Single-Cell Sequencing Reveals the Role of Radiation-Induced Stemness-Responsive Cancer Cells in the Development of Radioresistance
Abstract
1. Introduction
2. Results
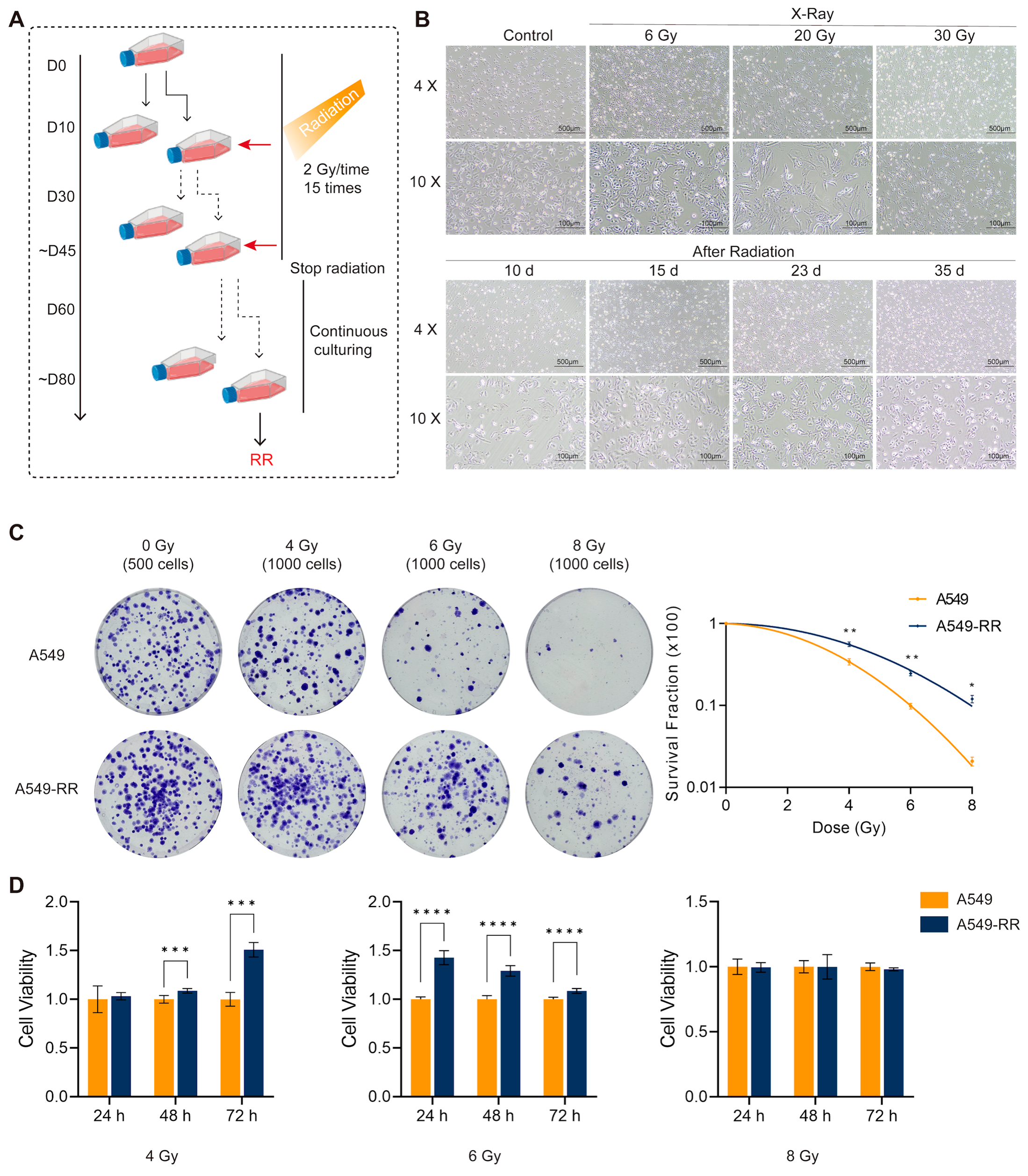
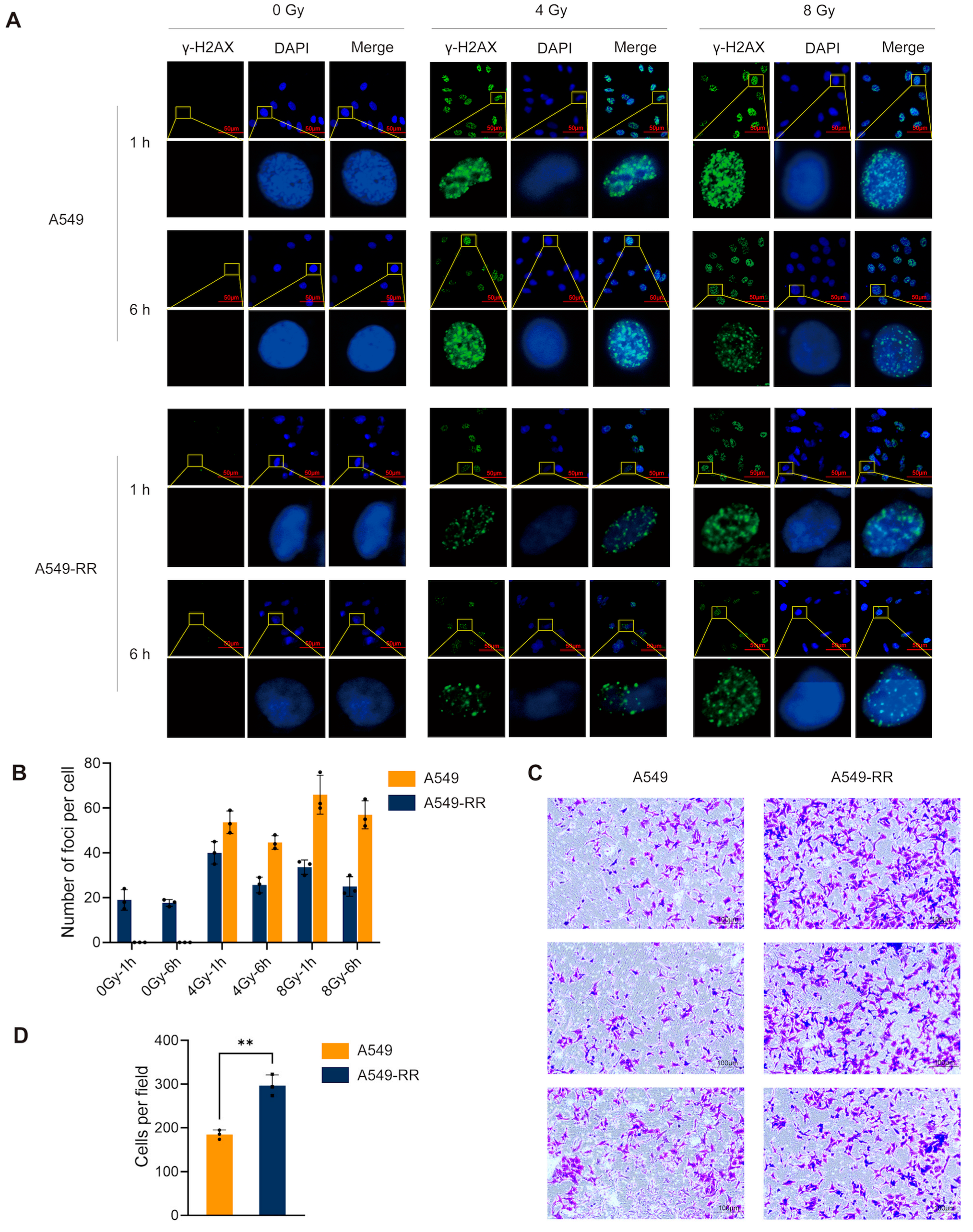
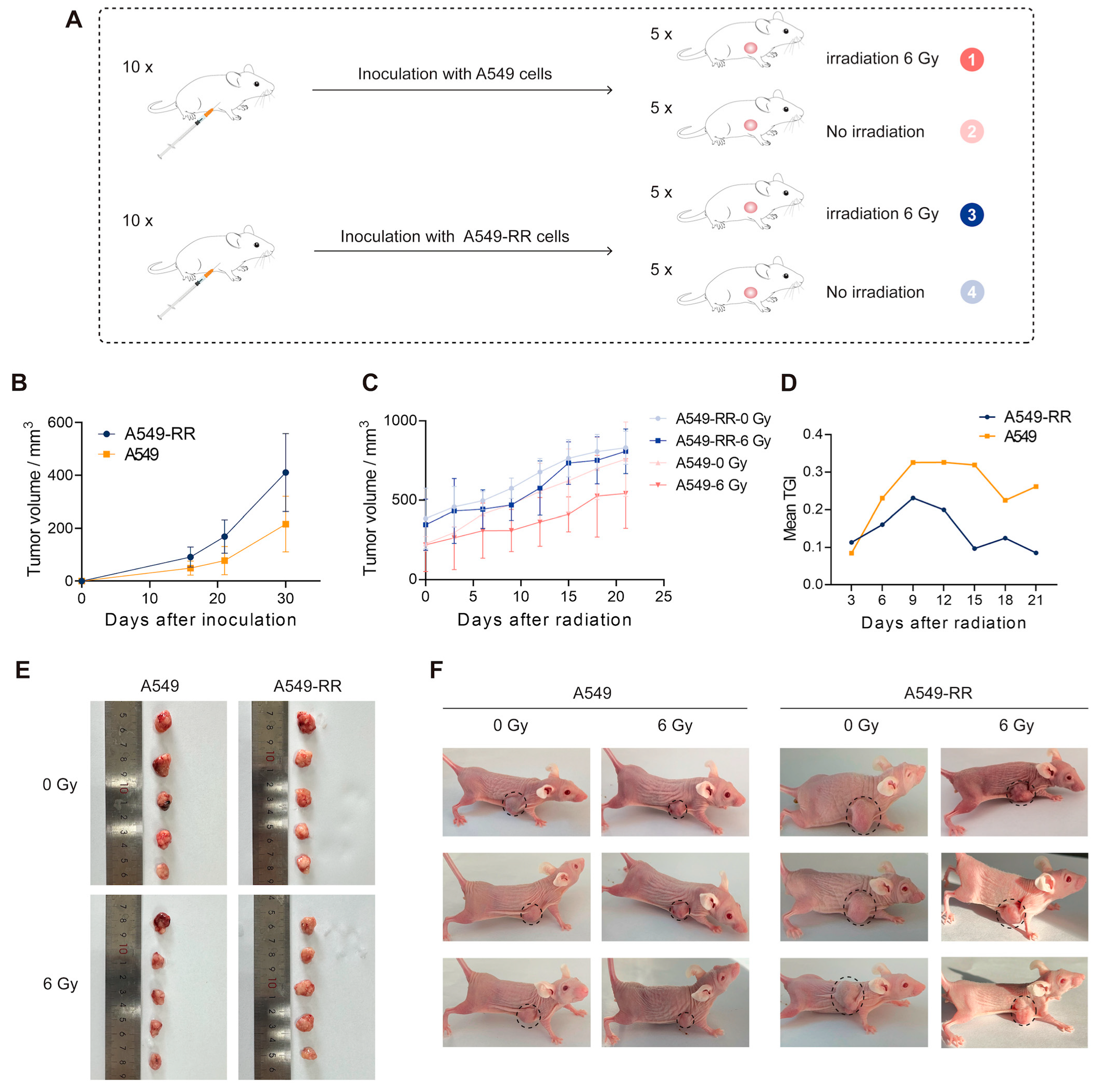
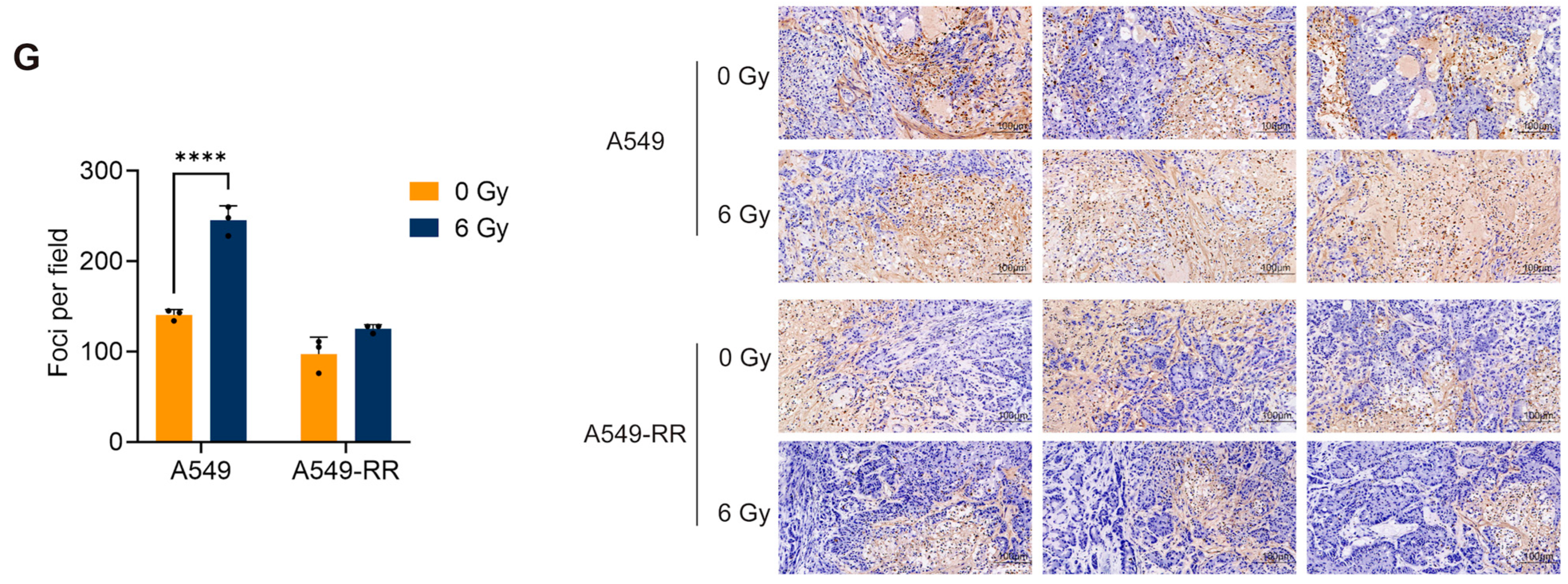
2.1. Modeling and Validation of Radioresistance in A549-RR Cells
2.1.1. In Vitro Experimental Procedure and Validation
2.1.2. In Vivo Validation
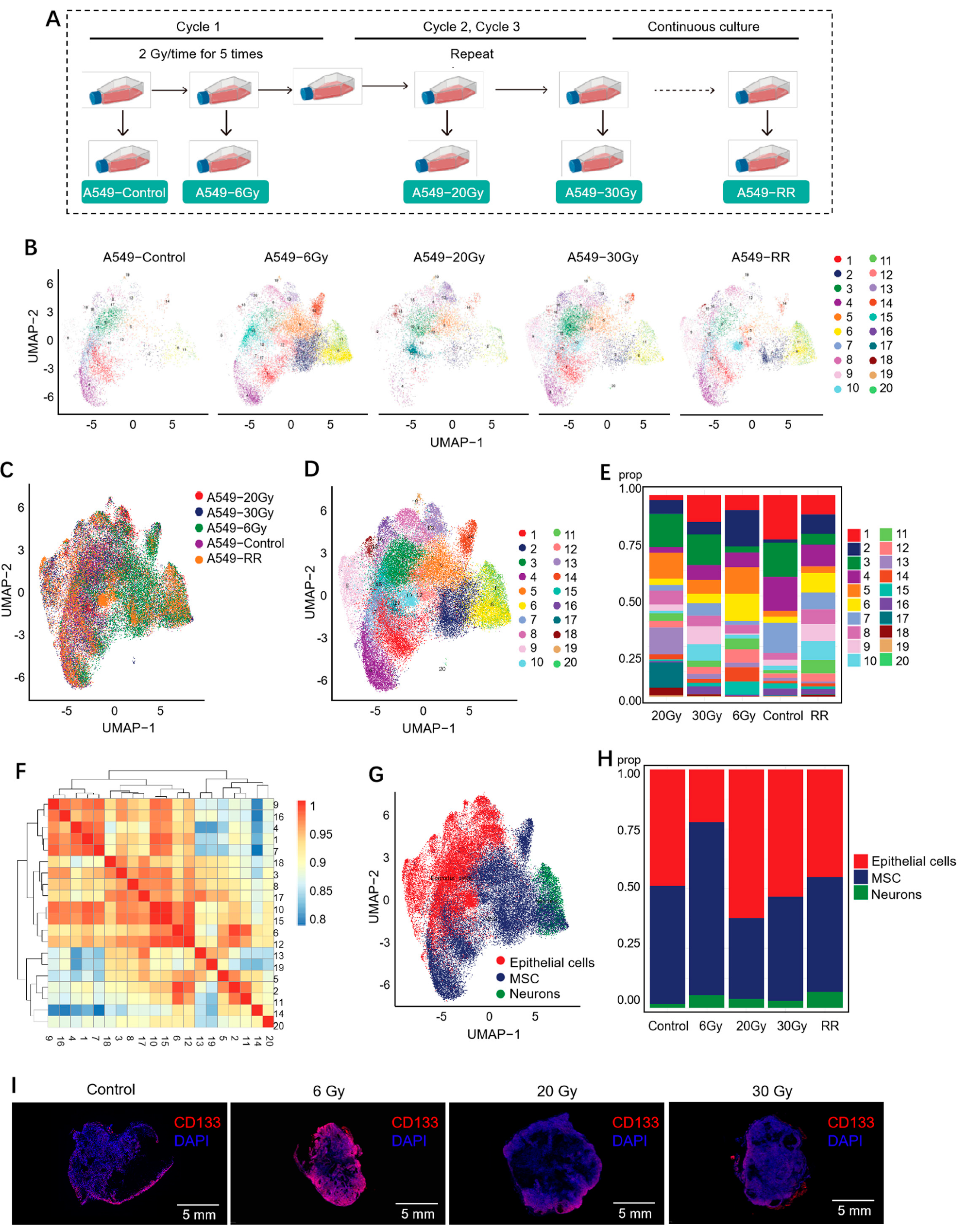
2.2. Single-Cell Sequencing Revealed the Role of Radiation-Induced Stemness-Responsive Cancer Cells in the Development of Radioresistance
2.2.1. Cell Clustering and Cell Type Analysis
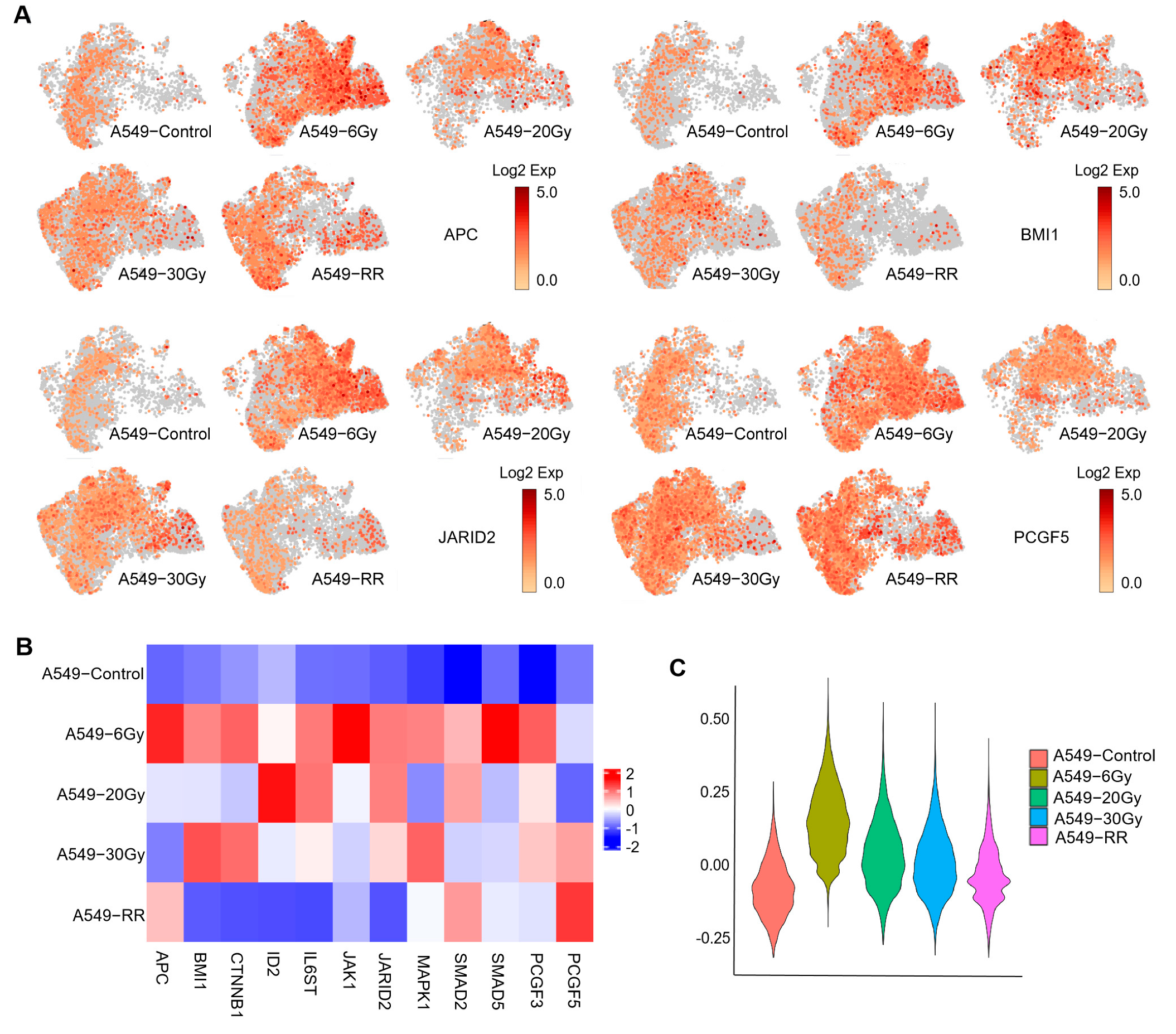
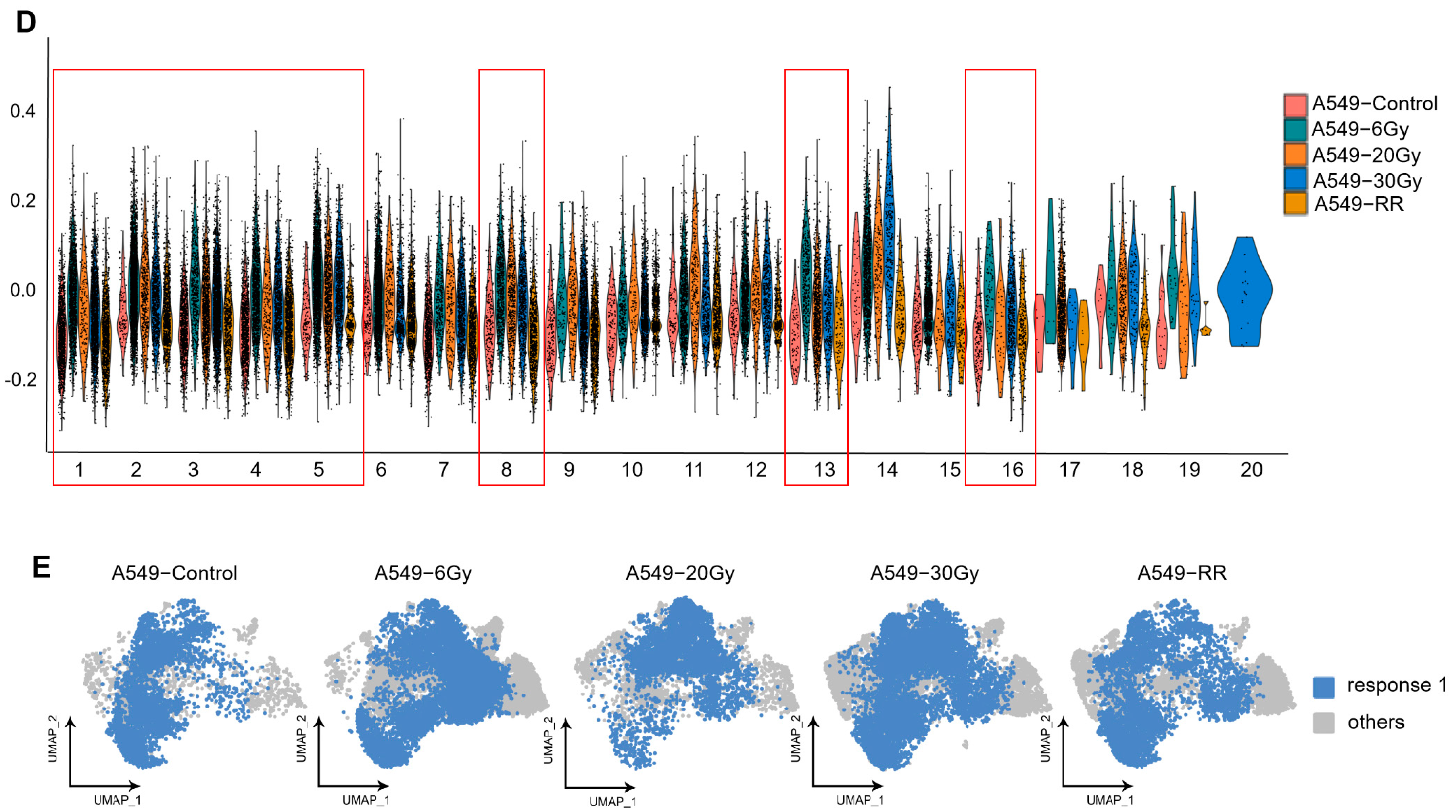
2.2.2. Exploration of Stemness-Responsive Cell Subpopulations
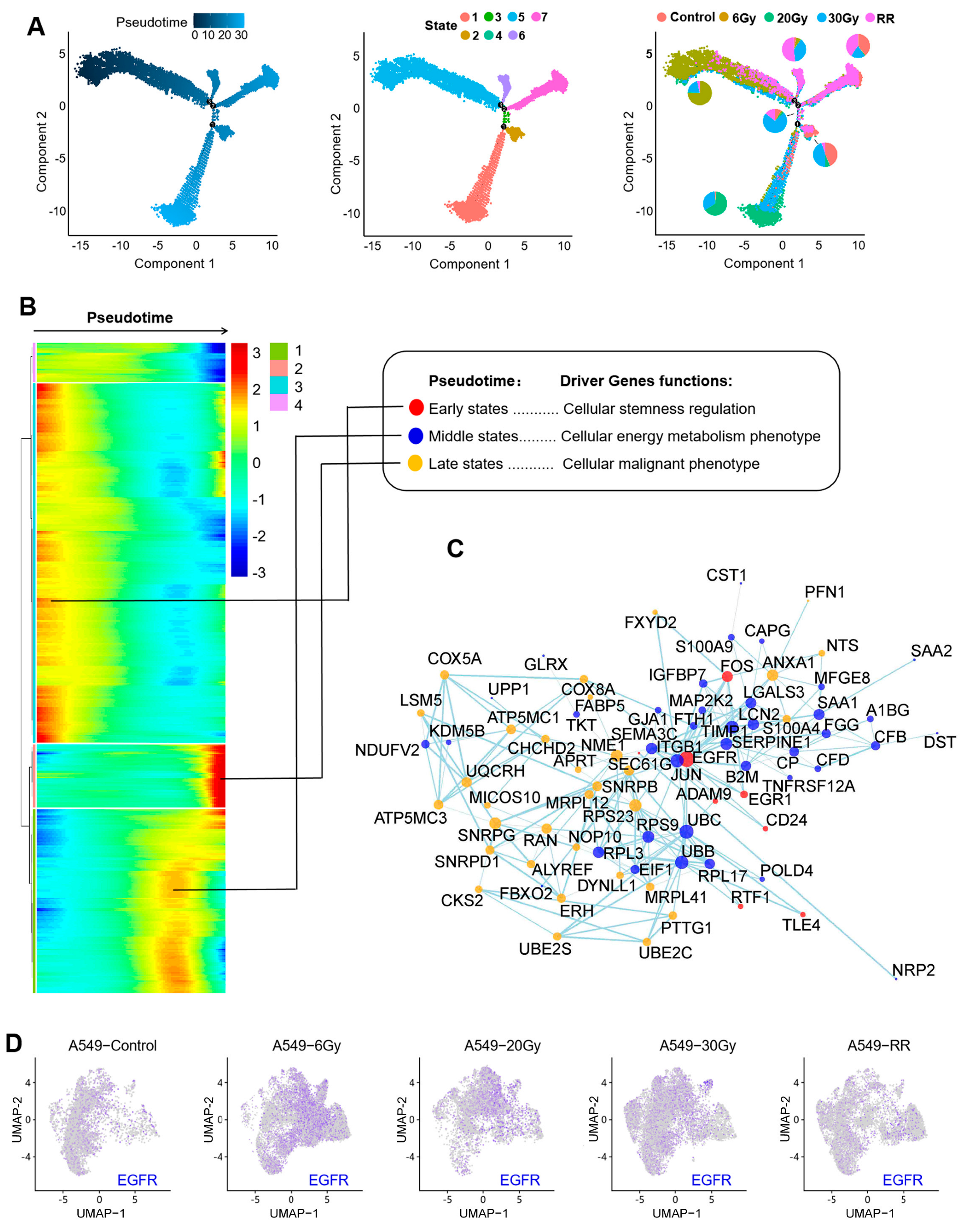
2.2.3. Pseudotime Analysis of Response 1
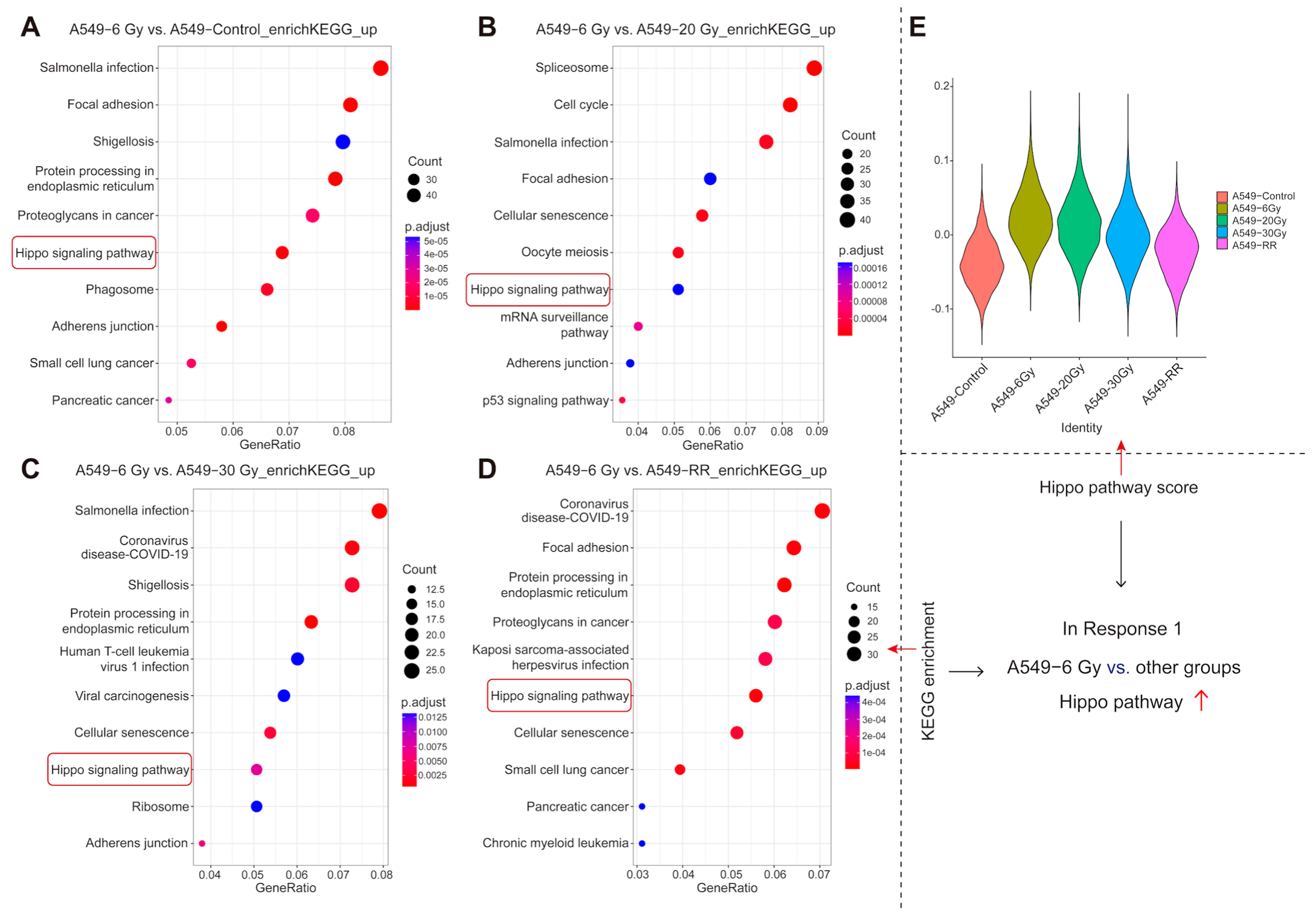
2.2.4. Functional Enrichment of Response 1
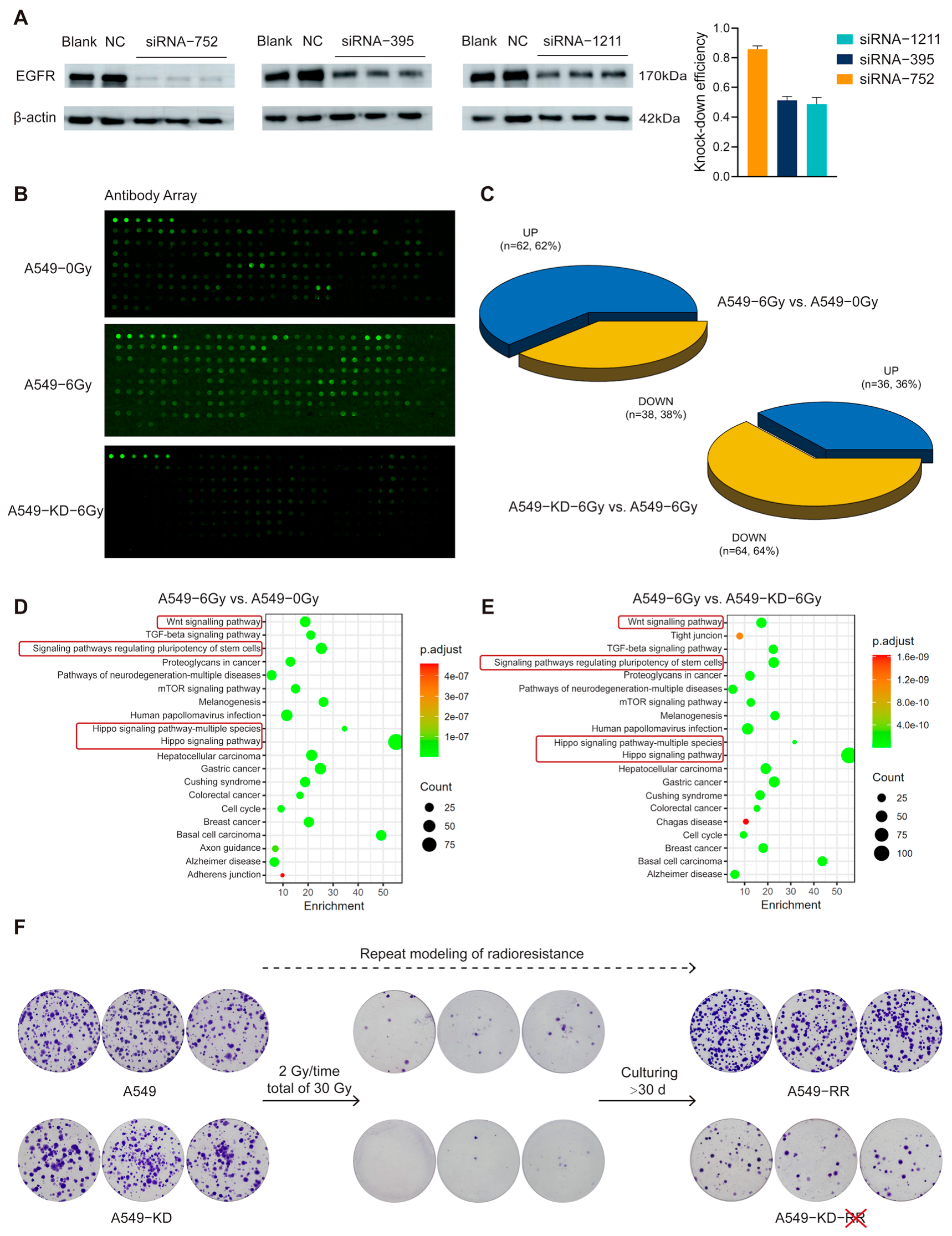
2.3. The EGFR-Hippo Pathway Regulated the Radiation-Induced Stemness Response of A549 Cells Resulting in Radioresistance
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. X-Ray Irradiation
4.3. Cell Viability Assay
4.4. Transwell Assay
4.5. Cell Transfection
4.6. γ-H2AX Foci Test
4.7. Western Blot Assay
4.8. In Vivo Experiments
4.9. Single-Cell Sequencing
4.10. Seahorse Analysis for Measuring Cellular Energy Metabolism Phenotypes
4.11. Protein Screening Array
4.12. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSC | Cancer stem cell |
| ECAR | Extracellular acidification rate |
| EGFR | Epidermal growth factor receptor |
| EGFR-TKI | Epidermal growth factor receptor inhibitor |
| FBS | Fetal bovine serum |
| GO | Gene Ontology |
| GSH | Glutathione |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MSC | Mesenchymal stem cell |
| NSCLC | Non-small cell lung cancer |
| OCR | Oxygen consumption rate |
| PBS | Phosphate-buffered saline |
| PFS | Progression-free survival |
| ROS | Reactive oxygen species |
| SBRT | Stereotactic body radiation therapy |
| TAMs | Tumor-associated macrophages |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TGI | Tumor growth inhibition |
| TME | Tumor microenvironment |
| UMAP | Uniform Manifold Approximation and Projection |
| WBRT | Whole brain radiation therapy |
| YAP | Yes-Associated Protein |
References
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Sui, X.; Geng, J.H.; Li, Y.H.; Zhu, G.Y.; Wang, W.H. Calcium channel α2δ1 subunit (CACNA2D1) enhances radioresistance in cancer stem-like cells in non-small cell lung cancer cell lines. Cancer Manag. Res. 2018, 10, 5009–5018. [Google Scholar] [CrossRef]
- Tu, S.M.; Lin, S.H.; Logothetis, C.J. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002, 3, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Iwatsuki, M.; Ieta, K.; Ohta, D.; Haraguchi, N.; Mimori, K.; Mori, M. Cancer stem cells and chemoradiation resistance. Cancer Sci. 2008, 99, 1871–1877. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.W.; Clarke, M.F. Recent advances in cancer stem cells. Curr. Opin. Genet. Dev. 2008, 18, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gibson, P.; Currle, D.S.; Tong, Y.; Richardson, R.J.; Bayazitov, I.T.; Poppleton, H.; Zakharenko, S.; Ellison, D.W.; Gilbertson, R.J. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009, 457, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.; Yakimova, A.; Matchuk, O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Webb, B.; Gerson, S.L. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother. Oncol. 2014, 110, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M. CD44: A cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin. Cancer Res. 2010, 16, 5091–5093. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Chen, Y.; Liang, N.; Xie, J.; Deng, G.; Chen, F.; Wang, X.; Liu, F.; Li, Y.; Zhang, J. Targeting Epithelial-to-Mesenchymal Transition in Radioresistance: Crosslinked Mechanisms and Strategies. Front. Oncol. 2022, 12, 775238. [Google Scholar] [CrossRef]
- Nantajit, D.; Lin, D.; Li, J.J. The network of epithelial-mesenchymal transition: Potential new targets for tumor resistance. J. Cancer Res. Clin. Oncol. 2015, 141, 1697–1713. [Google Scholar] [CrossRef]
- Zou, K.; Li, Z.; Zhang, Y.; Mu, L.; Chen, M.; Wang, R.; Deng, W.; Zou, L.; Liu, J. β-Elemene enhances radiosensitivity in non-small-cell lung cancer by inhibiting epithelial-mesenchymal transition and cancer stem cell traits via Prx-1/NF-kB/iNOS signaling pathway. Aging 2020, 13, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.H.; Cui, Y.; Qiu, X.N.; Zhang, L.Z.; Zhang, W.; Li, H.; Yu, J.M. Attenuated LKB1-SIK1 signaling promotes epithelial-mesenchymal transition and radioresistance of non-small cell lung cancer cells. Chin. J. Cancer 2016, 35, 50. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, J.; Hu, W.; Dai, L.; Jiang, Z.; Zhong, S.; Deng, B.; Huang, Y.; Wu, W.; Yin, J. LINC01224/ZNF91 Promote Stem Cell-Like Properties and Drive Radioresistance in Non-Small Cell Lung Cancer. Cancer Manag. Res. 2021, 13, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Li, P.; Li, L.; Liu, Y.; Feng, Y.; Li, R.; Xia, S. SOX2 Promotes Radioresistance in Non-small Cell Lung Cancer by Regulating Tumor Cells Dedifferentiation. Int. J. Med. Sci. 2023, 20, 781–796. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 2003, 21, 2787–2799. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Du, W.; Ni, L.; Liu, B.; Wei, Y.; Lv, Y.; Qiang, S.; Dong, J.; Liu, X. Upregulation of SALL4 by EGFR activation regulates the stemness of CD44-positive lung cancer. Oncogenesis 2018, 7, 36. [Google Scholar] [CrossRef]
- Chen, X.; Liang, R.; Lin, H.; Chen, K.; Chen, L.; Tian, G.; Zhu, X. CD166 promotes cancer stem cell-like phenotype via the EGFR/ERK1/2 pathway in the nasopharyngeal carcinoma cell line CNE-2R. Life Sci. 2021, 267, 118983. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Emdad, L.; Das, S.K.; Fisher, P.B. EGFR: An essential receptor tyrosine kinase-regulator of cancer stem cells. Adv. Cancer Res. 2020, 147, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef]
- Greenall, S.A.; Johns, T.G. EGFRvIII: The promiscuous mutation. Cell Death Discov. 2016, 2, 16049. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Asl, A.; Yin, H.; Soleimani, V.D.; Haque, T.; Luchman, H.A.; Chang, N.C.; Sincennes, M.C.; Puram, S.V.; Scott, A.M.; Lorimer, I.A.; et al. Control of glioblastoma tumorigenesis by feed-forward cytokine signaling. Nat. Neurosci. 2016, 19, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Galoczova, M.; Coates, P.; Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell Mol. Biol. Lett. 2018, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.P.; Tang, D. SOX2 plays a critical role in EGFR-mediated self-renewal of human prostate cancer stem-like cells. Cell Signal 2013, 25, 2734–2742. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Marcar, L.; Black, J.; Liu, Q.; Li, X.; Nagulapalli, K.; Sequist, L.V.; Mak, R.H.; Benes, C.H.; et al. Radiation Resistance in KRAS-Mutated Lung Cancer Is Enabled by Stem-like Properties Mediated by an Osteopontin-EGFR Pathway. Cancer Res. 2017, 77, 2018–2028. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, N.; Lai, W.; Yan, B.; Chen, L.; Liu, S.; Liu, S.; Wang, X.; Xiao, D.; Liu, X.; et al. Nuclear EGFR-PKM2 axis induces cancer stem cell-like characteristics in irradiation-resistant cells. Cancer Lett. 2018, 422, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Dong, W.; Zhang, C.; Hu, M.; Ma, W.; Jiang, X.; Li, H.; Yang, P.; Xiang, D. N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells’ Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways. Gastroenterology 2023, 164, 990–1005. [Google Scholar] [CrossRef]
- Thirusangu, P.; Ray, U.; Sarkar Bhattacharya, S.; Oien, D.B.; Jin, L.; Staub, J.; Kannan, N.; Molina, J.R.; Shridhar, V. PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma. Oncogene 2022, 41, 4003–4017. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Chen, P.; Li, S.; Cheng, Y. Thymine DNA glycosylase-regulated TAZ promotes radioresistance by targeting nonhomologous end joining and tumor progression in esophageal cancer. Cancer Sci. 2020, 111, 3613–3625. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, Y.; Chen, P.; Yin, T.; Wang, M.; Liu, T.; Wen, Z.; Cheng, Y. CD155 promotes radioresistance and malignancy of esophageal cancer by regulating Hippo-YAP pathway. Discov. Oncol. 2022, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhou, X.; Xie, Q.; Sun, H.; Huang, K.; Chen, H.; Wang, W.; Zhou, B.; Wei, X.; Zeng, D.; et al. CD146 interaction with integrin β1 activates LATS1-YAP signaling and induces radiation-resistance in breast cancer cells. Cancer Lett. 2022, 546, 215856. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Kwon, H.; Han, C.; Zhang, J.; Dash, S.; Lim, K.; Wu, T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: Regulation by MIR-122. Oncotarget 2015, 6, 40822–40835. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Uthaya Kumar, D.B.; Punj, V.; Xu, J.; Sher, L.; Tahara, S.M.; Hess, S.; Machida, K. NANOG Metabolically Reprograms Tumor-Initiating Stem-like Cells through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell Metab. 2016, 23, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Ren, Y.; Feng, M.; Meng, P.; Wang, Q.; Chen, W.; Jiao, Q.; Wang, Y.; Du, L.; Zhou, F.; et al. HDAC11 Regulates Glycolysis through the LKB1/AMPK Signaling Pathway to Maintain Hepatocellular Carcinoma Stemness. Cancer Res. 2021, 81, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Palorini, R.; Votta, G.; Balestrieri, C.; Monestiroli, A.; Olivieri, S.; Vento, R.; Chiaradonna, F. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J. Cell Biochem. 2014, 115, 368–379. [Google Scholar] [CrossRef]
- Loh, J.J.; Ma, S. Hallmarks of cancer stemness. Cell Stem Cell 2024, 31, 617–639. [Google Scholar] [CrossRef]
- Stouras, I.; Vasileiou, M.; Kanatas, P.F.; Tziona, E.; Tsianava, C.; Theocharis, S. Metabolic Profiles of Cancer Stem Cells and Normal Stem Cells and Their Therapeutic Significance. Cells 2023, 12, 2686. [Google Scholar] [CrossRef] [PubMed]
- Erkisa, M.; Karakas, D.; Ulukaya, E. Cancer Stem Cells: Root of the Evil. Crit. Rev. Oncog. 2019, 24, 69–87. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Zhang, X.; Yan, L.; Chen, Y.; Chen, H.; Liu, J.; Liu, J.; Wu, Y. PTEN-mediated AKT/β-catenin signaling enhances the proliferation and expansion of Lgr5+ hepatocytes. Int. J. Biol. Sci. 2021, 17, 861–868. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Han, J.; Lin, K.; Wang, J.; Li, G.; Li, X.; Gao, Y. PTEN/AKT and Wnt/β-catenin signaling pathways regulate the proliferation of Lgr5+ cells in liver cancer. Biochem. Biophys. Res. Commun. 2023, 683, 149117. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, C.; Zheng, X.; Sun, C.; Li, Q. Feedback loop between hypoxia and energy metabolic reprogramming aggravates the radioresistance of cancer cells. Exp. Hematol. Oncol. 2024, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Bensimon, J.; Biard, D.; Paget, V.; Goislard, M.; Morel-Altmeyer, S.; Konge, J.; Chevillard, S.; Lebeau, J. Forced extinction of CD24 stem-like breast cancer marker alone promotes radiation resistance through the control of oxidative stress. Mol. Carcinog. 2016, 55, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Zhang, Y.; Martin, S.G. Redox proteins and radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2014, 26, 289–300. [Google Scholar] [CrossRef]
- Moncharmont, C.; Levy, A.; Gilormini, M.; Bertrand, G.; Chargari, C.; Alphonse, G.; Ardail, D.; Rodriguez-Lafrasse, C.; Magné, N. Targeting a cornerstone of radiation resistance: Cancer stem cell. Cancer Lett. 2012, 322, 139–147. [Google Scholar] [CrossRef]
- Bütof, R.; Dubrovska, A.; Baumann, M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother. Oncol. 2013, 108, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Fukui, R.; Saga, R.; Matsuya, Y.; Tomita, K.; Kuwahara, Y.; Ohuchi, K.; Sato, T.; Okumura, K.; Date, H.; Fukumoto, M.; et al. Tumor radioresistance caused by radiation-induced changes of stem-like cell content and sub-lethal damage repair capability. Sci. Rep. 2022, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Arang, N.; Wang, Z.; Costea, D.E.; Feng, X.; Goto, Y.; Izumi, H.; Gilardi, M.; Ando, K.; Gutkind, J.S. EGFR Regulates the Hippo pathway by promoting the tyrosine phosphorylation of MOB1. Commun. Biol. 2021, 4, 1237. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.; Irvine, K.D. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev. Cell 2013, 24, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tsarouhas, V.; Samakovlis, C. WASH activation controls endosomal recycling and EGFR and Hippo signaling during tumor-suppressive cell competition. Nat. Commun. 2022, 13, 6243. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Alqahtani, A.M.; Youssif, B.G.M.; Gouda, A.M. Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.H.; Huang, J.W.; Tseng, J.S.; Chen, K.W.; Weng, Y.C.; Yu, S.L.; Yang, T.Y.; Huang, Y.H.; Chen, J.J.W.; Chen, K.C.; et al. Primary Tumor Radiotherapy During EGFR-TKI Disease Control Improves Survival of Treatment Naïve Advanced EGFR-Mutant Lung Adenocarcinoma Patients. Onco Targets Ther. 2021, 14, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, Z.; Cai, J.; Xu, P.; Liang, P.; Luo, Y.; Liu, A. Efficacy and acquired resistance for EGFR-TKI plus thoracic SBRT in patients with advanced EGFR-mutant non-small-cell lung cancer: A propensity-matched retrospective study. BMC Cancer 2021, 21, 482. [Google Scholar] [CrossRef]
- Jia, W.; Guo, H.; Jing, W.; Jing, X.; Li, J.; Wang, M.; Yu, J.; Zhu, H. An especially high rate of radiation pneumonitis observed in patients treated with thoracic radiotherapy and simultaneous osimertinib. Radiother. Oncol. 2020, 152, 96–100. [Google Scholar] [CrossRef]
- Zhou, K.; Cai, X.; Wang, X.; Lan, X.; Zhang, X. Efficacy and safety of WBRT+EGFR-TKI versus WBRT only in the treatment of NSCLC patients with brain metastasis: An updated meta-analysis. Thorac. Cancer 2022, 13, 563–570. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Li, M.F.; Lin, J.H.; Lin, D.; Lin, R.J. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: A retrospective cohort study. Cancer Manag. Res. 2019, 11, 2129–2138. [Google Scholar] [CrossRef]
- Marampon, F.; Gelibter, A.J.; Cicco, P.R.; Parisi, M.; Serpone, M.; De Felice, F.; Bulzonetti, N.; Musio, D.; Cortesi, E.; Tombolini, V. Safety and efficacy of combining afatinib and whole-brain radiation therapy in treating brain metastases from EGFR-mutated NSCLC: A case report and literature review. BJR Case Rep. 2022, 8, 20200134. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Jiang, M.; Li, L.; Wang, D.; Li, Y. Advancements in a novel model of autophagy and immune network regulation in radioresistance of cancer stem cells. Biomed. Pharmacother. 2024, 179, 117420. [Google Scholar] [CrossRef] [PubMed]
- Golino, J.L.; Bian, J.; Wang, X.; Fu, J.; Zhu, X.B.; Yeo, J.; Kelly, M.; Escorcia, F.E.; Cam, M.; Xie, C. Single-cell RNA sequencing reveals cancer stem-like cells and dynamics in tumor microenvironment during cholangiocarcinoma progression. Front. Cell Dev. Biol. 2023, 11, 1250215. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein enhances the radiosensitivity of breast cancer cells via G₂/M cell cycle arrest and apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef]
- Liu, X.X.; Sun, C.; Jin, X.D.; Li, P.; Zheng, X.G.; Zhao, T.; Li, Q. Genistein sensitizes sarcoma cells in vitro and in vivo by enhancing apoptosis and by inhibiting DSB repair pathways. J. Radiat. Res. 2016, 57, 227–237. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Hu, C.; Liu, J.; Cheng, W.; Chen, X.; Liu, X.; Bao, Y.; Tian, H.; Yu, B.; Gao, F.; et al. Single-Cell Sequencing Reveals the Role of Radiation-Induced Stemness-Responsive Cancer Cells in the Development of Radioresistance. Int. J. Mol. Sci. 2025, 26, 1433. https://doi.org/10.3390/ijms26041433
Shi Z, Hu C, Liu J, Cheng W, Chen X, Liu X, Bao Y, Tian H, Yu B, Gao F, et al. Single-Cell Sequencing Reveals the Role of Radiation-Induced Stemness-Responsive Cancer Cells in the Development of Radioresistance. International Journal of Molecular Sciences. 2025; 26(4):1433. https://doi.org/10.3390/ijms26041433
Chicago/Turabian StyleShi, Zheng, Cuilan Hu, Jiadi Liu, Wei Cheng, Xiaohua Chen, Xiongxiong Liu, Yanyu Bao, Haidong Tian, Boyi Yu, Feifei Gao, and et al. 2025. "Single-Cell Sequencing Reveals the Role of Radiation-Induced Stemness-Responsive Cancer Cells in the Development of Radioresistance" International Journal of Molecular Sciences 26, no. 4: 1433. https://doi.org/10.3390/ijms26041433
APA StyleShi, Z., Hu, C., Liu, J., Cheng, W., Chen, X., Liu, X., Bao, Y., Tian, H., Yu, B., Gao, F., Ye, F., Jin, X., Sun, C., & Li, Q. (2025). Single-Cell Sequencing Reveals the Role of Radiation-Induced Stemness-Responsive Cancer Cells in the Development of Radioresistance. International Journal of Molecular Sciences, 26(4), 1433. https://doi.org/10.3390/ijms26041433





