Vitisin A Outperforms Cyanidin-3-O-Glucoside in Triglyceride Reduction by Modulating Hepatic Lipogenesis and Fatty Acid β-Oxidation
Abstract
1. Introduction
2. Results
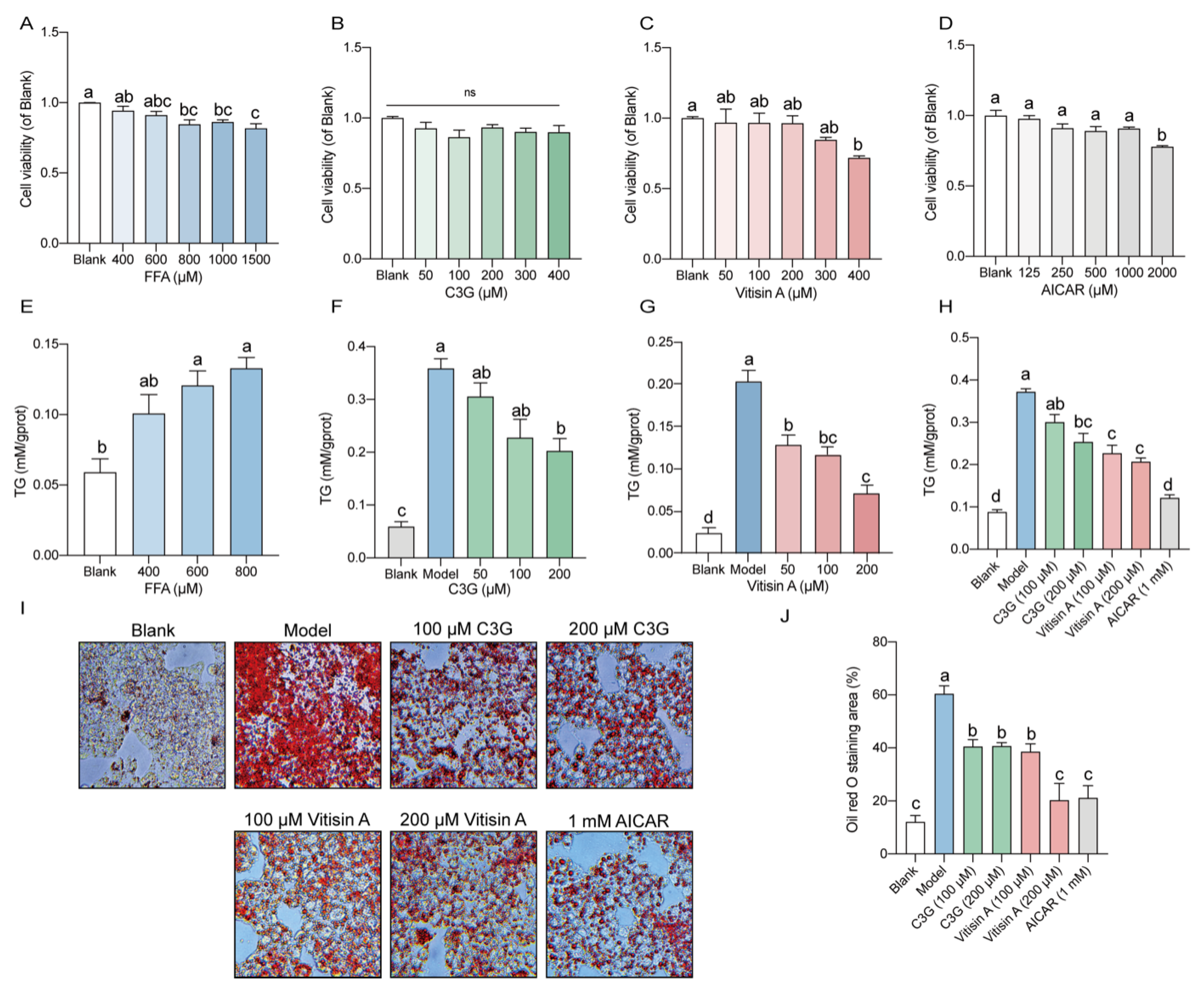
2.1. Vitisin A Decreased Intracellular TG Levels and Lipid Accumulation in HepG2 Cells
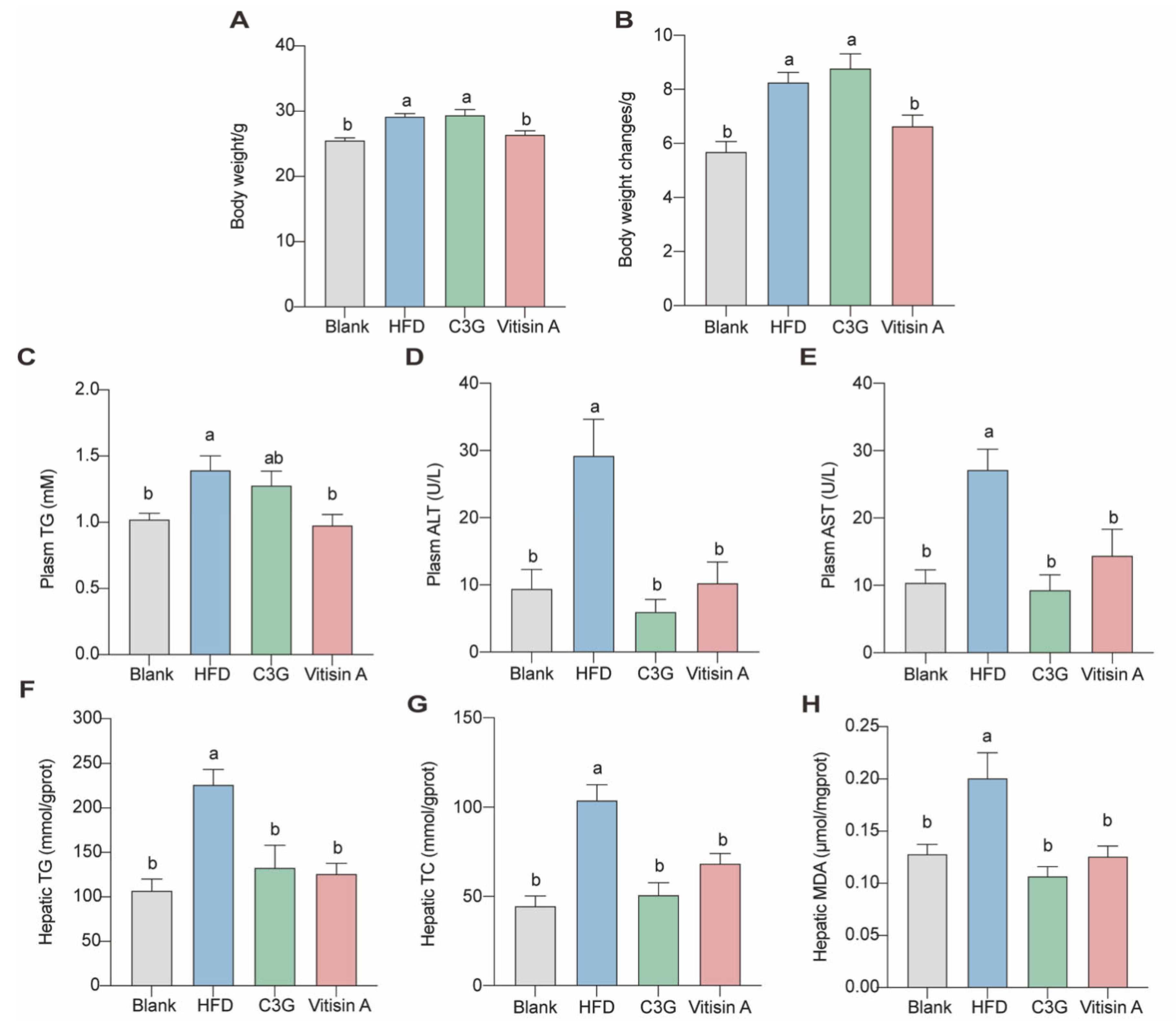
2.2. Effect of Vitisin A on Body Weight, Plasma, and Liver Parameters in ApoE−/− Mice
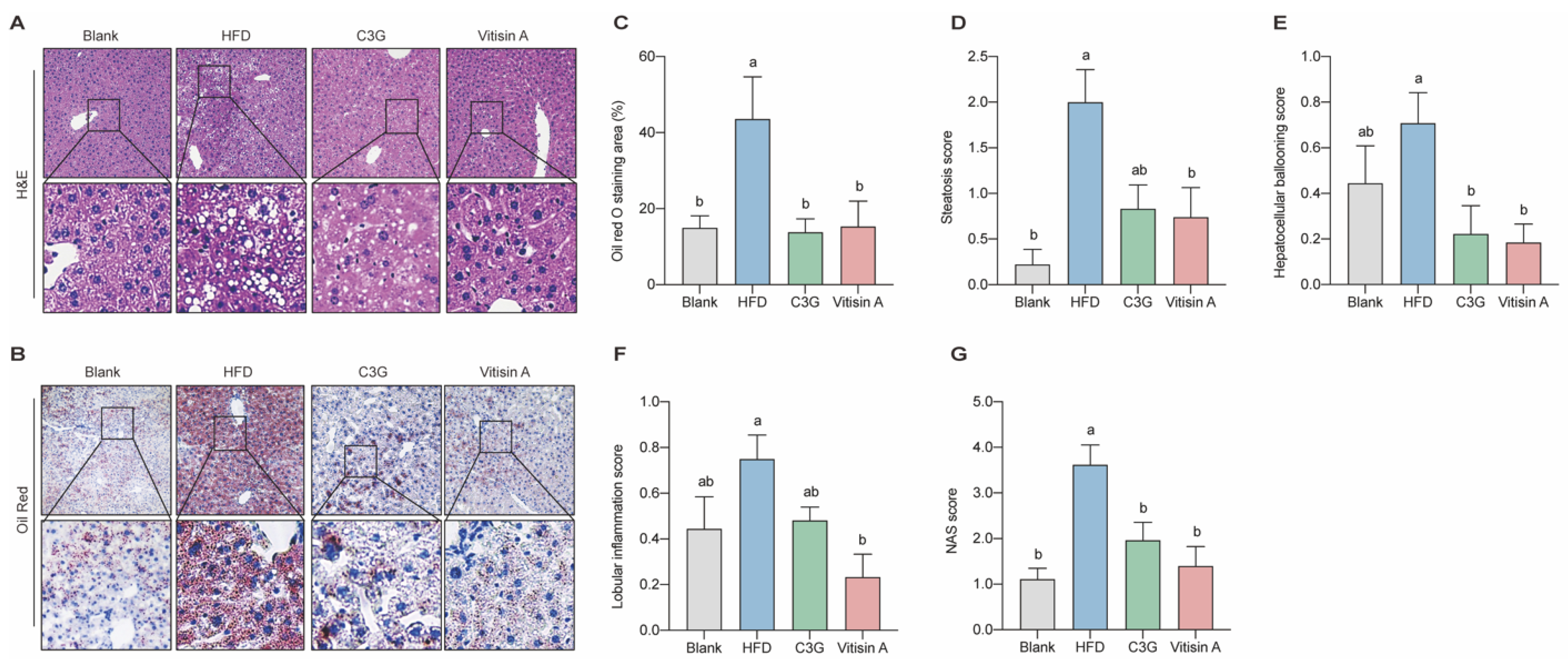
2.3. Effect of Vitisin A on NAS Score and Liver Lipid Droplet Accumulation
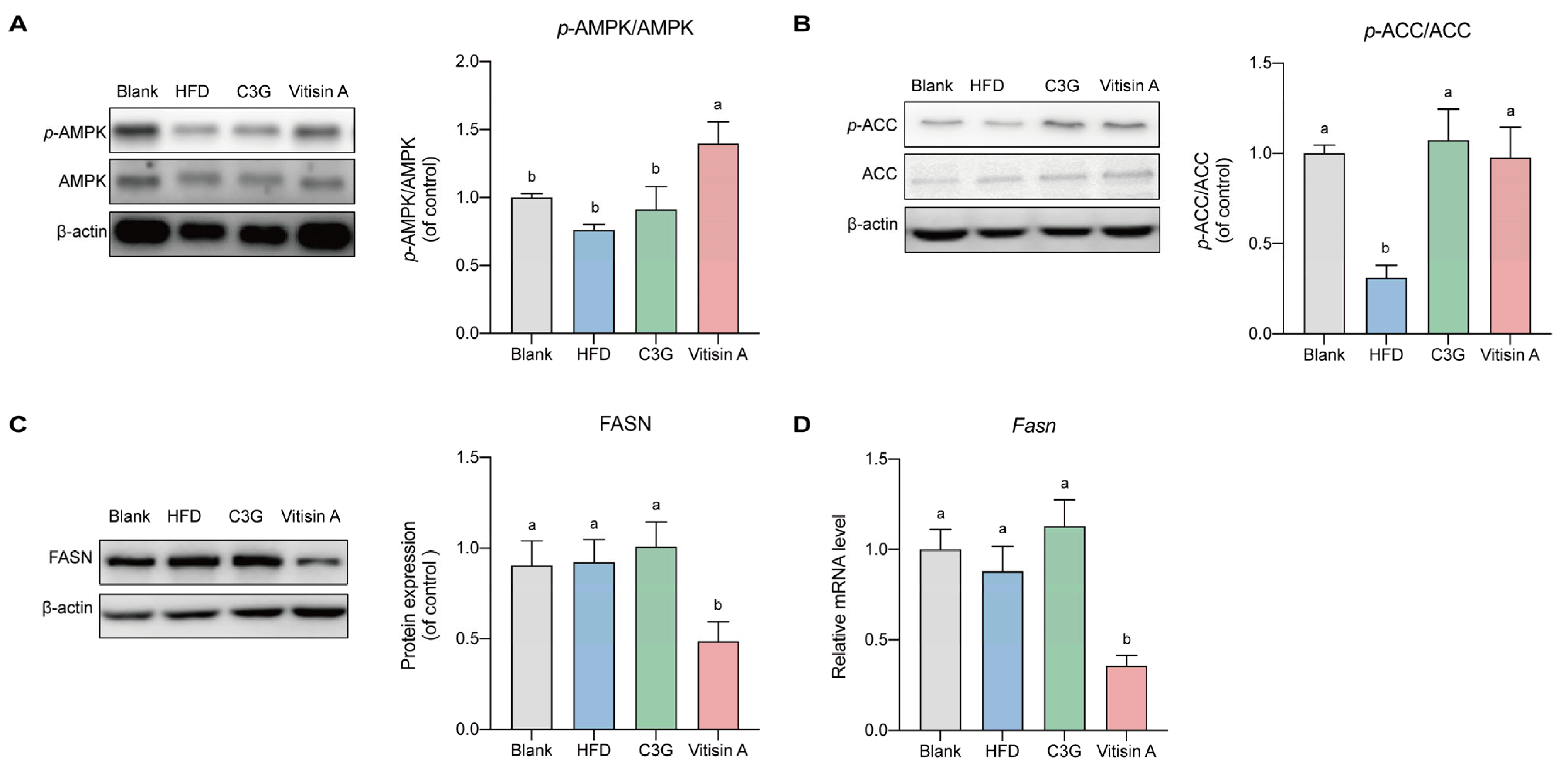
2.4. Effect of Vitisin A on the Fatty Acid Synthesis Pathway in the Liver
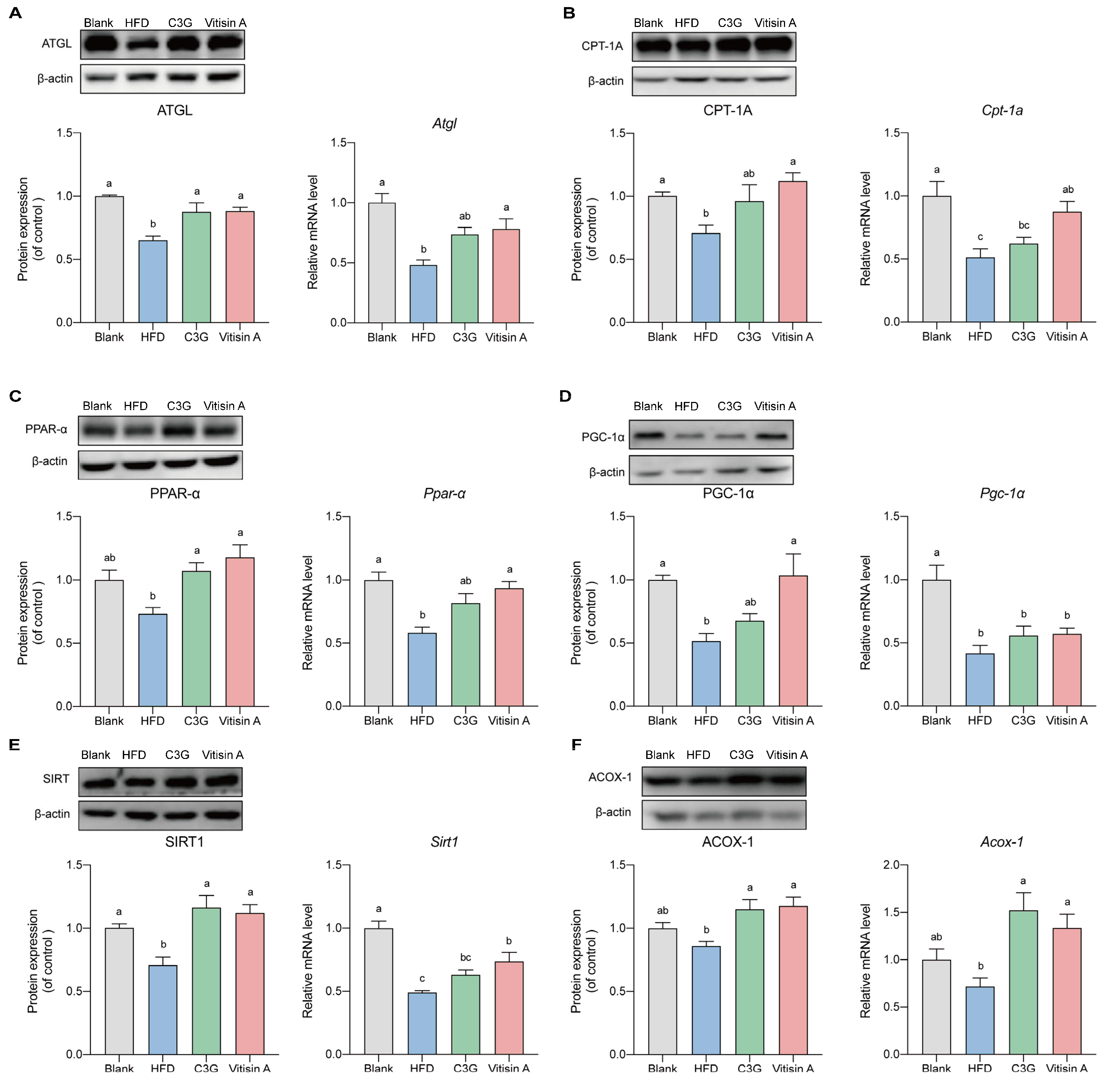
2.5. Effect of Vitisin A on the β-Oxidation Pathway in the Liver
3. Discussion
- (1)
- Inhibition of Hepatic DNL via the AMPK/ACC Pathway and Downregulation of FASN
- (2)
- Enhancement of Fatty Acid β-Oxidation
4. Materials and Methods
4.1. Reagents and Antibodies
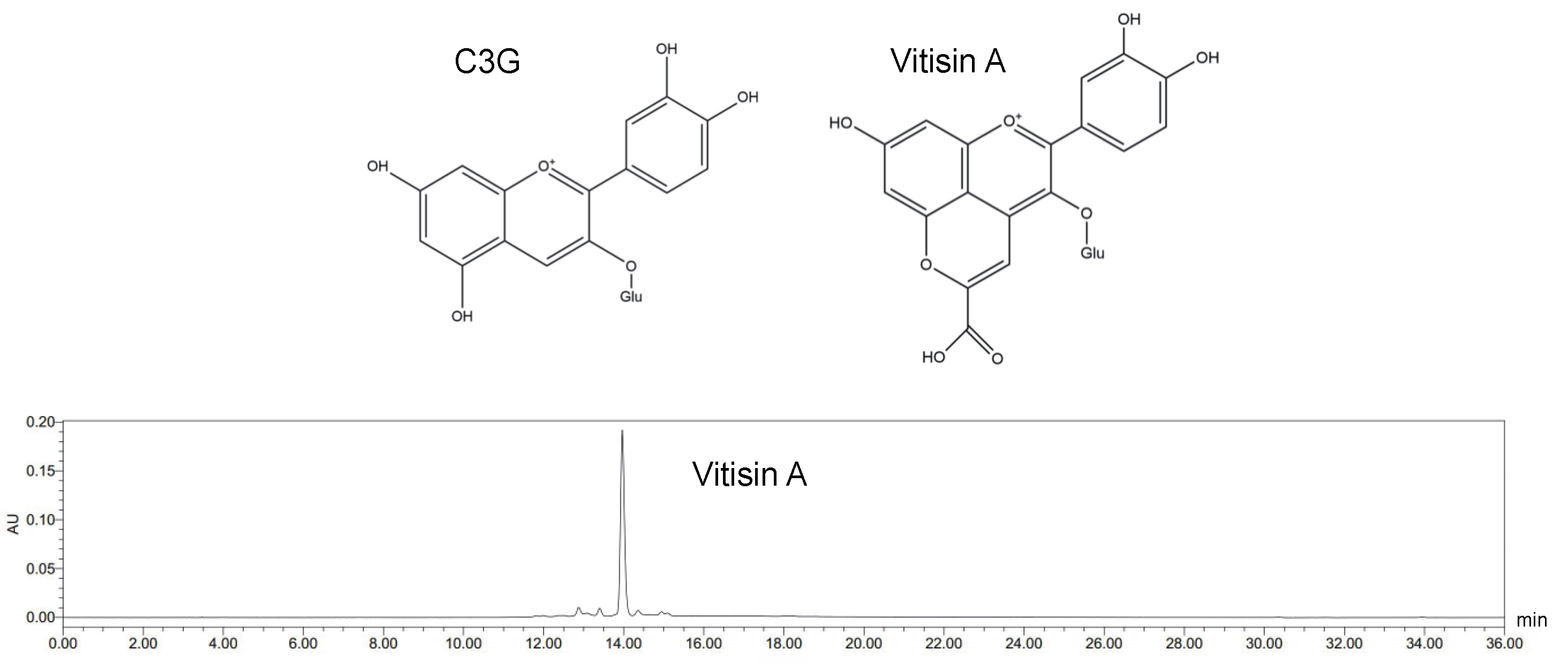
4.2. Vitisin A Preparation and Purification
4.3. In Vitro Study
4.3.1. Cell Culture and Treatments
4.3.2. CCK-8 Assay
4.3.3. Triglyceride Determination in Cells
4.3.4. Oil Red O Staining in HepG2 Cells
4.4. Animals and Treatment
4.5. Biochemical Analyses
4.6. Histopathological Examination of Hepatic Tissue, NAS Score Evaluation, and Hepatic Lipid Droplet Analysis
4.7. RNA Extraction and Gene Expression Assays
4.8. Protein Extraction and Western Blot
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2022, 78, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Che, B.; Zhong, C.; Zhang, R.; Pu, L.; Zhao, T.; Zhang, Y.; Han, L. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Xin, Q.Q.; Shi, W.L.; Miao, Y.; Zhu, Z.C.; Yuan, Y.H.; Chen, Y.; Chen, X.N.; Leng, S.X.; Chen, K.J.; et al. Tetramethylpyrazine and paeoniflorin combination (TMP-PF) alleviates atherosclerosis progress by reducing hyperlipemia and inhibiting plaque angiogenesis via the NR4A1/VEGFR2 pathway. Food Sci. Hum. Wellness 2024, 13, 2642–2652. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, T.; Jiang, Z.; Guo, Y.; Qiu, F.; Liu, R.; Zhang, L.; Chang, M.; Liu, R.; Jin, Q.; et al. Physical properties and cellular antioxidant activity of vegetable oil emulsions with different chain lengths and saturation of triglycerides. LWT 2019, 121, 108948. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Wang, J.; Li, Y.; Feng, F.; Zhao, M. Digestive behavior of unemulsified triglycerides with different chain lengths: In vitro dynamic and static simulated digestion models. LWT 2021, 149, 112006. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Z.; Qin, B.; Ju, X.; Wang, L. Evaluation of the intracellular lipid-lowering effect of polyphenols extract from highland barley in HepG2 cells. Food Sci. Hum. Wellness 2024, 13, 454–461. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Wang, Y.; Leng, L.; Xu, J.; Feng, L.; Jiang, S.; Wang, J.; Yang, Y.; Pan, G.; et al. The impact of triglyceride-glucose index on ischemic stroke: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 2. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, L.; Tai, F.; Zhang, L.; Zhang, X.; Chen, Y. Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis. Food Sci. Hum. Wellness 2024, 13, 482–493. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Z.; Huang, Q.; Wang, X.; Huang, F.; Deng, Q. Targeted microbiome metabolomics reveals flaxseed oil supplementation regulated the gut microbiota and farnesoid X receptor pathway in high-fat diet mice. Food Sci. Hum. Wellness 2023, 12, 2324–2335. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Polanco, S.R.; Bousvarou, M.D.; Papakonstantinou, E.J.; Genao, E.P.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Schwarz, M.; Quast, P.; von Baer, D.; Winterhalter, P. Vitisin A Content in Chilean Wines from Vitis vinifera Cv. Cabernet Sauvignon and Contribution to the Color of Aged Red Wines. J. Agric. Food Chem. 2003, 51, 6261–6267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Cai, D.; Li, Y.; Zhu, Y.; Jiao, R.; Lai, C.; Sun, J.; Bai, W. Vitisin A, as a Type of Pyranoanthocyanin, Suppresses Inflammation by Restricting Hematopoietic Stem Cell Differentiation toward Monocytes in Bone Marrow. J. Agric. Food Chem. 2023, 71, 15048–15063. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Lan, Y.-B.; Huang, Y.; Zhao, X.; Duan, C.-Q. Targeted metabolomics of anthocyanin derivatives during prolonged wine aging: Evolution, color contribution and aging prediction. Food Chem. 2020, 339, 127795. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and Mulberry Juice Prevent Obesity Development in C57BL/6 Mice. PLOS ONE. 2013, 8, e77585. [Google Scholar] [CrossRef]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, Y.; Li, Y.; Li, X.; Jiao, R.; Bai, W. Cholesterol-Lowering Activity of Vitisin A Is Mediated by Inhibiting Cholesterol Biosynthesis and Enhancing LDL Uptake in HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 3301. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, F.; Hou, X.; Xia, D. Dietary kaempferol exerts anti-obesity effects by inducing the browing of white adipocytes via the AMPK/SIRT1/PGC-1α signaling pathway. Curr. Res. Food Sci. 2024, 8, 100728. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, G.-L.; Qiu, M.-H.; Cao, J.; Xiong, W.-Y. Coffee, tea, and cocoa in obesity prevention: Mechanisms of action and future prospects. Curr. Res. Food Sci. 2024, 8, 100741. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.L.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hassing, H.C.; Surendran, R.P.; Mooij, H.L.; Stroes, E.S.; Nieuwdorp, M.; Dallinga-Thie, G.M. Pathophysiology of hypertriglyceridemia. Biochim. Biophys. Acta 2012, 1821, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Brunzell, J.D. Hypertriglyceridemia. N. Engl. J. Med. 2007, 357, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Fazio, S.; Wong, N.D.; Hull, M.; Nichols, G.A. Risk of cardiovascular events in patients with hypertriglyceridaemia: A review of real-world evidence. Diabetes Obes. Metab. 2019, 22, 279–289. [Google Scholar] [CrossRef]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2019, 153, 1231–1240. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Loarca-Piña, G.; Gonzalez de Mejia, E. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 79, 108343. [Google Scholar] [CrossRef]
- Parra-Vargas, M.; Sandoval-Rodriguez, A.; Rodriguez-Echevarria, R.; Dominguez-Rosales, J.A.; Santos-Garcia, A.; Armendariz-Borunda, J. Delphinidin Ameliorates Hepatic Triglyceride Accumulation in Human HepG2 Cells, but Not in Diet-Induced Obese Mice. Nutrients 2018, 10, 1060. [Google Scholar] [CrossRef]
- Guo, H.; Guo, J.; Jiang, X.; Li, Z.; Ling, W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012, 50, 3040–3047. [Google Scholar] [CrossRef]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De nova lipogenesis in health and disease. Metab. Clin. Exp. 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Galic, S.; Loh, K.; Murray-Segal, L.; Steinberg, G.R.; Andrews, Z.B.; Kemp, B.E. AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. elife 2018, 7, e32656. [Google Scholar] [CrossRef]
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Ruiz, S.; Fornari, A.; Fiorentino, M.; Priolo, C.; Zadra, G.; Inazuka, F.; Grisanzio, C.; Palescandolo, E.; Shin, E.; et al. Fatty Acid Synthase: A Metabolic Enzyme and Candidate Oncogene in Prostate Cancer. JNCI J. Natl. Cancer Inst. 2009, 101, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Lepropre, S.; Kautbally, S.; Octave, M.; Ginion, A.; Onselaer, M.-B.; Steinberg, G.R.; Kemp, B.E.; Hego, A.; Wéra, O.; Brouns, S.; et al. AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood 2018, 132, 1180–1192. [Google Scholar] [CrossRef]
- Krishan, S.; Richardson, D.R.; Sahni, S. AMP kinase (PRKAA1). J. Clin. Pathol. 2014, 67, 758–763. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.F.; Zheng, G.H.; Wang, A.M.; Sun, C.H.; Qin, S.P.; Zhuang, J.; Lu, J.; Ma, D.F.; Zheng, Y.L. Attenuation of hepatic steatosis by purple sweet potato colour is associated with blocking Src/ERK/C/EBP beta signalling in high-fat-diet-treated. Appl. Physiol. Nutr. Metab. 2017, 42, 1082–1091. [Google Scholar] [CrossRef]
- Saito, T.; Nishida, M.; Saito, M.; Tanabe, A.; Eitsuka, T.; Yuan, S.H.; Ikekawa, N.; Nishida, H. The fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms improves insulin resistance and hepatic lipid accumulation by modulation of liver adenosine monophosphate-activated protein kinase activity and lipogenic gene expression in high-fat diet-fed obese mice. Nutr. Res. 2016, 36, 1090–1097. [Google Scholar]
- Kunau, W.H.; Dommes, V.; Schulz, H. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: A century of continued progress. Prog. Lipid Res. 1995, 34, 267–342. [Google Scholar] [CrossRef]
- Byrne, C.D. Fatty liver: Role of inflammation and fatty acid nutrition. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 265–271. [Google Scholar] [CrossRef]
- Mizutani, H.; Sako, T.; Takemura, N.; Koyama, H.; Yamaguchi, M.; Motoyoshi, S. Hepatic Carnitine Palmitoyltransferase Activity in Cattle. J. Vet. Med. Sci. 1997, 59, 1067–1069. [Google Scholar] [CrossRef]
- Li, X.; Yao, Z.; Yang, D.; Jiang, X.; Sun, J.; Tian, L.; Hu, J.; Wu, B.; Bai, W. Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium-exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J. Hazard. Mater. 2019, 387, 121706. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.; Wu, S.; Jiang, X.; Chen, G.; Hu, Y.; Sun, J.; Bai, W. Cyanidin-3-O-glucoside and its derivative vitisin A alleviate androgenetic alopecia by exerting anti-androgen effect and inhibiting dermal papilla cell apoptosis. Eur. J. Pharmacol. 2023, 963, 176237. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Luo, H.; Ding, L.; Jiang, X.; Li, X.; Jiao, R.; Bai, W. Comparative Study on the Stability and Antioxidant Activity of Six Pyranoanthocyanins Based on Malvidin-3-glucoside. J. Agric. Food Chem. 2020, 68, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Pang, Y.; Wang, X.; Wu, Q.; Liu, H.; Liu, B.; Liu, G.; Ye, M.; Kong, W.; Jiang, C. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm. Sin. B 2019, 9, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, W.; Zhu, C.; Hu, Y.; Jiang, X.; Ou, S.; Su, Z.; Huang, Y.; Jiao, R.; Bai, W. Cyanidin-3-O-Glucoside Protects against 1,3-Dichloro-2-Propanol-Induced Reduction of Progesterone by Up-regulation of Steroidogenic Enzymes and cAMP Level in Leydig Cells. Front. Pharmacol. 2016, 7, 399. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Liang, N.; Jiang, X.; Song, Y.; Ou, S.; Hu, Y.; Jiao, R.; Bai, W. 6-Gingerol Regulates Hepatic Cholesterol Metabolism by Up-regulation of LDLR and Cholesterol Efflux-Related Genes in HepG2 Cells. Front. Pharmacol. 2018, 9, 159. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, X.; Liu, J.; Jayavanth, P.; Bai, W.; Jiao, R. Vitisin A Outperforms Cyanidin-3-O-Glucoside in Triglyceride Reduction by Modulating Hepatic Lipogenesis and Fatty Acid β-Oxidation. Int. J. Mol. Sci. 2025, 26, 1521. https://doi.org/10.3390/ijms26041521
Li Y, Li X, Liu J, Jayavanth P, Bai W, Jiao R. Vitisin A Outperforms Cyanidin-3-O-Glucoside in Triglyceride Reduction by Modulating Hepatic Lipogenesis and Fatty Acid β-Oxidation. International Journal of Molecular Sciences. 2025; 26(4):1521. https://doi.org/10.3390/ijms26041521
Chicago/Turabian StyleLi, Yawen, Xusheng Li, Jia Liu, Pallavi Jayavanth, Weibin Bai, and Rui Jiao. 2025. "Vitisin A Outperforms Cyanidin-3-O-Glucoside in Triglyceride Reduction by Modulating Hepatic Lipogenesis and Fatty Acid β-Oxidation" International Journal of Molecular Sciences 26, no. 4: 1521. https://doi.org/10.3390/ijms26041521
APA StyleLi, Y., Li, X., Liu, J., Jayavanth, P., Bai, W., & Jiao, R. (2025). Vitisin A Outperforms Cyanidin-3-O-Glucoside in Triglyceride Reduction by Modulating Hepatic Lipogenesis and Fatty Acid β-Oxidation. International Journal of Molecular Sciences, 26(4), 1521. https://doi.org/10.3390/ijms26041521






