Physiological and Transcriptome Analysis Provide Insights into the Effects of Low and High Selenium on Methionine and Starch Metabolism in Rice Seedlings
Abstract
1. Introduction
2. Results
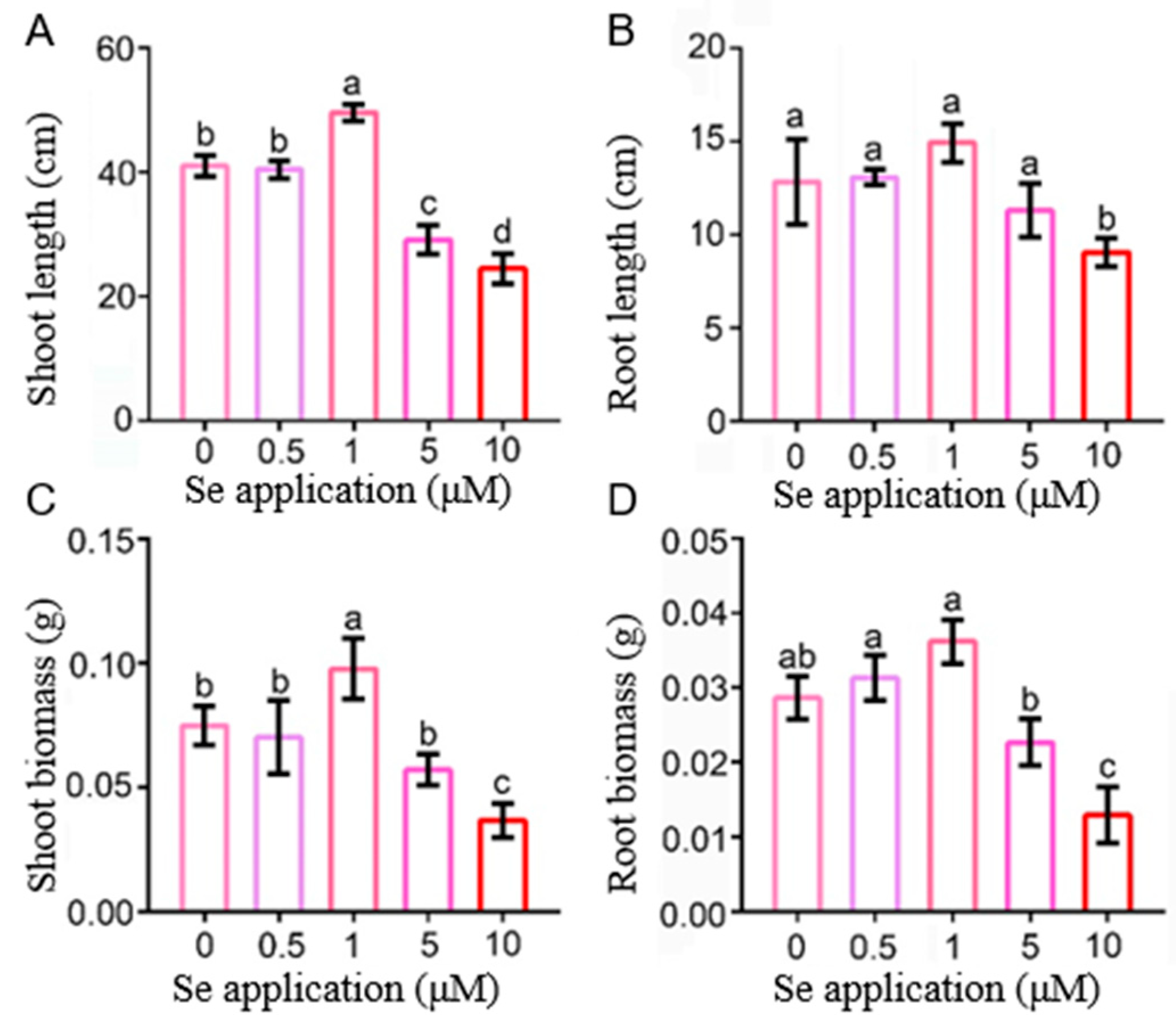
2.1. The Effects of Low and High Se on the Phenotype of Rice Seedlings
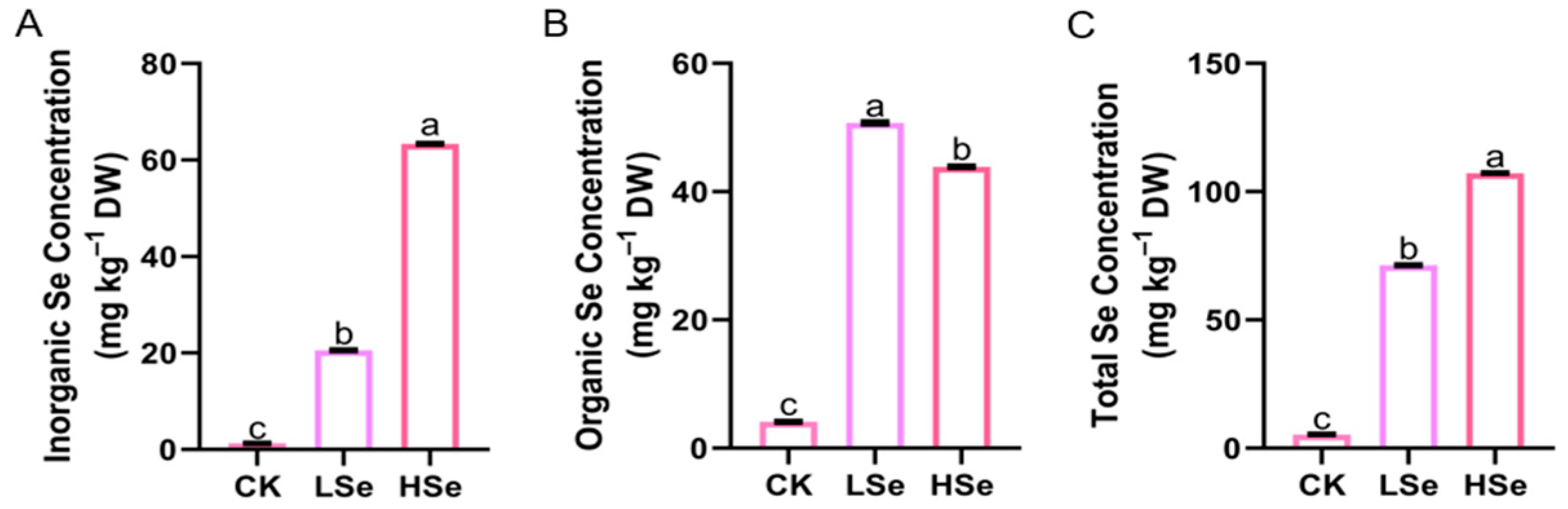
2.2. The Effects of Low and High Se on the Concentration of Se in Rice Seedings
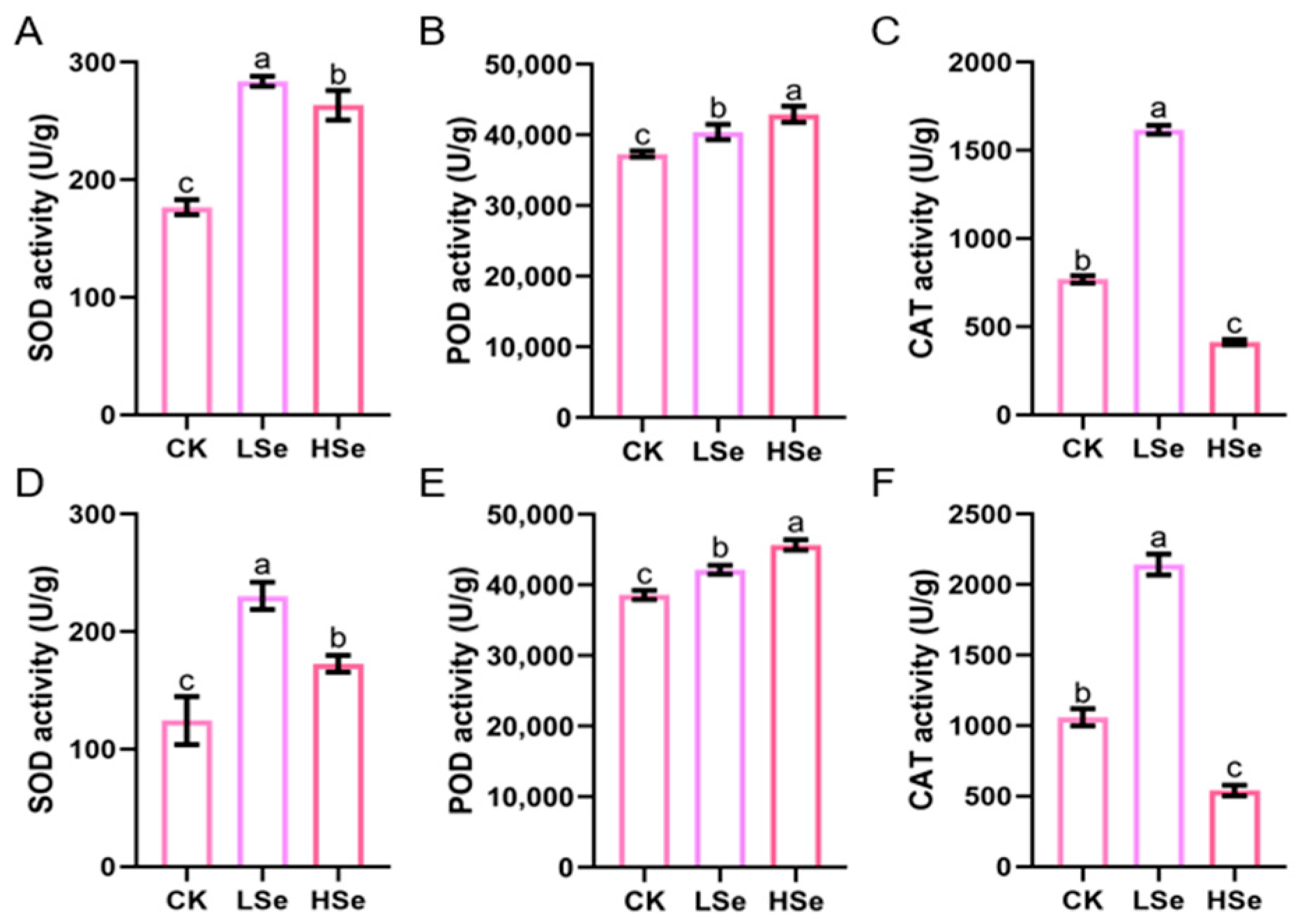
2.3. The Effects of Low and High Se on the Antioxidant Enzyme Activities of Rice Seedings
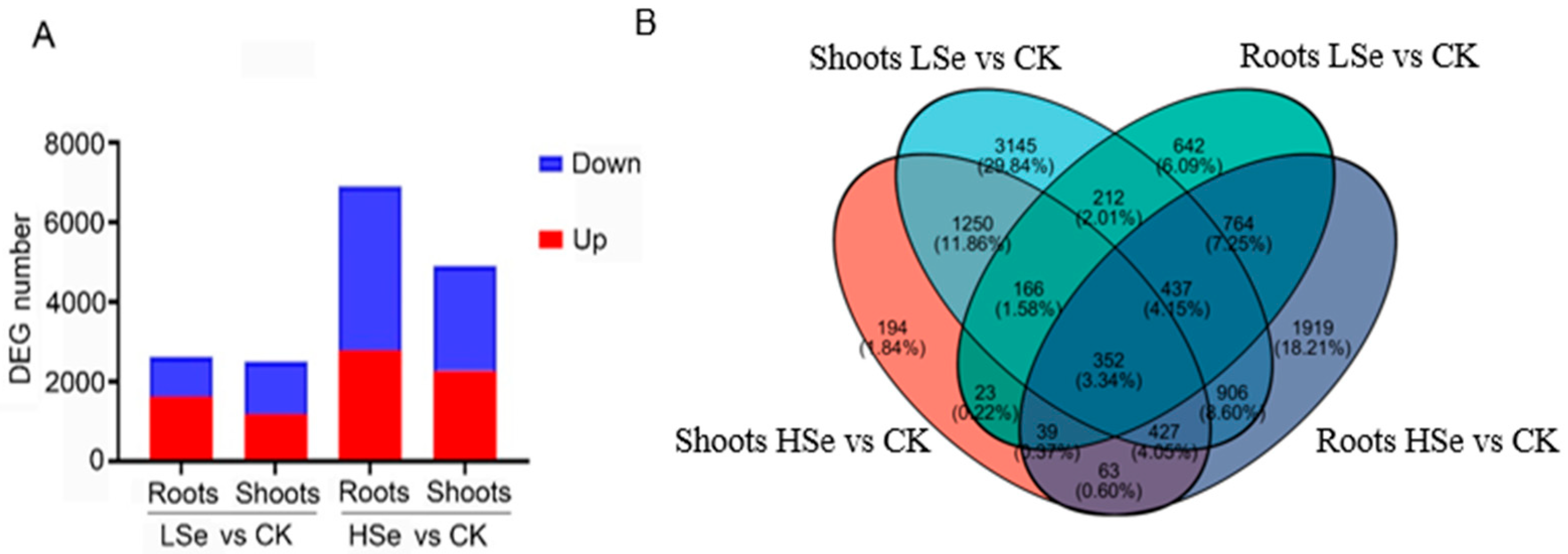
2.4. The Different Impacts of Low and High Se on the Differential Gene Expression in Rice Seedlings
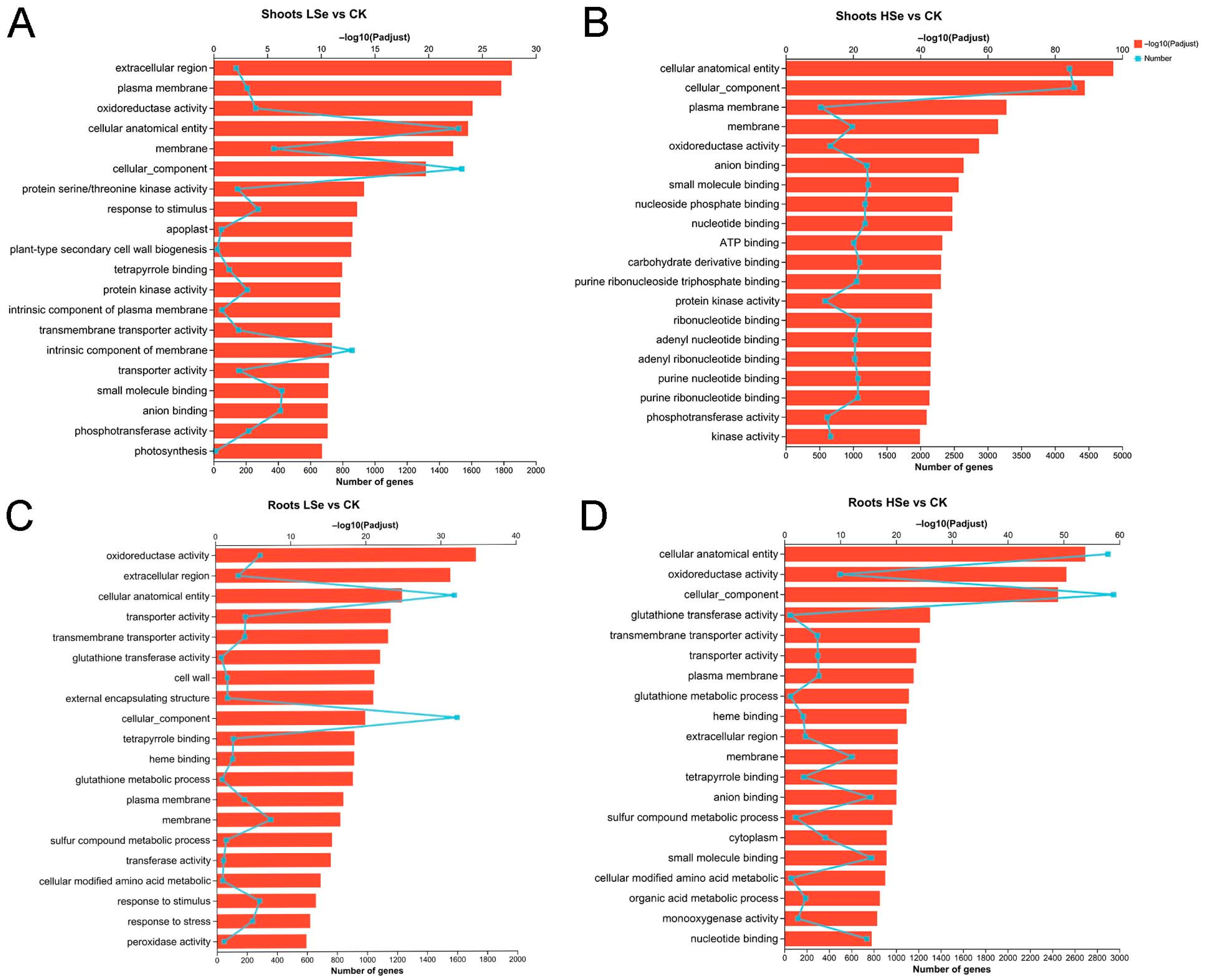
2.5. GO Enrichment Analysis of DEGs
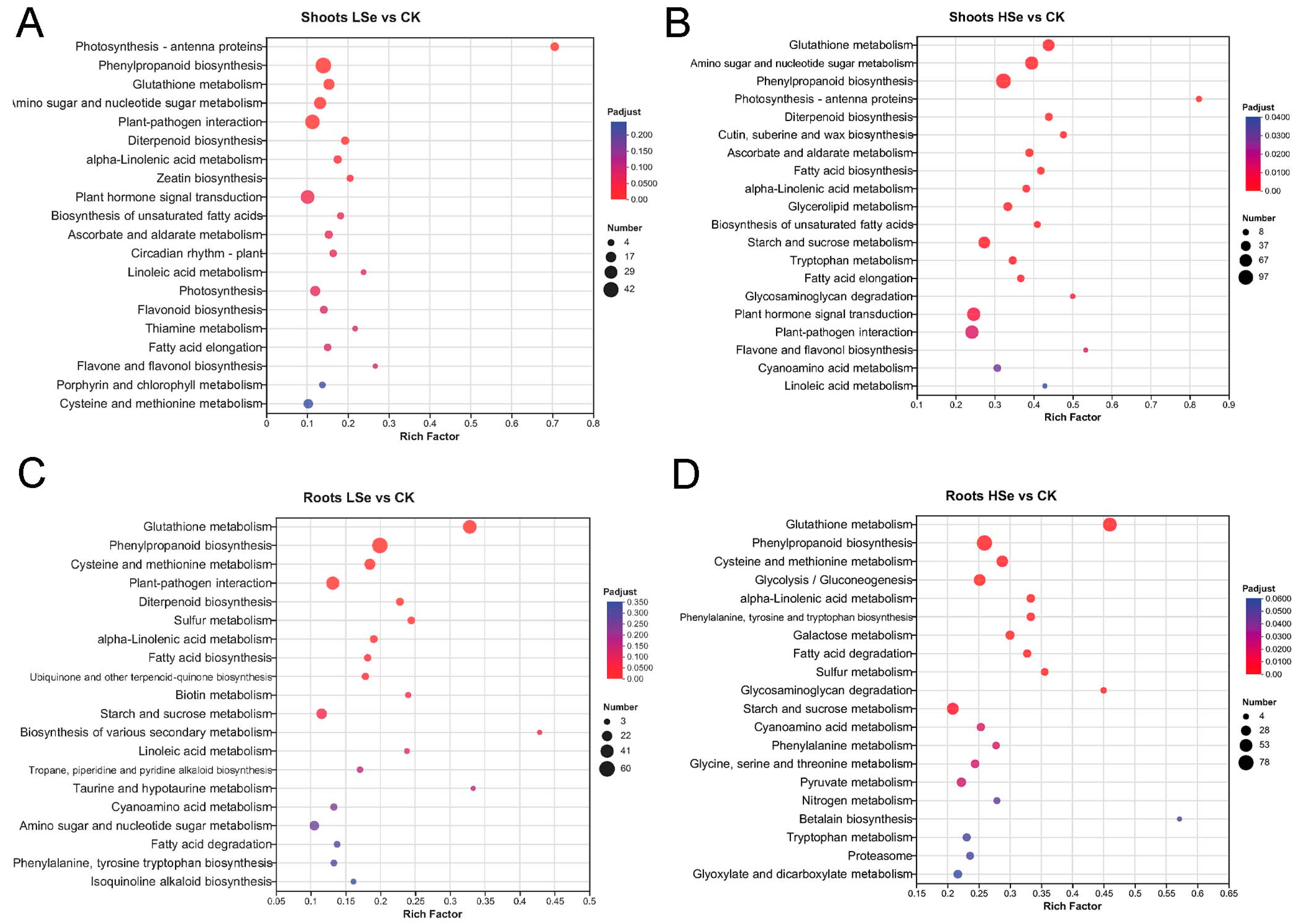
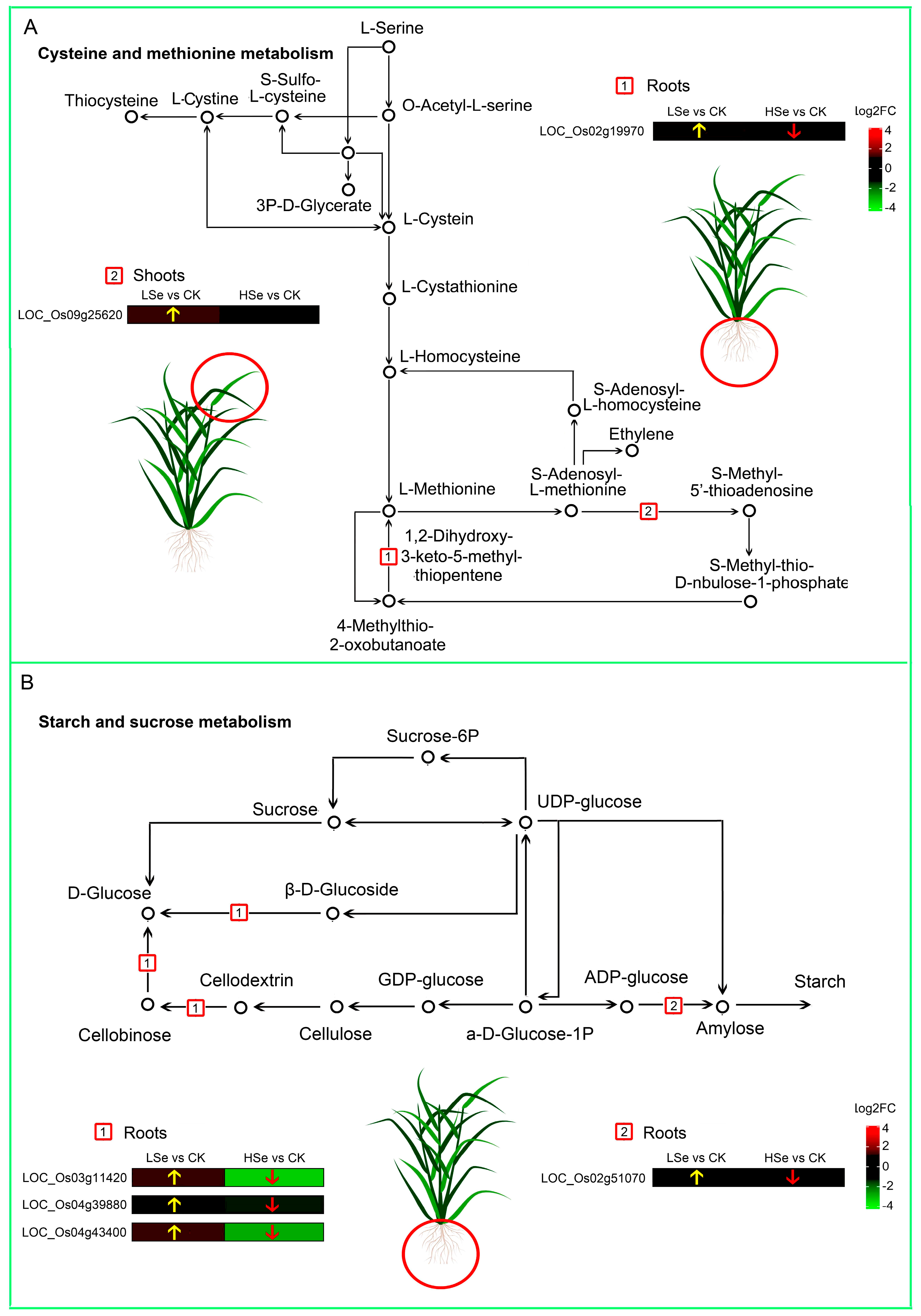
2.6. Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of DEGs
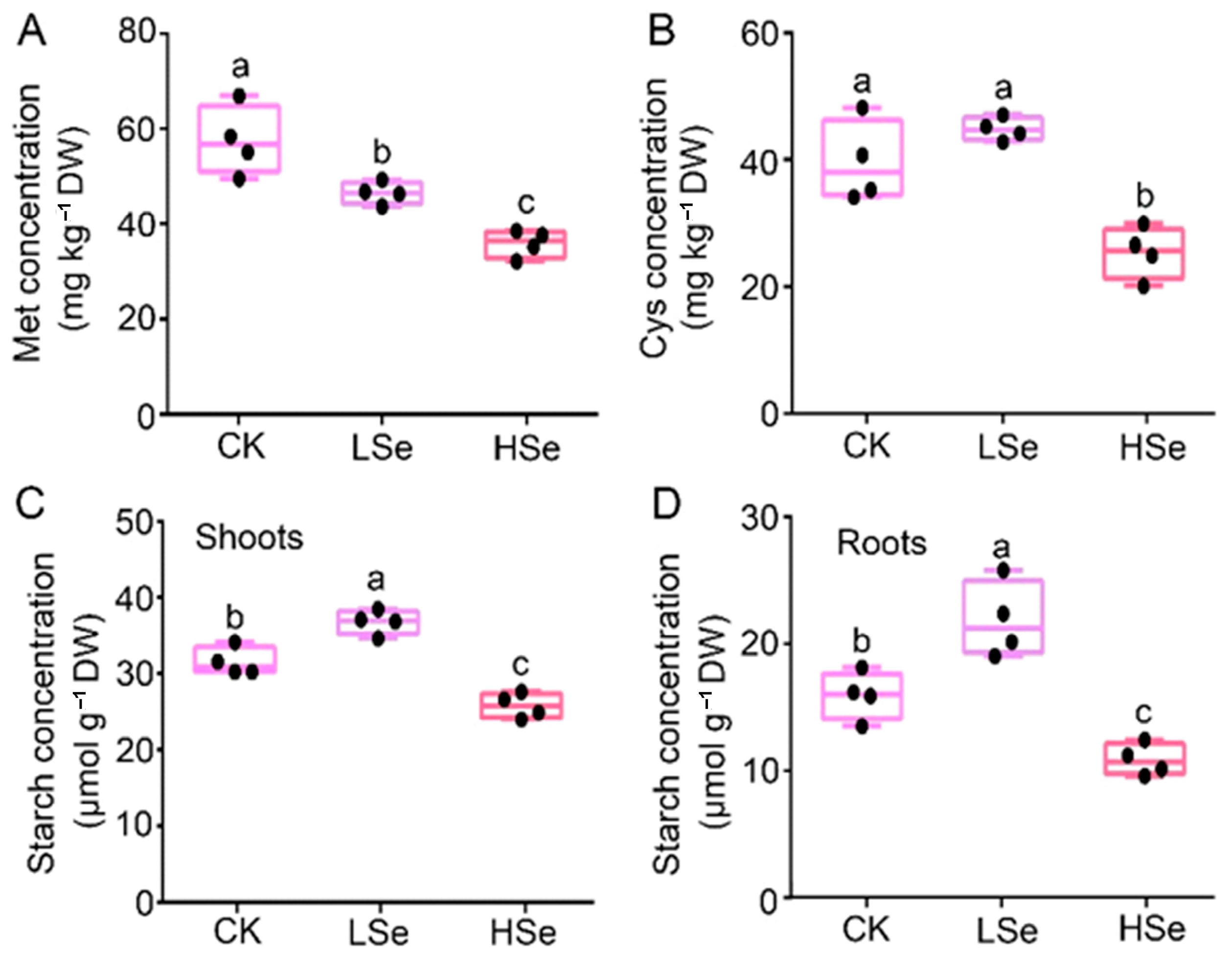
2.7. The Effects of High and Low Se on the Accumulation of Starch, Cys and Met
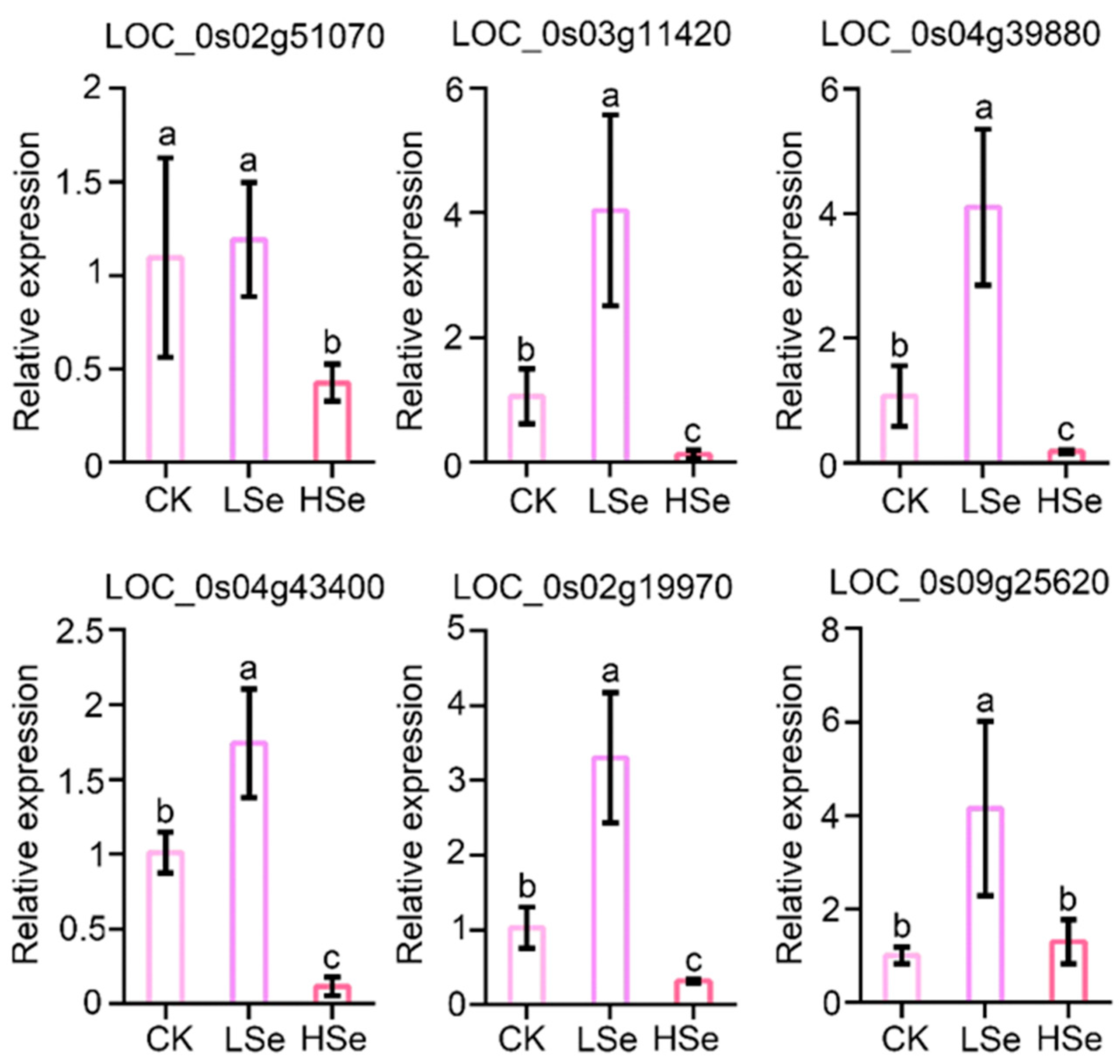
2.8. Validation of RNA Sequencing Date by Quantative Real-Time PCR
3. Discussion
4. Materials and Methods
4.1. Rice Cultivation and Treatment Methods
4.2. Detection of Total Se and Different Forms of Se in Rice Seedlings
4.3. Determination of Antioxidant Enzyme Activities
4.4. Transcriptome Sequencing Analysis
4.4.1. Sampling and RNA Extraction
4.4.2. cDNA Library Construction and RNA Sequencing
4.4.3. Sequence Assembly and Gene Annotation and Quantification
4.4.4. Differentially Expressed Genes (DEGs) and Their GO and KEGG Pathway Enrichment Analyses
4.5. qRT-PCR Analysis
4.6. The Analysis of Amino Acid and Starch of Rice Seedlings
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2013, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, H.; Guo, Y.; Fan, B.; Huang, Y.; Mao, X.; Liang, K.; Hu, Z.; Sun, X.; Fang, Y.; et al. Benefit-risk assessment of dietary selenium and its associated metals intake in China (2017–2019): Is current selenium-richagro-food safe enough? J. Hazard. Mater. 2020, 398, 123224. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, T.; Xu, Y.; Chen, W.; Liu, B.; Zhang, L.; Liu, D.; Zhang, H. Comparative transcriptome analysis of two selenium-accumulating genoty pes of Aegilops tauschii Coss. in response to selenium. BMC Genet. 2019, 20, 9. [Google Scholar] [CrossRef]
- Lucca, P.; Poletti, S.; Sautter, C. Genetic engineering approaches to enrich rice with iron and vitamin A. Physiol. Plant. 2006, 126, 291–303. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Yasumoto, K. Selenium contents of rice grown at various sites in Japan. J. Food Compos. Anal. 1987, 1, 71–75. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Supriatin, S.; Weng, L.; Comans, R.N. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total. Environ. 2015, 532, 368–382. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The role of selenium and nano selenium on physiological responses in p lant: A review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Effect of Selenium on the Responses Induced by Heat Stress in Plant Cell Cultures. Plants 2018, 7, 64. [Google Scholar] [CrossRef]
- Han, D.; Li, X.; Xiong, S.; Tu, S.; Chen, Z.; Li, J.; Xie, Z. Selenium uptake, speciation and stressed response of Nicotiana tabacum L. Environ. Exp. Bot. 2013, 95, 6–14. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Element Res. 2009, 130, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Gou, Y.; Yu, T.; Cong, X.; Gui, J.; Zhu, Z.; Zhang, W.; Liao, Y.; Ye, J.; Cheng, S.; et al. Effects of selenate on Se, flavonoid, and glucosinolate in broccoli florets by combined transcriptome and metabolome analyses. Food Res. Int. 2021, 146, 110463. [Google Scholar] [CrossRef]
- Yang, X.; Liao, X.; Yu, L.; Rao, S.; Chen, Q.; Zhu, Z.; Cong, X.; Zhang, W.; Ye, J.; Cheng, S.; et al. Combined metabolome and transcriptome analysis reveal the mechanism of selenate influence on the growth and quality of cabbage (Brassica oleracea var. capitata L.). Food Res. Int. 2022, 156, 111135. [Google Scholar] [CrossRef]
- Li, L.; Wu, S.; Wang, S.; Shi, X.; Cheng, S.; Cheng, H. Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard. Agronomy 2023, 13, 1425. [Google Scholar] [CrossRef]
- Rao, S.; Yu, T.; Cong, X.; Lai, X.; Xiang, J.; Cao, J.; Liao, X.; Gou, Y.; Chao, W.; Xue, H.; et al. Transcriptome, proteome, and metabolome reveal the mechanism of tolerance to selenate toxicity in Cardamine violifolia. J. Hazard. Mater. 2021, 406, 124283. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Siddiqui, N.; Fujita, M.; Tran, L.-S.P. Phenotypical, physiological and biochemical analyses provide insight into selenium-induced phytotoxicity in rice plants. Chemosphere 2017, 178, 212–223. [Google Scholar] [CrossRef]
- Gouveia, G.C.C.; Galindo, F.S.; Lanza, M.G.D.B.; Silva, A.C.d.R.; Mateus, M.P.d.B.; da Silva, M.S.; Tavanti, R.F.R.; Tavanti, T.R.; Lavres, J.; dos Reis, A.R. Selenium toxicity stress-induced phenotypical, biochemical and physiological responses in rice plants: Characterization of symptoms and plant metabolic adjustment. Ecotoxicol. Environ. Saf. 2020, 202, 110916. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Tananuwong, K.; Shoemaker, C.F.; Chun, A.; Tanadul, O.; Labavitch, J.M.; Beckles, D.M. Effects of timing and severity of salinity stress on rice (Oryza sativa L.) yield, grain composition, and starch functionality. J. Agric. Food Chem. 2015, 63, 2296–2304. [Google Scholar] [CrossRef]
- Kumari, M.; Asthir, B. Transformation of Sucrose to Starch and Protein in Rice Leaves and Grains under Two Establishment Methods. Rice Sci. 2016, 23, 255–265. [Google Scholar] [CrossRef]
- Li, G.; Hu, Q.; Shi, Y.; Cui, K.; Nie, L.; Huang, J.; Peng, S. Low Nitrogen Application Enhances Starch-Metabolizing Enzyme Activity and Improves Accumulation and Translocation of Non-structural Carbohydrates in Rice Stems. Front. Plant Sci. 2018, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Tian, J.; Long, Y.; Ma, H.; Tian, M.; Liu, X.; Yin, G.; Wang, L. Metabolomics and transcriptomics analyses provide new insights into the nutritional quality during the endosperm development of different ploidy rice. Front. Plant Sci. 2023, 14, 1210134. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Hoefgen, R.; Hesse, H. Improving the nutritive value of rice seeds: Elevation of cysteine and methionine contents in rice plants by ectopic expression of a bacterial serine acetyltransferase. J. Exp. Bot. 2012, 63, 5991–6001. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Xu, L.; Ma, A.; Zhuang, G.; Huo, S.; Zou, B.; Qian, J.; Cui, Y. Selenium volatilization in plants, microalgae, and microorganisms. Heliyon 2024, 10, e26023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, Z.; Zhan, C.; Qin, T.; Wang, L.; Zhang, X. Environmentally relevant concentrations of selenite trigger reproductive toxicity by affecting oocyte development and promoting larval apoptosis. Environ. Pollut. 2022, 316 Pt 1, 120648. [Google Scholar] [CrossRef]
- Chan, K.X.; Phua, S.Y.; Van Breusegem, F. Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 2019, 70, 4237–4250. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, V.; Takabe, T.; Rai, V.; Singh, N.K. Elucidation of salt-tolerance metabolic pathways in contrasting rice g enotypes and their segregating progenies. Plant Cell Rep. 2016, 35, 1273–1286. [Google Scholar] [CrossRef]
- Whitcomb, S.J.; Rakpenthai, A.; Brückner, F.; Fischer, A.; Parmar, S.; Erban, A.; Kopka, J.; Hawkesford, M.J.; Hoefgen, R. Cysteine and Methionine Biosynthetic Enzymes Have Distinct Effects on Seed Nutritional Quality and on Molecular Phenotypes Associated With Accumulation of a Methionine-Rich Seed Storage Protein in Rice. Front. Plant Sci. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Malheiros, R.S.P.; Costa, L.C.; Ávila, R.T.; Pimenta, T.M.; Teixeira, L.S.; Brito, F.A.L.; Zsögön, A.; Araújo, W.L.; Ribeiro, D.M. Selenium downregulates auxin and ethylene biosynthesis in rice seedlings to modify primary metabolism and root architecture. Planta 2019, 250, 333–345. [Google Scholar] [CrossRef]
- Chao, W.; Rao, S.; Chen, Q.; Zhang, W.; Liao, Y.; Ye, J.; Cheng, S.; Yang, X.; Xu, F. Advances in Research on the Involvement of Selenium in Regulating Plant Ecosystems. Plants 2022, 11, 2712. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, J.; Li, J. The alleviating effects and underlying mechanisms of exogenous selenium on both Sb(III) and Sb(V) toxicity in rice seedlings (Oryza sativa L.). Environ. Sci. Pollut. Res. 2023, 30, 89927–89941. [Google Scholar] [CrossRef]
- Yan, J.; Chen, X.; Zhu, T.; Zhang, Z.; Fan, J. Effects of Selenium Fertilizer Application on Yield and Selenium Accumulation Characteristics of Different Japonica Rice Varieties. Sustainability 2021, 13, 10284. [Google Scholar] [CrossRef]
- Liang, Y.; Su, Y.; Li, L.; Huang, X.; Panhwar, F.H.; Zheng, T.; Tang, Z.; Ei, H.H.; Farooq, M.U.; Zeng, R.; et al. Quick selenium accumulation in the selenium-rich rice and its physiological responses in changing selenium environments. BMC Plant Biol. 2019, 19, 559. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, X.; Li, L.; Muhammad, Z.A.; Li, M.; Zheng, T.; Guo, Z.; Zhang, Y.; Luo, D.; Ye, X.; et al. Comparative Responses of Silicon to Reduce Cadmium and Enrich Selenium in Rice Varieties. Foods 2023, 12, 1656. [Google Scholar] [CrossRef]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress to lerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Luo, H.; Xing, P.; Liu, J.; Pan, S.; Tang, X.; Duan, M. Selenium improved antioxidant response and photosynthesis in fragrant rice (Oryza sativa L.) seedlings during drought stress. Physiol. Mol. Biol. Plants 2021, 27, 2849–2858. [Google Scholar] [CrossRef]
- Guo, K.; Yao, Y.; Yang, M.; Li, Y.; Wu, B.; Lin, X. Transcriptome sequencing and analysis reveals the molecular response to selenium stimuli in Pueraria lobata (willd.) Ohwi. PeerJ 2020, 8, e8768. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, B.; Zhao, C.-R.; Sun, J.; Lai, Q.; Yu, C. Comparative de novo transcriptomics and untargeted metabolomic analyses elucidate complicated mechanisms regulating celery (Apium graveolens L.) responses to selenium stimuli. PLoS ONE 2019, 14, e0226752. [Google Scholar] [CrossRef]
- Ren, H.; Li, X.; Guo, L.; Wang, L.; Hao, X.; Zeng, J. Integrative Transcriptome and Proteome Analysis Reveals the Absorption and Metabolism of Selenium in Tea Plants [Camellia sinensis (L.) O. Kuntze]. Front. Plant Sci. 2022, 13, 848349. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Liu, Q.; Liu, H.; Yang, C.; Zhao, Q.; Xu, Y.; Wu, W. Structure characterization and antioxidant activity of abalone viscera l peptides-selenium in vitro. Food Chem. 2024, 433, 137398. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Hua, Y.; Wang, K.; Guo, Y.; Lu, Y.; Zhu, S.; Zhang, P.; Hu, G. Transcriptome and Metabolome Analysis of Selenium Treated Alfalfa Reve als Influence on Phenylpropanoid Biosynthesis to Enhance Growth. Plants 2023, 12, 2038. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhang, Y.; Zhang, W.; Lang, D.; Zhang, X.; Li, Z.; Zhang, X. Response of Carbon and Nitrogen Metabolism and Secondary Metabolites to Drought Stress and Salt Stress in Plants. J. Plant Biol. 2019, 62, 387–399. [Google Scholar] [CrossRef]
- Turakainen, M.; Hartikainen, H.; Seppänen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2024, 52, 5378–5382. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Nicolás, J.J.M.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Ingrisano, R.; Tosato, E.; Trost, P.; Gurrieri, L.; Sparla, F. Proline, Cysteine and Branched-Chain Amino Acids in Abiotic Stress Res ponse of Land Plants and Microalgae. Plants 2023, 12, 3410. [Google Scholar] [CrossRef]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine salvage and S-adenosylmethionine: Essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef]
- Morita, R.; Crofts, N.; Miura, S.; Ikeda, K.-I.; Aoki, N.; Fukayama, H.; Fujita, N. Characterization of the Functions of Starch Synthase IIIb Expressed in the Vegetative Organs of Rice (Oryza sativa L.). Plant Cell Physiol. 2022, 64, 94–106. [Google Scholar] [CrossRef]
- Prathap, V.; Tyagi, A. Correlation between expression and activity of ADP glucose pyrophosphorylase and starch synthase and their role in starch accumulation during grain filling under drought stress in rice. Plant Physiol. Biochem. 2020, 157, 239–243. [Google Scholar]
- Opassiri, R.; Pomthong, B.; Onkoksoong, T.; Akiyama, T.; Esen, A.; Cairns, J.R.K. Analysis of rice glycosyl hydrolase family 1 and expression of Os4bglu 12 beta-glucosidase. BMC Plant Biol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Akiyama, T.; Opassiri, R.; Kuaprasert, B.; Cairns, J.K. Structural and enzymatic characterization of Os3BGlu6, a rice beta-glucosidase hydrolyzing hydrophobic glycosides and (1->3)- and (1->2)-linked disaccharides. Plant Physiol. 2009, 151, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-Y.; Yang, J.-F.; Liu, T.-Y.; Su, Z.-F.; Zhu, F.-Y.; Chen, M.-X.; Fan, T.; Ye, N.-H.; Feng, Z.; Wang, L.-J.; et al. A Phylogenetically Informed Comparison of GH1 Hydrolases between Arabidopsis and Rice Response to Stressors. Front. Plant Sci. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Dong, Y.; Ren, R.; Chen, D.; Chen, X. Chloroplastic Os3BGlu6 contributes significantly to cellular ABA pools and impacts drought tolerance and photosynthesis in rice. New Phytol. 2020, 226, 1042–1054. [Google Scholar] [CrossRef]
- Opassiri, R.; Maneesan, J.; Akiyama, T.; Pomthong, B.; Jin, S.; Kimura, A.; Cairns, J.R.K. Rice Os4BGlu12 is a wound-induced β-glucosidase that hydrolyzes cell wall-β-glucan-derived oligosaccharides and glycosides. Plant Sci. 2010, 179, 273–280. [Google Scholar] [CrossRef]
- Ge, G.; Liu, Z.; Yu, T.; Zhou, L.; Sun, X.; Li, Z.; Zheng, Y. Aminotransferase Class I and II Gene Family in the Jinjiang Oyster (Crassostrea ariakensis): Genomewide Identification, Phylogenetic Analysis and Expression Profiles after Salinity Stress. Fishes 2023, 8, 459. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Du, B.; Pan, S.; Mo, Z.; Duan, M.; Tian, H.; Tang, X. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crop. Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Zeng, R.; Farooq, M.U.; Wang, L.; Su, Y.; Zheng, T.; Ye, X.; Jia, X.; Zhu, J. Study on Differential Protein Expression in Natural Selenium-Enriched and Non-Selenium-Enriched Rice Based on iTRAQ Quantitative Proteomics. Biomolecules 2019, 9, 130. [Google Scholar] [CrossRef]
- Khaliq, A.; Aslam, F.; Matloob, A.; Hussain, S.; Geng, M.; Wahid, A.; Rehman, H.U. Seed Priming with Selenium: Consequences for Emergence, Seedling Growth, and Biochemical Attributes of Rice. Biol. Trace Element Res. 2015, 166, 236–244. [Google Scholar] [CrossRef]
- A Hayden, C.; Bosco, G. Comparative genomic analysis of novel conserved peptide upstream open reading frames in Drosophila melanogaster and other dipteran species. BMC Genom. 2008, 9, 61. [Google Scholar] [CrossRef]
- Causier, B.; Hopes, T.; McKay, M.; Paling, Z.; Davies, B. Plants utilise ancient conserved peptide upstream open reading frames in stress-responsive translational regulation. Plant Cell Environ. 2022, 45, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-D.; Wang, X.; Wong, Y.-S. Generation of selenium-enriched rice with enhanced grain yield, seleni um content and bioavailability through fertilisation with selenite. Food Chem. 2013, 141, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or wit hout a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, Q.; Song, K.; Sun, L.; Jiang, T.; Yang, S.; Li, Z.; Xu, G.; Sun, S.; Xue, Y. Does Sulfoquinovosyl Diacylglycerol Synthase OsSQD1 Affect the Composition of Lipids in Rice Phosphate-Deprived Root? Int. J. Mol. Sci. 2022, 24, 114. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, J.; Sun, L.; Qin, Q.; Yang, S.; Wang, J.; Sun, Y.; Xue, Y. Physiological and Transcriptome Analysis Provide Insights into the Effects of Low and High Selenium on Methionine and Starch Metabolism in Rice Seedlings. Int. J. Mol. Sci. 2025, 26, 1596. https://doi.org/10.3390/ijms26041596
Yang Y, Zhang J, Sun L, Qin Q, Yang S, Wang J, Sun Y, Xue Y. Physiological and Transcriptome Analysis Provide Insights into the Effects of Low and High Selenium on Methionine and Starch Metabolism in Rice Seedlings. International Journal of Molecular Sciences. 2025; 26(4):1596. https://doi.org/10.3390/ijms26041596
Chicago/Turabian StyleYang, Yang, Jiarui Zhang, Lijuan Sun, Qin Qin, Shiyan Yang, Jun Wang, Yafei Sun, and Yong Xue. 2025. "Physiological and Transcriptome Analysis Provide Insights into the Effects of Low and High Selenium on Methionine and Starch Metabolism in Rice Seedlings" International Journal of Molecular Sciences 26, no. 4: 1596. https://doi.org/10.3390/ijms26041596
APA StyleYang, Y., Zhang, J., Sun, L., Qin, Q., Yang, S., Wang, J., Sun, Y., & Xue, Y. (2025). Physiological and Transcriptome Analysis Provide Insights into the Effects of Low and High Selenium on Methionine and Starch Metabolism in Rice Seedlings. International Journal of Molecular Sciences, 26(4), 1596. https://doi.org/10.3390/ijms26041596





