G-Quadruplex Structures Formed by Human Telomere and C9orf72 GGGGCC Repeats
Abstract
1. Introduction
2. Human Telomeric G4 Structures
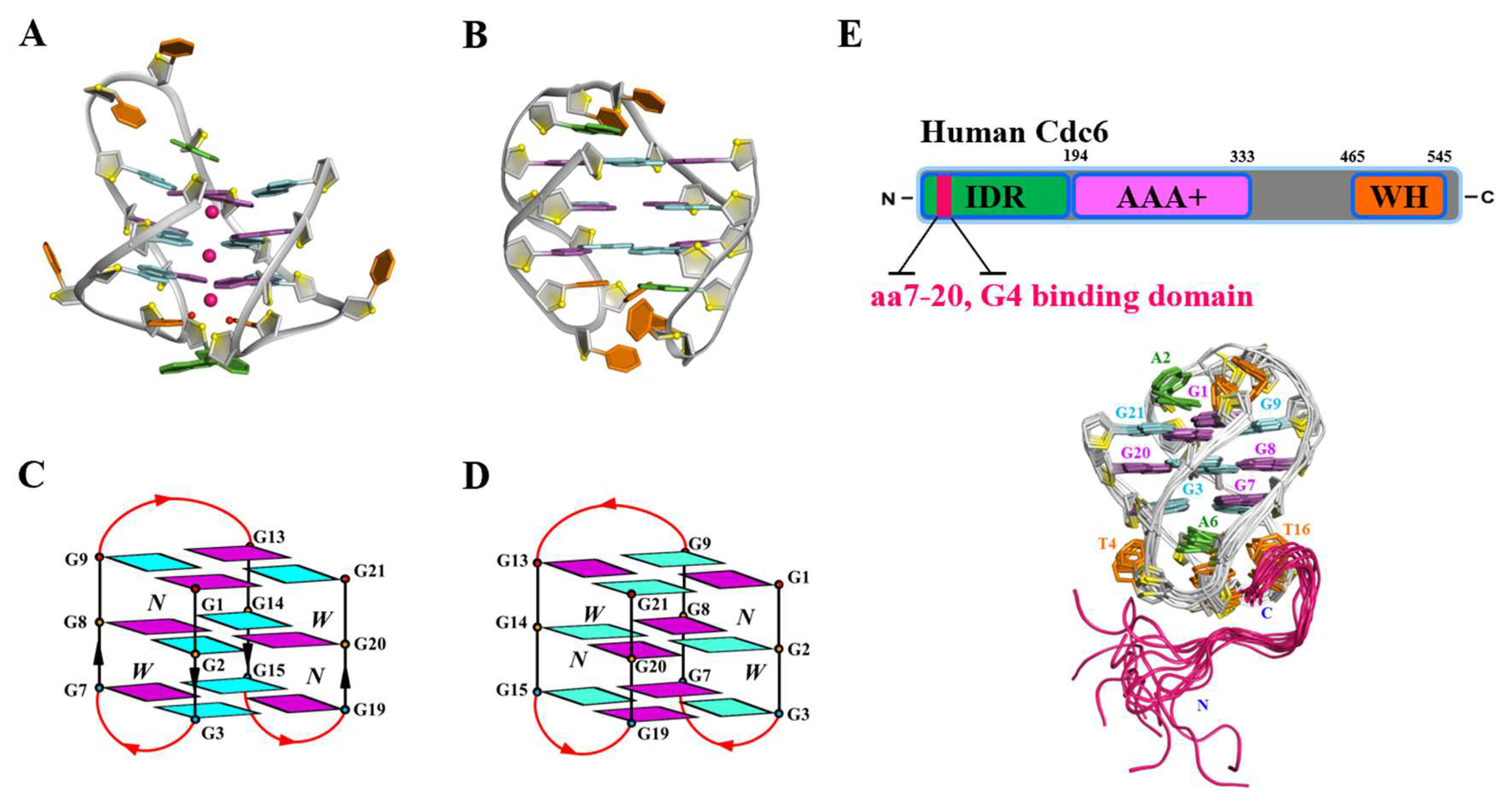
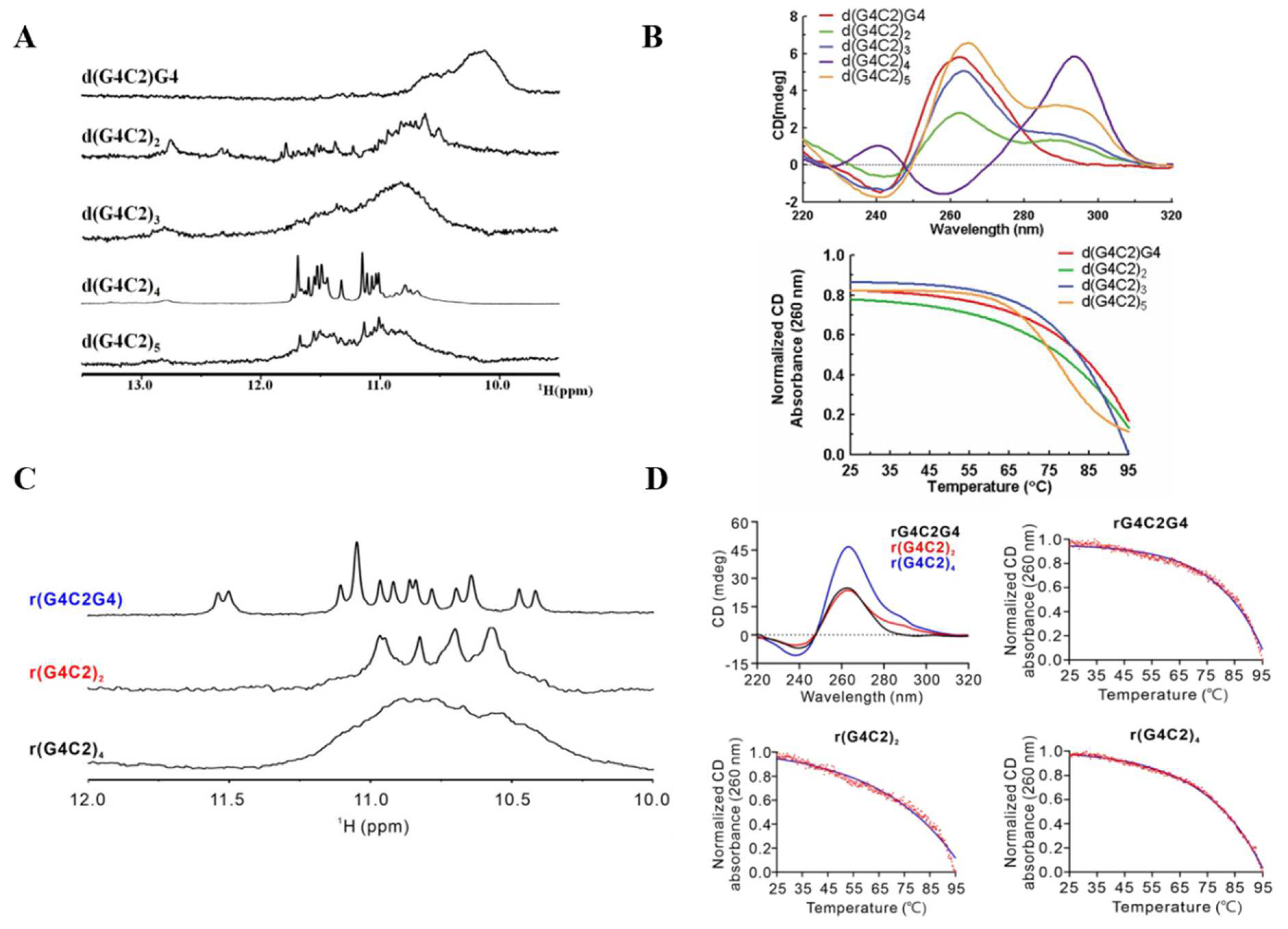
3. G4s Formed by C9orf72 Hexanucleotide Repeats (G4C2)n
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Largy, E.; Mergny, J.L.; Gabelica, V. Role of Alkali Metal Ions in G-Quadruplex Nucleic Acid Structure and Stability. Met. Ions Life Sci. 2016, 16, 203–258. [Google Scholar] [PubMed]
- Neidle, S.; Balasubramanian, S. Quadruplex Nucleic Acids; Royal Society of Chemistry: London, UK, 2006; Volume 7. [Google Scholar]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, H.L.; Hagen, T.; Tatum, N.J.; Hall, J. The diverse structural landscape of quadruplexes. FEBS Lett. 2019, 593, 2083–2102. [Google Scholar] [CrossRef]
- Phan, A.T.; Kuryavyi, V.; Darnell, J.C.; Serganov, A.; Majumdar, A.; Ilin, S.; Raslin, T.; Polonskaia, A.; Chen, C.; Clain, D.; et al. Structure-function studies of FMRP RGG peptide recognition of an RNA duplex-quadruplex junction. Nat. Struct. Mol. Biol. 2011, 18, 796–804. [Google Scholar] [CrossRef]
- Hazel, P.; Huppert, J.; Balasubramanian, S.; Neidle, S. Loop-length-dependent folding of G-quadruplexes. J. Am. Chem. Soc. 2004, 126, 16405–16415. [Google Scholar] [CrossRef]
- Heddi, B.; Phan, A.T. Structure of human telomeric DNA in crowded solution. J. Am. Chem. Soc. 2011, 133, 9824–9833. [Google Scholar] [CrossRef]
- Ambrus, A.; Chen, D.; Dai, J.; Bialis, T.; Jones, R.A.; Yang, D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006, 34, 2723–2735. [Google Scholar] [CrossRef]
- Murat, P.; Balasubramanian, S. Existence and consequences of G-quadruplex structures in DNA. Curr. Opin. Genet. Dev. 2014, 25, 22–29. [Google Scholar] [CrossRef]
- Pavlova, A.V.; Kubareva, E.A.; Monakhova, M.V.; Zvereva, M.I.; Dolinnaya, N.G. Impact of G-Quadruplexes on the Regulation of Genome Integrity, DNA Damage and Repair. Biomolecules 2021, 11, 1284. [Google Scholar] [CrossRef]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef]
- Di Antonio, M.; Ponjavic, A.; Radzevicius, A.; Ranasinghe, R.T.; Catalano, M.; Zhang, X.Y.; Shen, J.Z.; Needham, L.M.; Lee, S.F.; Klenerman, D.; et al. Single-molecule visualization of DNA G-quadruplex formation in live cells. Nat. Chem. 2020, 12, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Hansel-Hertsch, R.; Di Antonio, M.; Balasubramanian, S. DNA G-quadruplexes in the human genome: Detection, functions and therapeutic potential. Nat. Rev. Mol. Cell. Biol. 2017, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Iida, K.; Nagasawa, K. Topologies of G-quadruplex: Biological functions and regulation by ligands. Biochem. Biophys. Res. Commun. 2020, 531, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Prioleau, M.N. G-Quadruplexes and DNA Replication Origins. Adv. Exp. Med. Biol. 2017, 1042, 273–286. [Google Scholar] [PubMed]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef]
- Simone, R.; Fratta, P.; Neidle, S.; Parkinson, G.N.; Isaacs, A.M. G-quadruplexes: Emerging roles in neurodegenerative diseases and the non-coding transcriptome. FEBS Lett. 2015, 589, 1653–1668. [Google Scholar] [CrossRef]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-quadruplexes: A promising target for cancer therapy. Mol. Cancer 2021, 20, 40. [Google Scholar] [CrossRef]
- Cammas, A.; Millevoi, S. RNA G-quadruplexes: Emerging mechanisms in disease. Nucleic Acids Res. 2017, 45, 1584–1595. [Google Scholar] [CrossRef]
- Wu, Y.; Brosh, R.M., Jr. G-quadruplex nucleic acids and human disease. FEBS J. 2010, 277, 3470–3488. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Yang, D.; Okamoto, K. Structural insights into G-quadruplexes: Towards new anticancer drugs. Future Med. Chem. 2010, 2, 619–646. [Google Scholar] [CrossRef] [PubMed]
- Metifiot, M.; Amrane, S.; Litvak, S.; Andreola, M.L. G-quadruplexes in viruses: Function and potential therapeutic applications. Nucleic Acids Res. 2014, 42, 12352–12366. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, L. G-Quadruplexes Are Present in Human Coronaviruses Including SARS-CoV-2. Front. Microbiol. 2020, 11, 567317. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, G.; Niu, J.; Wang, Z.; Wang, C.; Ren, J.; Qu, X. Targeting RNA G-Quadruplex in SARS-CoV-2: A Promising Therapeutic Target for COVID-19? Angew. Chem. Int. Ed. Engl. 2021, 60, 432–438. [Google Scholar] [CrossRef]
- Ruggiero, E.; Richter, S.N. Viral G-quadruplexes: New frontiers in virus pathogenesis and antiviral therapy. Annu. Rep. Med. Chem 2020, 54, 101–131. [Google Scholar]
- Cheng, A.; Liu, C.; Ye, W.; Huang, D.; She, W.; Liu, X.; Fung, C.P.; Xu, N.; Suen, M.C.; Ye, W.; et al. Selective C9orf72 G-Quadruplex-Binding Small Molecules Ameliorate Pathological Signatures of ALS/FTD Models. J. Med. Chem. 2022, 65, 12825–12837. [Google Scholar] [CrossRef]
- Neidle, S. Quadruplex nucleic acids as targets for anticancer therapeutics. Nat. Rev. Chem. 2017, 1, 41. [Google Scholar] [CrossRef]
- Wang, E.; Thombre, R.; Shah, Y.; Latanich, R.; Wang, J.O. G-Quadruplexes as pathogenic drivers in neurodegenerative disorders. Nucleic Acids Res. 2021, 49, 4816–4830. [Google Scholar] [CrossRef]
- Zamiri, B.; Reddy, K.; Macgregor, R.B., Jr.; Pearson, C.E. TMPyP4 porphyrin distorts RNA G-quadruplex structures of the disease-associated r(GGGGCC)n repeat of the C9orf72 gene and blocks interaction of RNA-binding proteins. J. Biol. Chem. 2014, 289, 4653–4659. [Google Scholar] [CrossRef]
- Platella, C.; Napolitano, E.; Riccardi, C.; Musumeci, D.; Montesarchio, D. Disentangling the Structure-Activity Relationships of Naphthalene Diimides as Anticancer G-Quadruplex-Targeting Drugs. J. Med. Chem. 2021, 64, 3578–3603. [Google Scholar] [CrossRef]
- Figueiredo, J.; Mergny, J.L.; Cruz, C. G-quadruplex ligands in cancer therapy: Progress, challenges, and clinical perspectives. Life Sci. 2024, 340, 122481. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Komiyama, M. G-Quadruplexes in Human Telomere: Structures, Properties, and Applications. Molecules 2023, 29, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, C.D.; Geng, Y.Y.; Zhu, G. Topology of a G-quadruplex DNA formed by C9orf72 hexanucleotide repeats associated with ALS and FTD. Sci. Rep. 2015, 5, 16673. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Liu, C.; Xu, N.; Suen, M.C.; Miao, H.; Xie, Y.; Zhang, B.; Chen, X.; Song, Y.; Wang, Z.; et al. Crystal structure of a tetrameric RNA G-quadruplex formed by hexanucleotide repeat expansions of C9orf72 in ALS/FTD. Nucleic Acids Res. 2024, 52, 7961–7970. [Google Scholar] [CrossRef]
- Raguseo, F.; Wang, Y.; Li, J.; Petric Howe, M.; Balendra, R.; Huyghebaert, A.; Vadukul, D.M.; Tanase, D.A.; Maher, T.E.; Malouf, L.; et al. The ALS/FTD-related C9orf72 hexanucleotide repeat expansion forms RNA condensates through multimolecular G-quadruplexes. Nat. Commun. 2023, 14, 8272. [Google Scholar] [CrossRef]
- Bailey, S.M.; Murnane, J.P. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006, 34, 2408–2417. [Google Scholar] [CrossRef]
- Bryan, T.M. G-Quadruplexes at Telomeres: Friend or Foe? Molecules 2020, 25, 3686. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L. Aging, Cancer, and Inflammation: The Telomerase Connection. Int. J. Mol. Sci. 2024, 25, 8542. [Google Scholar] [CrossRef]
- Dai, J.X.; Carver, M.; Punchihewa, C.; Jones, R.A.; Yang, D.Z. Structure of the Hybrid-2 type intramolecular human telomeric G-quadruplex in K+ solution: Insights into structure polymorphism of the human telomeric sequence. Nucleic Acids Res. 2007, 35, 4927–4940. [Google Scholar] [CrossRef]
- Dai, J.X.; Punchihewa, C.; Ambrus, A.; Chen, D.; Jones, R.A.; Yang, D.Z. Structure of the intramolecular human telomeric G-quadruplex in potassium solution: A novel adenine triple formation. Nucleic Acids Res. 2007, 35, 2440–2450. [Google Scholar] [CrossRef]
- Lim, K.W.; Amrane, S.; Bouaziz, S.; Xu, W.X.; Mu, Y.G.; Patel, D.J.; Luu, K.N.; Phan, A.T. Structure of the Human Telomere in K+ Solution: A Stable Basket-Type G-Quadruplex with Only Two G-Tetrad Layers. J. Am. Chem. Soc. 2009, 131, 4301–4309. [Google Scholar] [CrossRef]
- Lim, K.W.; Ng, V.C.M.; Martin-Pintado, N.; Heddi, B.; Phan, A.T. Structure of the human telomere in Na+ solution: An antiparallel (2+2) G-quadruplex scaffold reveals additional diversity. Nucleic Acids Res. 2013, 41, 10556–10562. [Google Scholar] [CrossRef]
- Liu, C.; Geng, Y.; Miao, H.; Shi, X.; You, Y.; Xu, N.; Zhou, B.; Zhu, G. G-quadruplex structures formed by human telomeric DNA and C9orf72 hexanucleotide repeats. Biophys. Rev. 2019, 11, 389–393. [Google Scholar] [CrossRef]
- Parkinson, G.N.; Lee, M.P.H.; Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature 2002, 417, 876–880. [Google Scholar] [CrossRef]
- Phan, A.T.; Kuryavyi, V.; Luu, K.N.; Patel, D.J. Structure of two intramolecular G-quadruplexes formed by natural human telomere sequences in K+ solution. Nucleic Acids Res. 2007, 35, 6517–6525. [Google Scholar] [CrossRef]
- Wang, Y.; Patel, D.J. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure 1993, 1, 263–282. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Dai, J.X.; Veliath, E.; Jones, R.A.; Yang, D.Z. Structure of a two-G-tetrad intramolecular G-quadruplex formed by a variant human telomeric sequence in K+ solution: Insights into the interconversion of human telomeric G-quadruplex structures. Nucleic Acids Res. 2010, 38, 1009–1021. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, C.; Zhou, B.; Cai, Q.; Miao, H.; Shi, X.; Xu, N.; You, Y.; Fung, C.P.; Din, R.U.; et al. The crystal structure of an antiparallel chair-type G-quadruplex formed by Bromo-substituted human telomeric DNA. Nucleic Acids Res. 2019, 47, 5395–5404. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, B.; Geng, Y.; Yan Tam, D.; Feng, R.; Miao, H.; Xu, N.; Shi, X.; You, Y.; Hong, Y.; et al. A chair-type G-quadruplex structure formed by a human telomeric variant DNA in K(+) solution. Chem. Sci. 2019, 10, 218–226. [Google Scholar] [CrossRef]
- Geng, Y.Y.; Liu, C.D.; Xu, N.N.; Shi, X.; Suen, M.C.; Zhou, B.; Yan, B.; Wu, C.M.; Li, H.; Song, Y.J.; et al. The N-terminal region of Cdc6 specifically recognizes human DNA G-quadruplex. Int. J. Biol. Macromol. 2024, 260, 129487. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, R.; Neumann, M.; Mackenzie, I.R. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2012, 8, 423–434. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef]
- Haeusler, A.R.; Donnelly, C.J.; Periz, G.; Simko, E.A.; Shaw, P.G.; Kim, M.S.; Maragakis, N.J.; Troncoso, J.C.; Pandey, A.; Sattler, R.; et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 2014, 507, 195–200. [Google Scholar] [CrossRef]
- Reddy, K.; Zamiri, B.; Stanley, S.Y.R.; Macgregor, R.B.; Pearson, C.E. The Disease-associated r(GGGGCC)(n) Repeat from the C9orf72 Gene Forms Tract Length-dependent Uni- and Multimolecular RNA G-quadruplex Structures. J. Biol. Chem. 2013, 288, 9860–9866. [Google Scholar] [CrossRef]
- Wang, Z.F.; Ursu, A.; Childs-Disney, J.L.; Guertler, R.; Yang, W.Y.; Bernat, V.; Rzuczek, S.G.; Fuerst, R.; Zhang, Y.J.; Gendron, T.F.; et al. The Hairpin Form of r(G(4)C(2))(exp) in c9ALS/FTD Is Repeat-Associated Non-ATG Translated and a Target for Bioactive Small Molecules. Cell Chem. Biol. 2019, 26, 179–190. [Google Scholar] [CrossRef]
- Brcic, J.; Plavec, J. NMR structure of a G-quadruplex formed by four d(G4C2) repeats: Insights into structural polymorphism. Nucleic Acids Res. 2018, 46, 11605–11617. [Google Scholar]
- Brcic, J.; Plavec, J. Solution structure of a DNA quadruplex containing ALS and FTD related GGGGCC repeat stabilized by 8-bromodeoxyguanosine substitution. Nucleic Acids Res. 2015, 43, 8590–8600. [Google Scholar] [CrossRef]
- Schmitz, A.; Pinheiro Marques, J.; Oertig, I.; Maharjan, N.; Saxena, S. Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD. Front. Cell. Neurosci. 2021, 15, 637548. [Google Scholar] [CrossRef]
- Conlon, E.G.; Lu, L.; Sharma, A.; Yamazaki, T.; Tang, T.; Shneider, N.A.; Manley, J.L. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. eLife 2016, 5, e17820. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W.; Van Den Bosch, L. RNA toxicity in non-coding repeat expansion disorders. EMBO J. 2020, 39, e101112. [Google Scholar] [CrossRef]
- Mirceta, M.; Schmidt, M.H.M.; Shum, N.; Prasolava, T.K.; Meikle, B.; Lanni, S.; Mohiuddin, M.; McKeever, P.M.; Zhang, M.; Liang, M.; et al. C9orf72 repeat expansion creates the unstable folate-sensitive fragile site FRA9A. NAR Mol. Med. 2024, 1, ugae019. [Google Scholar] [CrossRef]
- Fratta, P.; Mizielinska, S.; Nicoll, A.J.; Zloh, M.; Fisher, E.M.C.; Parkinson, G.; Isaacs, A.M. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci. Rep. 2012, 2, 1016. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, C.; Cai, Q.; Luo, Z.; Miao, H.; Shi, X.; Xu, N.; Fung, C.P.; Choy, T.T.; Yan, B.; et al. Crystal structure of parallel G-quadruplex formed by the two-repeat ALS- and FTD-related GGGGCC sequence. Nucleic Acids Res. 2021, 49, 5881–5890. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, B.; Xu, N.; Fung, C.P.; Yan, B.; Suen, M.C.; Huang, Z.; Zhu, G. The parallel tetrameric DNA G-quadruplex formed by the two-repeat C9orf72 GGGGCC sequence in solution. Magn. Reson. Lett. 2022, 2, 196–204. [Google Scholar] [CrossRef]

| PDB ID | Sequence | Structure Type | Solution Conditions | Method | Structure |
|---|---|---|---|---|---|
| 143D | AGGG(TTAGGG)3 | antiparallel basket-type | Na+ | NMR |  |
| 1KF1 | d[(AGGGTT)3AGGG] | parallel | K+ | XRD |  |
| 1K8P | d[BrUAGGGBrUTAGGGT] | parallel | K+/Na+ | XRD | 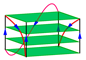 |
| 2GKU | d[TTGGG(TTAGGG)3A] | hybrid | K+ | NMR | 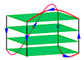 |
| 2HY9 | d[AAAGGG(TTAGGG)3AA] | hybrid-1 | K+ | NMR | 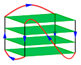 |
| 2JPZ | d[(TTAGGG)4TT] | hybrid-2 | K+ | NMR |  |
| 2JSM | d[TAGGG(TTAGGG)3] | hybrid-1 | K+ | NMR |  |
| 2JSL | d[TAGGG(TTAGGG)3TT] | hybrid-2 | K+ | NMR | 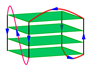 |
| 2JSK | d[TAGGGTTAGGGTTAG(BrG)GTTAGGG] | hybrid-1 | K+ | NMR | 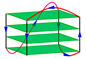 |
| 2JSQ | d[TAGGGTTAGGGTTA(BrG)GGTTAGGGTT] | hybrid-2 | K+ | NMR | 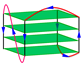 |
| 2KF8 | d[(GGGTTA)3GGGT] | basket-type | K+ | NMR | 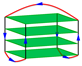 |
| 2KF7 | d[GGGTTA(BrG)GGTTAGGGTTAGGGT] | basket-type | K+ | NMR | 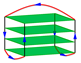 |
| 2KKA | d[(AGGGTT)2AIGGTTAGGGT] | basket-type | K+ | NMR | 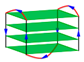 |
| 5YEY | d[(GGGTTA)2GGGTTTGGG] | chair-type | K+ | NMR | 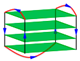 |
| 6JKN | d[GGGTTAG(BrG)GTTAGGGTTAG(BrG)G] | chair-type | K+ | XRD | 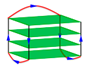 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Suen, M.C.; Xu, N.; Lu, C.; Liu, C.; Zhu, G. G-Quadruplex Structures Formed by Human Telomere and C9orf72 GGGGCC Repeats. Int. J. Mol. Sci. 2025, 26, 1591. https://doi.org/10.3390/ijms26041591
Yan B, Suen MC, Xu N, Lu C, Liu C, Zhu G. G-Quadruplex Structures Formed by Human Telomere and C9orf72 GGGGCC Repeats. International Journal of Molecular Sciences. 2025; 26(4):1591. https://doi.org/10.3390/ijms26041591
Chicago/Turabian StyleYan, Bing, Monica Ching Suen, Naining Xu, Chao Lu, Changdong Liu, and Guang Zhu. 2025. "G-Quadruplex Structures Formed by Human Telomere and C9orf72 GGGGCC Repeats" International Journal of Molecular Sciences 26, no. 4: 1591. https://doi.org/10.3390/ijms26041591
APA StyleYan, B., Suen, M. C., Xu, N., Lu, C., Liu, C., & Zhu, G. (2025). G-Quadruplex Structures Formed by Human Telomere and C9orf72 GGGGCC Repeats. International Journal of Molecular Sciences, 26(4), 1591. https://doi.org/10.3390/ijms26041591






