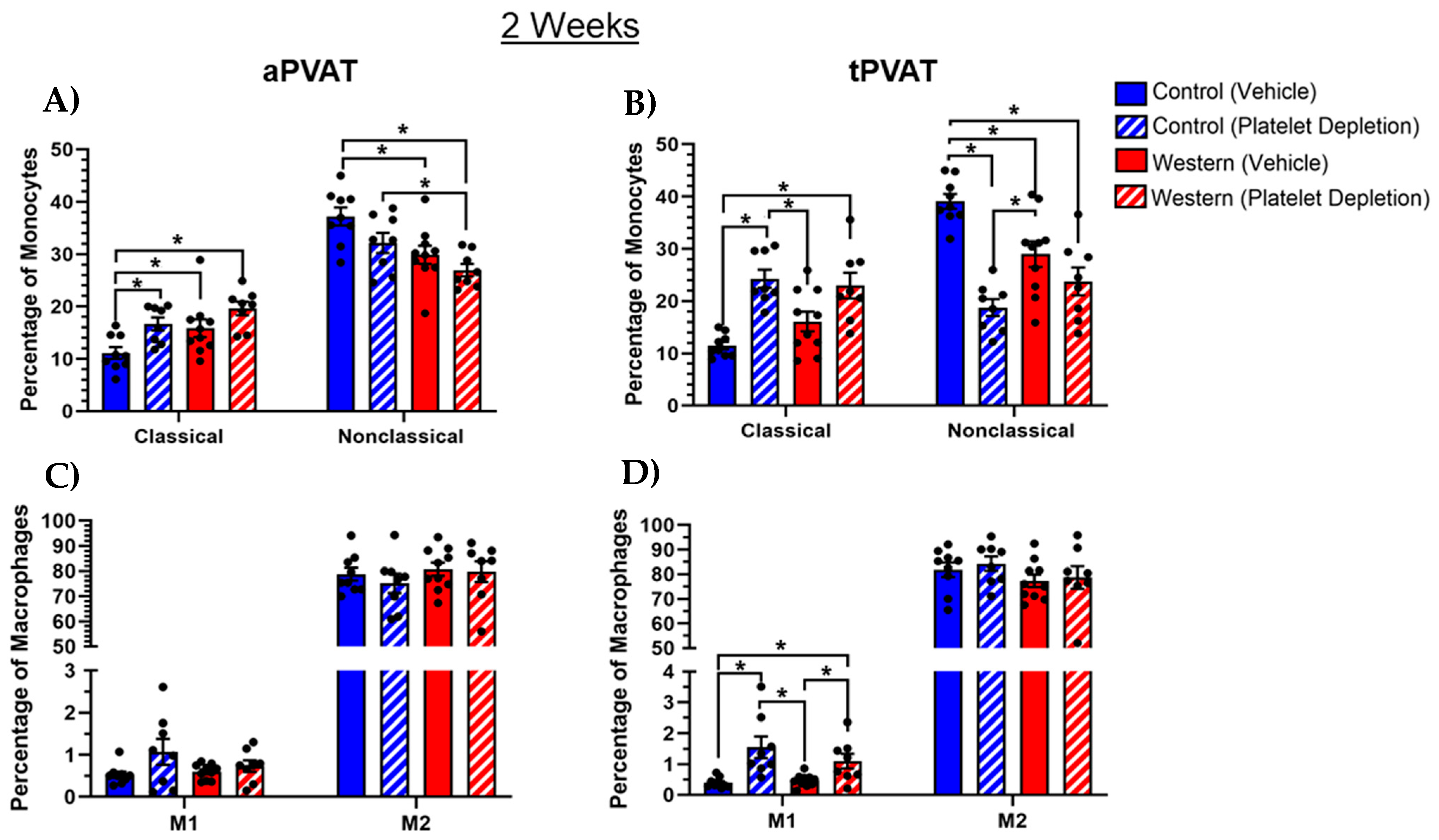
2.3. Platelet Modulation of Monocytes and Macrophages in PVAT After Acute Western Diet
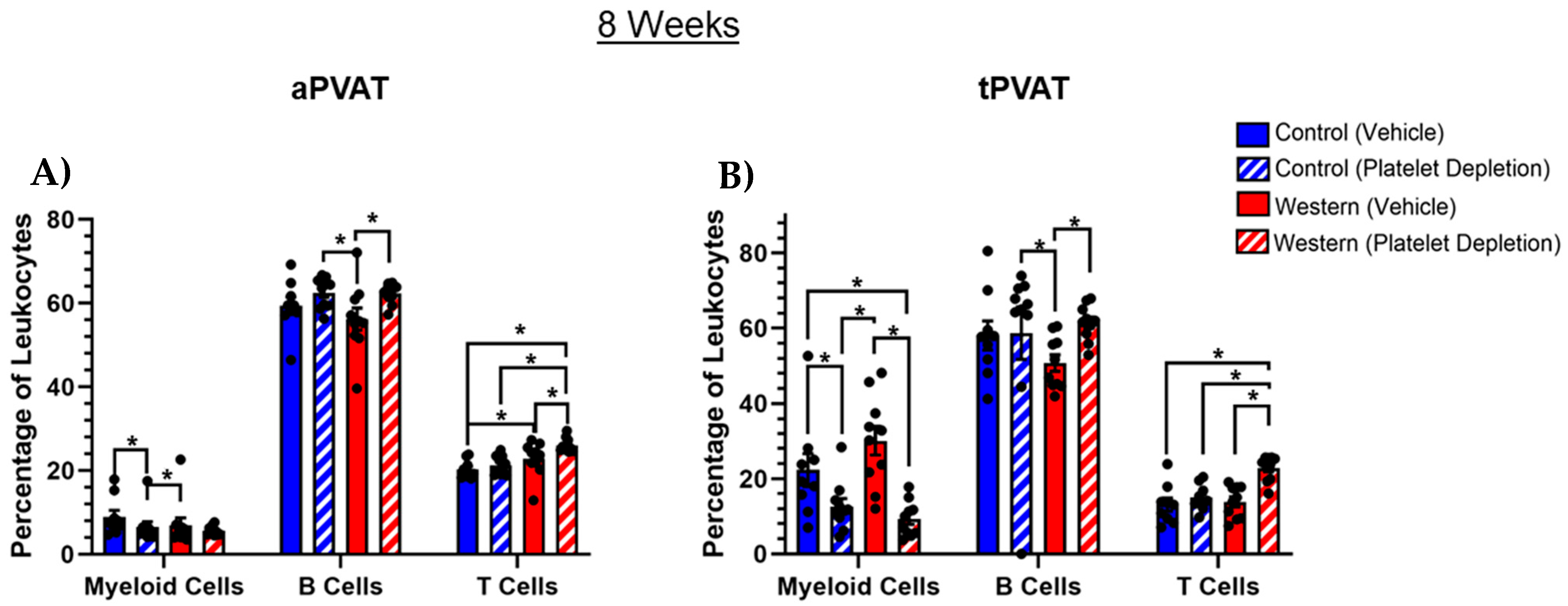
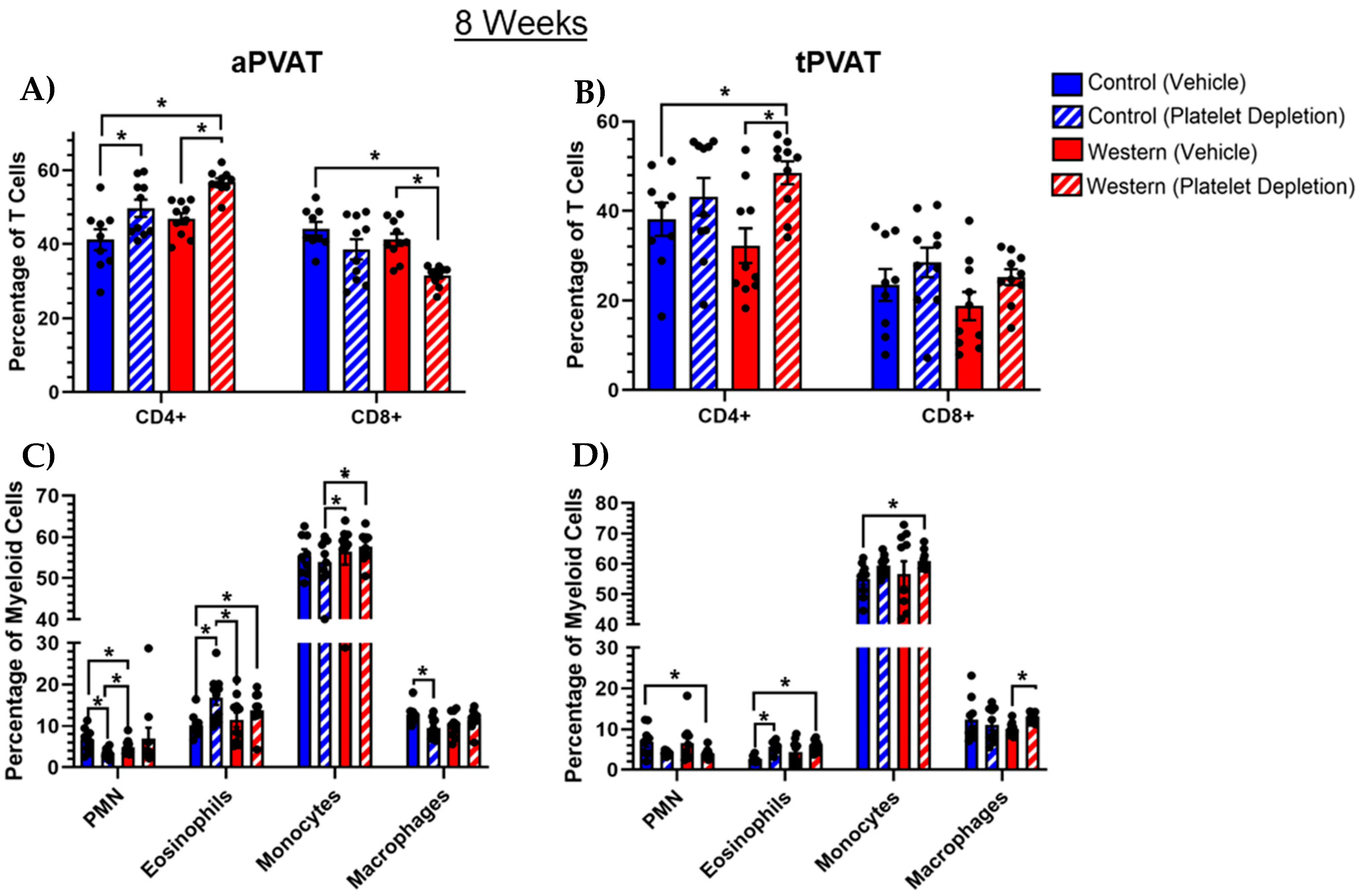
2.4. PVAT Leukocyte Content After Extended Western Diet Feeding
2.5. Myeloid Cell Subsets Shifted with Extended Western Diet Feeding and Platelet Depletion
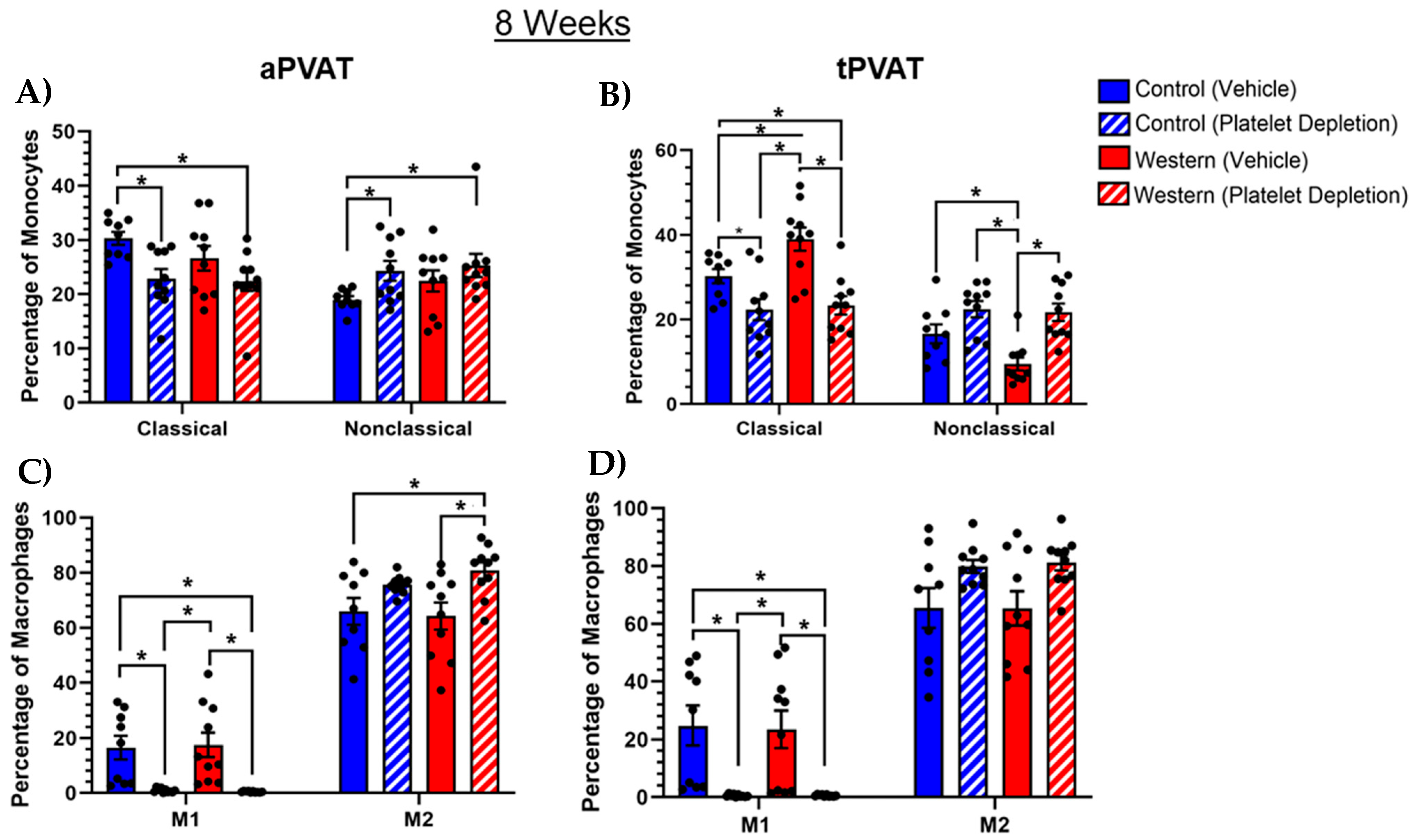
2.6. Platelets Influenced Monocyte and Macrophage Subsets with Extended Feeding
3. Discussion
4. Materials and Methods
4.1. Mouse Model and Diet Administration
4.2. Platelet Depletion
4.3. PVAT Collection and Processing
4.4. Flow Cytometry Preparation
4.5. Flow Cytometry Data Collection and Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.J. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr. Pharm. Des. 2007, 13, 2185–2192. [Google Scholar] [PubMed]
- Hillock-Watling, C.; Gotlieb, A.I. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc. Pathol. 2022, 61, 107459. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular adipose tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Flood, E.D.; Garver, H.; Fink, G.D.; Roccabianca, S. A New Function for Perivascular Adipose Tissue (PVAT): Assistance of Arterial Stress Relaxation. Sci. Rep. 2020, 10, 1807. [Google Scholar] [CrossRef]
- Zaborska, K.E.; Wareing, M.; Edwards, G.; Austin, C. Loss of anti-contractile effect of perivascular adipose tissue in offspring of obese rats. Int. J. Obes. 2016, 40, 1205–1214. [Google Scholar] [CrossRef]
- Li, H.-F.; Liu, H.-T.; Chen, P.-Y.; Lin, H.; Tseng, T.-L. Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3. iScience 2022, 25, 105631. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Zeng, Z.-h.; Teoh, K.; Sharma, A.M.; Abouzahr, L.; Cybulsky, I.; Lamy, A.; Semelhago, L.; Lee, R.M.K.W. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J. Thorac. Cardiovasc. Surg. 2005, 130, 1130–1136. [Google Scholar] [CrossRef]
- Bussey, C.E.; Withers, S.B.; Aldous, R.G.; Edwards, G.; Heagerty, A.M. Obesity-Related Perivascular Adipose Tissue Damage Is Reversed by Sustained Weight Loss in the Rat. Arter. Thromb. Vasc. Biol. 2016, 36, 1377–1385. [Google Scholar] [CrossRef]
- Karaca, Ü.; Schram, M.T.; Houben, A.J.; Muris, D.M.; Stehouwer, C.D. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res. Clin. Pract. 2014, 103, 382–387. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef]
- Dos Reis Costa, D.E.F.; Silveira, A.L.M.; Campos, G.P.; Nóbrega, N.R.C.; de Araújo, N.F.; de Figueiredo Borges, L.; Dos Santos Aggum Capettini, L.; Ferreira, A.V.M.; Bonaventura, D. High-Carbohydrate Diet Enhanced the Anticontractile Effect of Perivascular Adipose Tissue Through Activation of Renin-Angiotensin System. Front. Physiol. 2020, 11, 628101. [Google Scholar] [CrossRef] [PubMed]
- Sasoh, T.; Kugo, H.; Kondo, Y.; Miyamoto, K.; Minami, M.; Higashihara, M.; Kawamoto, H.; Takeshita, F.; Moriyama, T.; Zaima, N. Different effects of high-fat and high-sucrose diets on the physiology of perivascular adipose tissues of the thoracic and abdominal aorta. Adipocyte 2021, 10, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Fox, C.S. Perivascular adipose tissue and vascular disease. Clin. Lipidol. 2011, 6, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Matrougui, K.; Abd Elmageed, Z.; Kassan, M.; Choi, S.; Nair, D.; Gonzalez-Villalobos, R.A.; Chentoufi, A.A.; Kadowitz, P.; Belmadani, S.; Partyka, M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am. J. Pathol. 2011, 178, 434–441. [Google Scholar] [CrossRef]
- Itani, H.A.; McMaster, W.G., Jr.; Saleh, M.A.; Nazarewicz, R.R.; Mikolajczyk, T.P.; Kaszuba, A.M.; Konior, A.; Prejbisz, A.; Januszewicz, A.; Norlander, A.E.; et al. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension 2016, 68, 123–132. [Google Scholar] [CrossRef]
- Farias-Itao, D.S.; Pasqualucci, C.A.; de Andrade, R.A.; da Silva, L.F.F.; Yahagi-Estevam, M.; Lage, S.H.G.; Leite, R.E.P.; Campo, A.B.; Suemoto, C.K. Macrophage Polarization in the Perivascular Fat Was Associated With Coronary Atherosclerosis. J. Am. Heart Assoc. 2022, 11, e023274. [Google Scholar] [CrossRef]
- Shirai, T.; Hilhorst, M.; Harrison, D.G.; Goronzy, J.J.; Weyand, C.M. Macrophages in vascular inflammation—From atherosclerosis to vasculitis. Autoimmunity 2015, 48, 139–151. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Mishra, M. The heme oxygenase system selectively suppresses the proinflammatory macrophage m1 phenotype and potentiates insulin signaling in spontaneously hypertensive rats. Am. J. Hypertens. 2013, 26, 1123–1131. [Google Scholar] [CrossRef][Green Version]
- Stöger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- Kumar, R.K.; Yang, Y.; Contreras, A.G.; Garver, H.; Bhattacharya, S.; Fink, G.D.; Rockwell, C.E.; Watts, S.W. Phenotypic Changes in T Cell and Macrophage Subtypes in Perivascular Adipose Tissues Precede High-Fat Diet-Induced Hypertension. Front. Physiol. 2021, 12, 616055. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Sonmez, O.; Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Mussbacher, M.; Salzmann, M.; Assinger, A. Platelet-leukocyte interplay during vascular disease. Atherosclerosis 2020, 307, 109–120. [Google Scholar] [CrossRef]
- Li, C.; Ture, S.K.; Nieves-Lopez, B.; Blick-Nitko, S.K.; Maurya, P.; Livada, A.C.; Stahl, T.J.; Kim, M.; Pietropaoli, A.P.; Morrell, C.N. Thrombocytopenia Independently Leads to Changes in Monocyte Immune Function. Circ. Res. 2024, 134, 970–986. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef]
- Rolling, C.C.; Barrett, T.J.; Berger, J.S. Platelet-monocyte aggregates: Molecular mediators of thromboinflammation. Front. Cardiovasc. Med. 2023, 10, 960398. [Google Scholar] [CrossRef]
- Corken, A.; Russell, S.; Dent, J.; Post, S.R.; Ware, J. Platelet glycoprotein Ib-IX as a regulator of systemic inflammation. Arter. Thromb. Vasc. Biol. 2014, 34, 996–1001. [Google Scholar] [CrossRef]
- Carestia, A.; Mena, H.A.; Olexen, C.M.; Ortiz Wilczyñski, J.M.; Negrotto, S.; Errasti, A.E.; Gómez, R.M.; Jenne, C.N.; Carrera Silva, E.A.; Schattner, M. Platelets Promote Macrophage Polarization toward Pro-inflammatory Phenotype and Increase Survival of Septic Mice. Cell Rep. 2019, 28, 896–908.e5. [Google Scholar] [CrossRef]
- Zuchtriegel, G.; Uhl, B.; Puhr-Westerheide, D.; Pörnbacher, M.; Lauber, K.; Krombach, F.; Reichel, C.A. Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol. 2016, 14, e1002459. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol. 2018, 9, 2712. [Google Scholar] [CrossRef] [PubMed]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef] [PubMed]
- Graca, F.A.; Stephan, A.; Minden-Birkenmaier, B.A.; Shirinifard, A.; Wang, Y.D.; Demontis, F.; Labelle, M. Platelet-derived chemokines promote skeletal muscle regeneration by guiding neutrophil recruitment to injured muscles. Nat. Commun. 2023, 14, 2900. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Rua, R.; McGavern, D.B. Elucidation of monocyte/macrophage dynamics and function by intravital imaging. J. Leukoc. Biol. 2015, 98, 319–332. [Google Scholar] [CrossRef]
- Xu, Z.J.; Gu, Y.; Wang, C.Z.; Jin, Y.; Wen, X.M.; Ma, J.C.; Tang, L.J.; Mao, Z.W.; Qian, J.; Lin, J. The M2 macrophage marker CD206: A novel prognostic indicator for acute myeloid leukemia. Oncoimmunology 2020, 9, 1683347. [Google Scholar] [CrossRef]
- Nawaz, A.; Bilal, M.; Fujisaka, S.; Kado, T.; Aslam, M.R.; Ahmed, S.; Okabe, K.; Igarashi, Y.; Watanabe, Y.; Kuwano, T.; et al. Depletion of CD206+ M2-like macrophages induces fibro-adipogenic progenitors activation and muscle regeneration. Nat. Commun. 2022, 13, 7058. [Google Scholar] [CrossRef]
- Lisi, L.; Ciotti, G.M.; Braun, D.; Kalinin, S.; Currò, D.; Dello Russo, C.; Coli, A.; Mangiola, A.; Anile, C.; Feinstein, D.L.; et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 2017, 645, 106–112. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, R.; Geng, S.; Peng, L.; Jayaraman, P.; Chen, C.; Xu, F.; Yang, J.; Li, Q.; Zheng, H.; et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 2015, 6, 6676. [Google Scholar] [CrossRef]
- Hildebrand, S.; Stümer, J.; Pfeifer, A. PVAT and Its Relation to Brown, Beige, and White Adipose Tissue in Development and Function. Front. Physiol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, S.; Pan, D.; Welch, H.C. Platelets in neutrophil recruitment to sites of inflammation. Curr. Opin. Hematol. 2017, 24, 23–31. [Google Scholar] [CrossRef]
- Lutz, M.S.; Klimovich, B.; Maurer, S.; Heitmann, J.S.; Märklin, M.; Zekri, L.; Jung, G.; Salih, H.R.; Hinterleitner, C. Platelets subvert antitumor efficacy of T cell-recruiting bispecific antibodies. J. Immunother. Cancer 2022, 10, e003655. [Google Scholar] [CrossRef]
- Schulz, C.; von Brühl, M.L.; Barocke, V.; Cullen, P.; Mayer, K.; Okrojek, R.; Steinhart, A.; Ahmad, Z.; Kremmer, E.; Nieswandt, B.; et al. EMMPRIN (CD147/basigin) mediates platelet-monocyte interactions in vivo and augments monocyte recruitment to the vascular wall. J. Thromb. Haemost. 2011, 9, 1007–1019. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokari, R.; Matsunaga, H.; Takebayashi, K.; Watanabe, C.; Komoto, S.; Okada, Y.; Kurihara, C.; Kawaguchi, A.; Nagao, S.; et al. P-selectin-dependent monocyte recruitment through platelet interaction in intestinal microvessels of LPS-treated mice. Microcirculation 2008, 15, 441–450. [Google Scholar] [CrossRef]
- Nishio, H.; Saita, Y.; Kobayashi, Y.; Takaku, T.; Fukusato, S.; Uchino, S.; Wakayama, T.; Ikeda, H.; Kaneko, K. Platelet-rich plasma promotes recruitment of macrophages in the process of tendon healing. Regen. Ther. 2020, 14, 262–270. [Google Scholar] [CrossRef]
- Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 2336. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Hawwari, I.; Rossnagel, L.; Rosero, N.; Maasewerd, S.; Vasconcelos, M.B.; Jentzsch, M.; Demczuk, A.; Teichmann, L.L.; Meffert, L.; Bertheloot, D.; et al. Platelet transcription factors license the pro-inflammatory cytokine response of human monocytes. EMBO Mol. Med. 2024, 16, 1901–1929. [Google Scholar] [CrossRef]
- Fu, G.; Deng, M.; Neal, M.D.; Billiar, T.R.; Scott, M.J. Platelet-Monocyte Aggregates: Understanding Mechanisms and Functions in Sepsis. Shock 2021, 55, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Martins-Gonçalves, R.; Palhinha, L.; Azevedo-Quintanilha, I.G.; de Campos, M.M.; Sacramento, C.Q.; Temerozo, J.R.; Soares, V.C.; Dias, S.S.G.; Teixeira, L.; et al. Platelet-monocyte interaction amplifies thromboinflammation through tissue factor signaling in COVID-19. Blood Adv. 2022, 6, 5085–5099. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Maurya, P.; Nieves-Lopez, B.; Ture, S.; Morrell, C.N. Platelet Mediated Monocyte/Macrophage Immune Training. Blood 2021, 138, 3127. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C. Platelet-macrophage partnership in innate immunity and inflammation. Nat. Immunol. 2013, 14, 768–770. [Google Scholar] [CrossRef]
- Okubo, K.; Kurosawa, M.; Kamiya, M.; Urano, Y.; Suzuki, A.; Yamamoto, K.; Hase, K.; Homma, K.; Sasaki, J.; Miyauchi, H.; et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat. Med. 2018, 24, 232–238. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Griffin, K.A.; Bidani, A.K.; Davisson, R.L.; Hall, J.E. Recommendations for blood pressure measurement in humans and experimental animals: Part 2: Blood pressure measurement in experimental animals: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arter. Thromb. Vasc. Biol. 2005, 25, e22–e33. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H77–H111. [Google Scholar] [CrossRef]
- Salzmann, M.; Schrottmaier, W.C.; Kral-Pointner, J.B.; Mussbacher, M.; Volz, J.; Hoesel, B.; Moser, B.; Bleichert, S.; Morava, S.; Nieswandt, B.; et al. Genetic platelet depletion is superior in platelet transfusion compared to current models. Haematologica 2020, 105, 1738–1749. [Google Scholar] [CrossRef]
- Steubing, R.D.; Szepanowski, F.; David, C.; Mohamud Yusuf, A.; Mencl, S.; Mausberg, A.-K.; Langer, H.F.; Sauter, M.; Deuschl, C.; Forsting, M.; et al. Platelet depletion does not alter long-term functional outcome after cerebral ischaemia in mice. Brain Behav. Immun.—Health 2022, 24, 100493. [Google Scholar] [CrossRef]
- Morodomi, Y.; Kanaji, S.; Won, E.; Ruggeri, Z.M.; Kanaji, T. Mechanisms of anti-GPIbα antibody-induced thrombocytopenia in mice. Blood 2020, 135, 2292–2301. [Google Scholar] [CrossRef]
- Luis, T.C.; Barkas, N.; Carrelha, J.; Giustacchini, A.; Mazzi, S.; Norfo, R.; Wu, B.; Aliouat, A.; Guerrero, J.A.; Rodriguez-Meira, A.; et al. Perivascular niche cells sense thrombocytopenia and activate hematopoietic stem cells in an IL-1 dependent manner. Nat. Commun. 2023, 14, 6062. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Huang, Y.; He, B. New Insights into Adipose Tissue Macrophages in Obesity and Insulin Resistance. Cells 2022, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kim, H.W.; Weintraub, N.L. Macrophage immunometabolism in perivascular adipose tissue. Arter. Thromb. Vasc. Biol. 2021, 41, 731–733. [Google Scholar] [CrossRef]
- Bergmeier, W.; Rackebrandt, K.; Schröder, W.; Zirngibl, H.; Nieswandt, B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood 2000, 95, 886–893. [Google Scholar] [CrossRef]
- Paul, D.S.; Bergmeier, W. Novel Mouse Model for Studying Hemostatic Function of Human Platelets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1891–1904. [Google Scholar] [CrossRef]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-Mediated Modulation of Adaptive Immunity: A Communication Link between Innate and Adaptive Immune Compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corken, A.; Weinkopff, T.; Wahl, E.C.; Sikes, J.D.; Thakali, K.M. Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue. Int. J. Mol. Sci. 2025, 26, 1625. https://doi.org/10.3390/ijms26041625
Corken A, Weinkopff T, Wahl EC, Sikes JD, Thakali KM. Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue. International Journal of Molecular Sciences. 2025; 26(4):1625. https://doi.org/10.3390/ijms26041625
Chicago/Turabian StyleCorken, Adam, Tiffany Weinkopff, Elizabeth C. Wahl, James D. Sikes, and Keshari M. Thakali. 2025. "Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue" International Journal of Molecular Sciences 26, no. 4: 1625. https://doi.org/10.3390/ijms26041625
APA StyleCorken, A., Weinkopff, T., Wahl, E. C., Sikes, J. D., & Thakali, K. M. (2025). Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue. International Journal of Molecular Sciences, 26(4), 1625. https://doi.org/10.3390/ijms26041625




