The Cortisol Effect on the NO/cGMP Pathway
Abstract
1. Introduction
2. Results
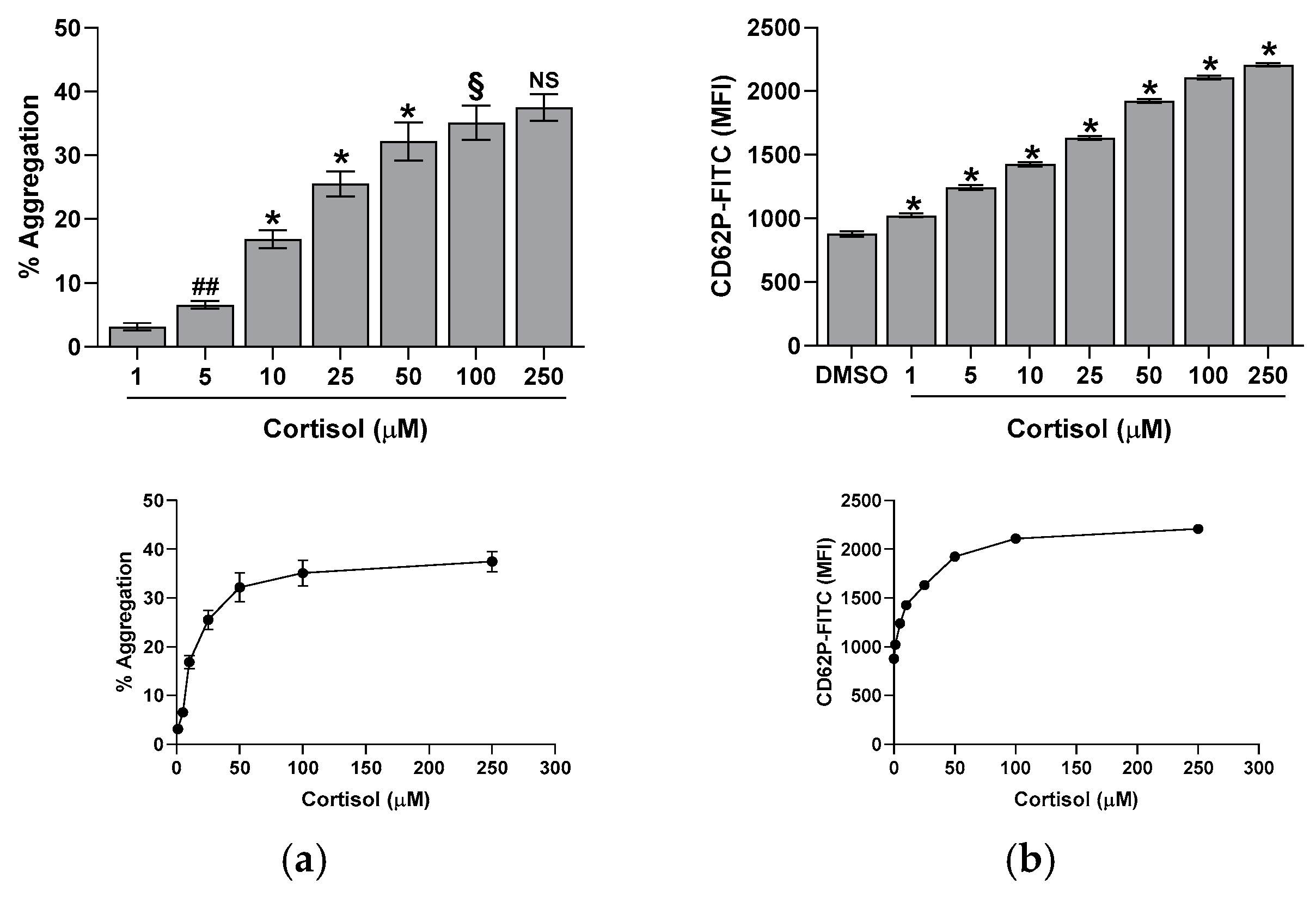
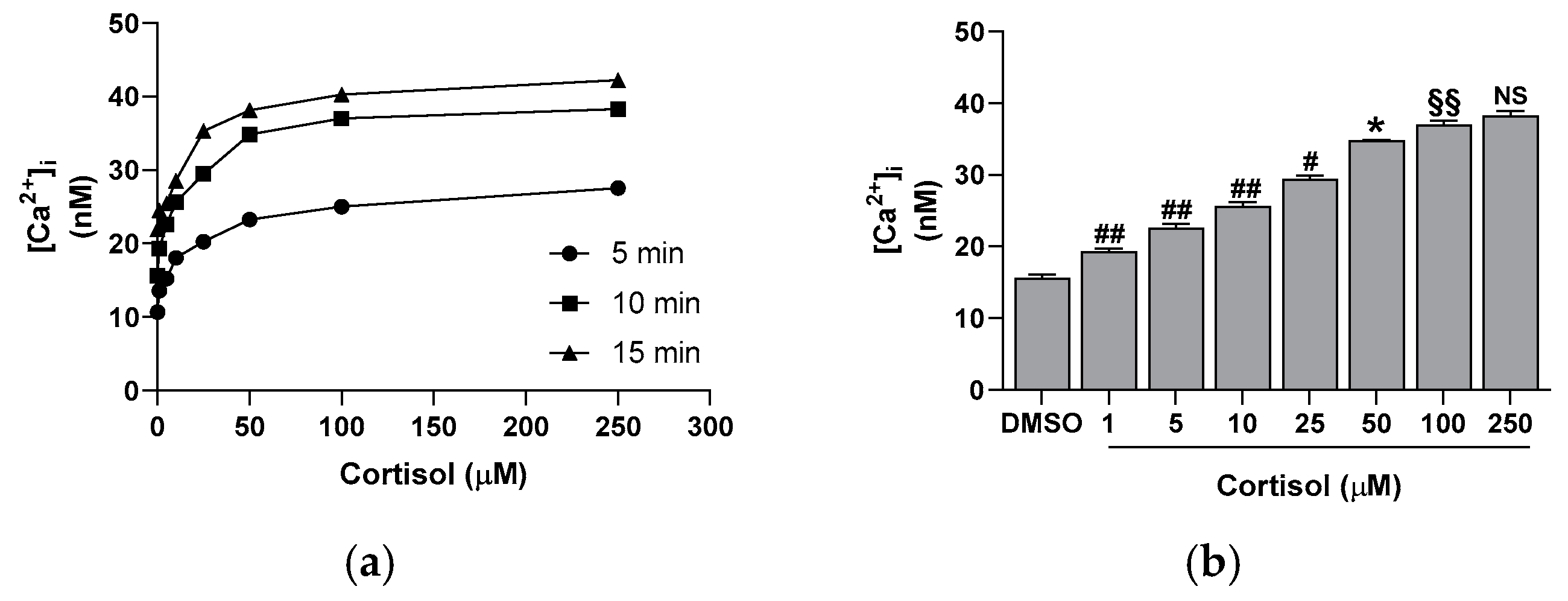
2.1. Effect of Cortisol on Platelet Aggregation, CD62P Exposure, and Calcium Elevation
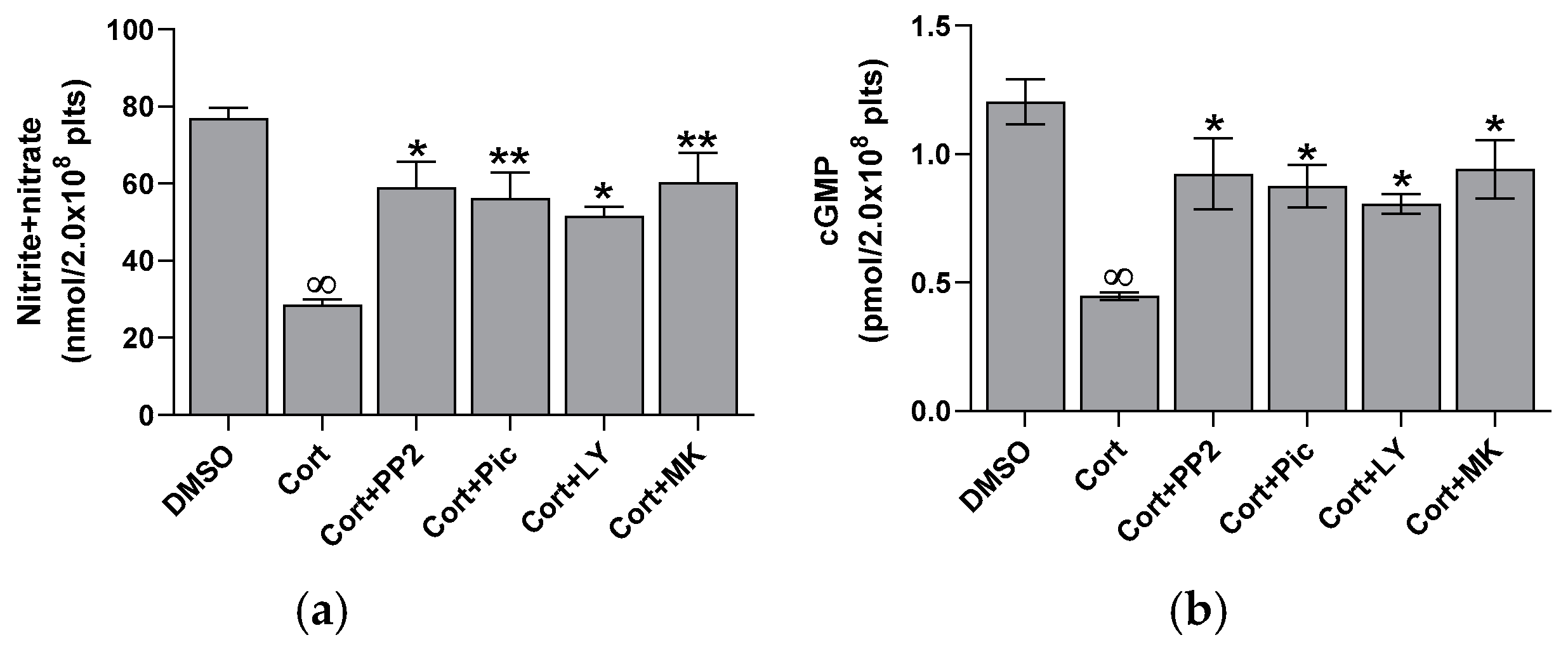
2.2. Effect of Cortisol on NO and cGMP Basal Levels
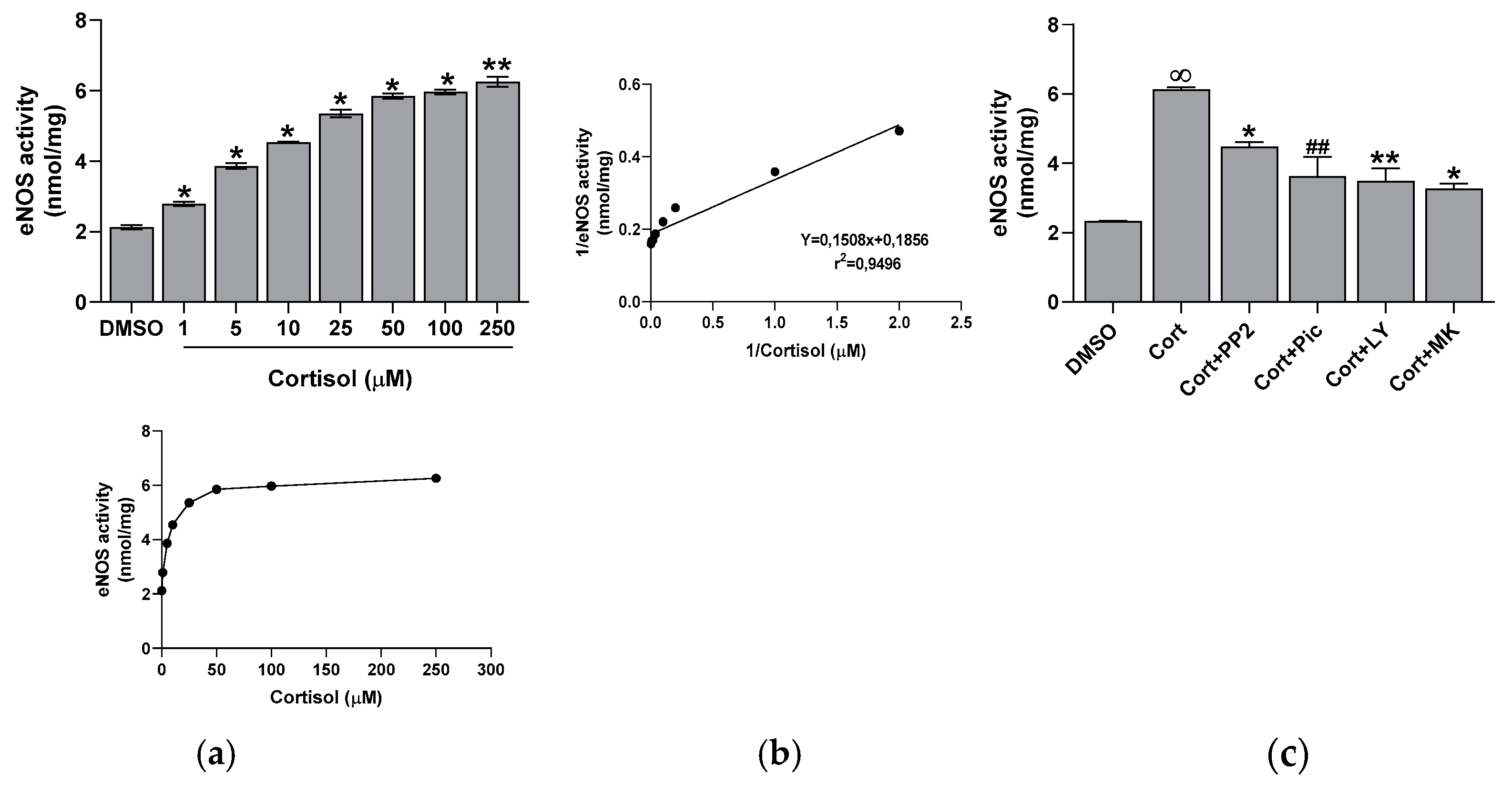
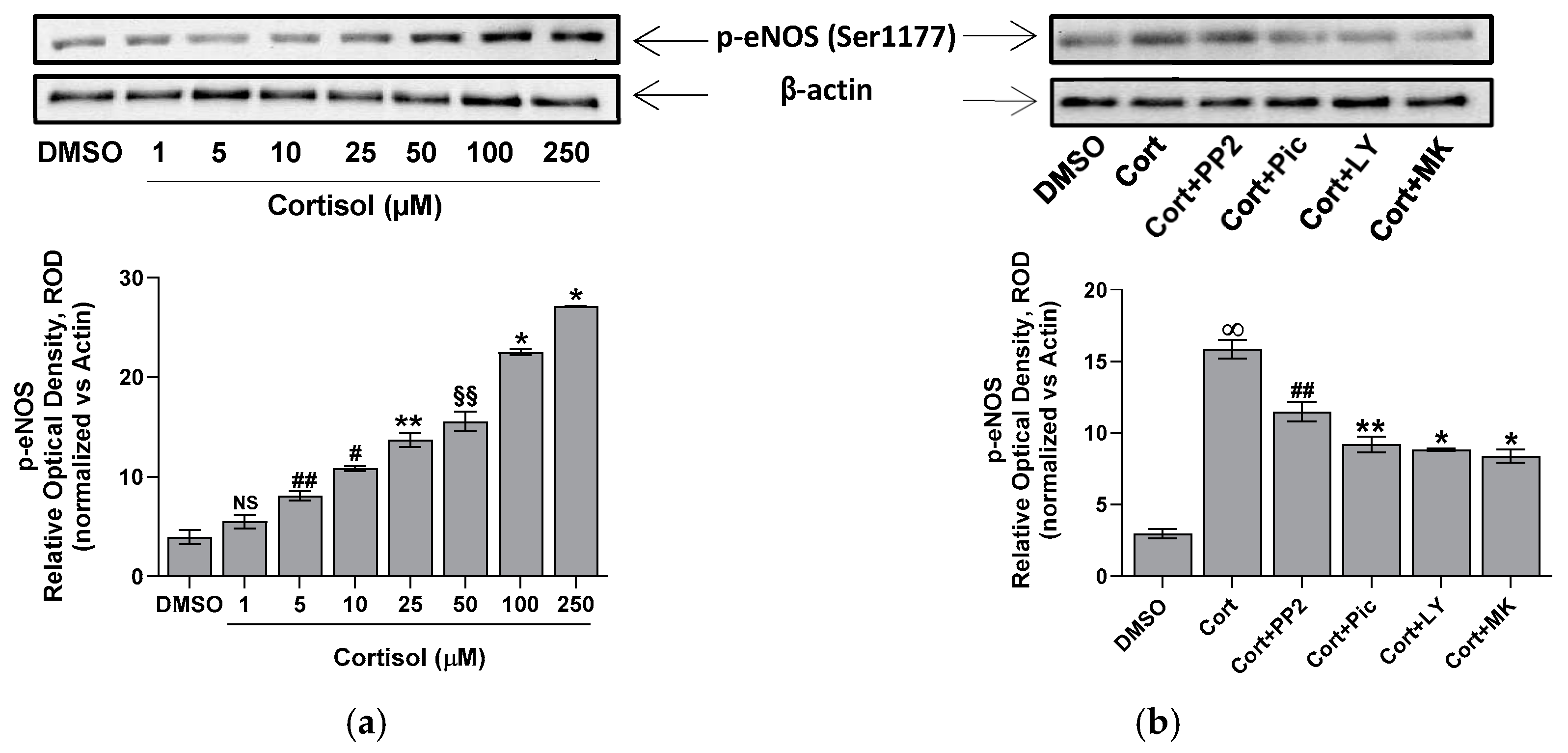
2.3. Effect of Cortisol on eNOS Activity
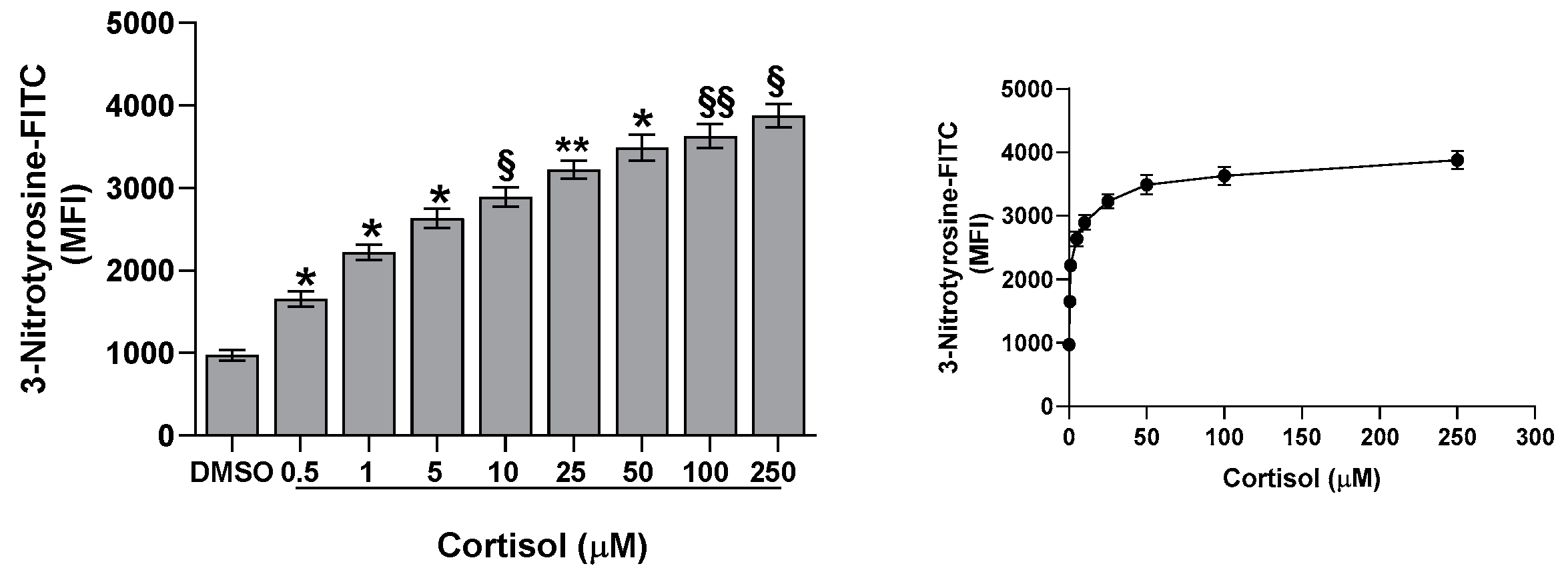
2.4. Effect of Cortisol on Nitrotyrosine Formation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Blood Collection and Preparative Procedure
4.3. Aggregation Studies
4.4. Flow Cytometric Analysis of CD62P
4.5. Intracellular [Ca2+] Measurement
4.6. Nitrite + Nitrate Measurement
4.7. cGMP Determination
4.8. eNOS Assay in a Cell-Free System
4.9. Immunoblotting Analysis
4.10. Nitrotyrosine Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelier, F.; Wingfield, J.C. Importance of the glucocorticoid stress response in a changing world: Theory, hypotheses and perspectives. Gen. Comp. Endocrinol. 2013, 190, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Van Zaane, B.; Nur, E.; Squizzato, A.; Dekkers, O.M.; Twickler, M.B.; Fliers, E.; Gerdes, V.E.; Buller, H.R.; Brandjes, D.P. Hypercoagulable state in Cushing’s syndrome: A systematic review. J. Clin. Endocrinol. Metab. 2009, 94, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Stuijver, D.J.F.; Van Zaane, B.; Feelders, R.A.; Debeij, J.; Cannegieter, S.C.; Hermus, A.R.; Berg, G.v.D.; Pereira, A.M.; de Herder, W.W.; Wagenmakers, M.A.E.M.; et al. Incidence of venous thromboembolism in patients with Cushing’s syndrome: A multicenter cohort study. J. Clin. Endocrinol. Metab. 2011, 96, 3525–3532. [Google Scholar] [CrossRef]
- Graversen, D.; Vestergaard, P.; Stochholm, K.; Gravholt, C.H.; Jørgensen, J.O.L. Mortality in Cushing’s syndrome: A systematic review and meta-analysis. Eur. J. Intern. Med. 2012, 23, 278–282. [Google Scholar] [CrossRef]
- Karamouzis, I.; Berardelli, R.; D’Angelo, V.; Fussotto, B.; Zichi, C.; Giordano, R.; Settanni, F.; Maccario, M.; Ghigo, E.; Arvat, E. Enhanced oxidative stress and platelet activation in patients with Cushing’s syndrome. Clin. Endocrinol. 2015, 82, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Casonato, A.; Pontara, E.; Boscaro, M.; Sonino, N.; Sartorello, F.; Ferasin, S.; Girolami, A. Abnormalities of von Willebrand factor are also part of the prothrombotic state of Cushing’s syndrome. Blood Coagul. Fibrinolysis 1999, 10, 145–151. [Google Scholar] [CrossRef]
- Unsworth, A.J.; Flora, G.D.; Gibbins, J.M. Non-genomic effects of nuclear receptors: Insights from the anucleate platelet. Cardiovasc. Res. 2018, 114, 645–655. [Google Scholar] [CrossRef]
- Glass, F.L.; Lippton, H.L.; Kadovitz, P.J. Effects of methylprednisolone and hydrocortisone on aggregation of rabbit platelets induced by arachidonic acid and other aggregating substances. Thromb. Haemost. 1981, 46, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Van Giezen, J.J.J.; Brakkee, J.G.P.; Dreteler, G.H.; Bouma, B.N.; Jansen, J.W.C.M. Dexamethasone affects platelet aggregation and fibrinolytic activity in rats at different doses which is reflected by their effect on arterial thrombosis. Blood Coagul. Fibrinolysis 1994, 5, 249–256. [Google Scholar] [CrossRef]
- Moraes, L.A.; Paul-Clark, M.J.; Rickman, A.; Flower, R.J.; Goulding, N.J.; Perretti, M. Ligand-specific glucocorticoid receptor activation in human platelets. Blood 2005, 106, 4167–4175. [Google Scholar] [CrossRef]
- Karolczak, K.; Konieczna, L.; Soltysik, B.; Kostka, T.; Witas, P.J.; Kostanek, J.; Baczek, T.; Watala, C. Plasma Concentration of Cortisol Negatively Associates with Platelet Reactivity in Older Subjects. Int. J. Mol. Sci. 2022, 24, 717. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA 1990, 87, 5193–5197. [Google Scholar] [CrossRef]
- Böhmer, A.; Gambaryan, S.; Tsikas, D. Human blood platelets lack nitric oxide synthase activity. Platelets 2015, 26, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, S.; Tsikas, D. A review and discussion of platelet nitric oxide and nitric oxide synthase: Do blood platelets produce nitric oxide from L-arginine or nitrite? Amino Acids 2015, 47, 1779–1793. [Google Scholar] [CrossRef]
- Chen, L.Y.; Mehta, J.L. Further evidence of the presence of constitutive and inducible nitric oxide synthase isoforms in human platelets. J. Cardiovasc. Pharmacol. 1996, 27, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Randriamboavonjy, V.; Fleming, I. Endothelial nitric oxide synthase (eNOS) in platelets: How is it regulated and what is it doing there? Pharmacol. Rep. 2005, 57, 59–65. [Google Scholar]
- Dangel, O.; Mergia, E.; Karlisch, K.; Groneberg, D.; Koesling, D.; Friebe, A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J. Thromb. Haemost. 2010, 8, 1343–1352. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [PubMed]
- Nakae, H.; Endo, S.; Inada, K.; Yamada, Y.; Nasu, W.; Taniguchi, S.; Ishikura, H.; Tanaka, T.; Wakabayashi, G.; Sato, S. Are nitrite/nitrate (NOx) levels elevated by inhalation injury? Burns 2000, 26, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Signorello, M.G.; Ravera, S.; Leoncini, G. Oxidative Stress Induced by Cortisol in Human Platelets. Int. J. Mol. Sci. 2024, 25, 3776. [Google Scholar] [CrossRef]
- Swiatkowska-Stodulska, R.; Babińska, A.; Sworczak, K. Hypercortisolism and hemostasis. Pol. Merkur. Lek. 2009, 26, 142–144. [Google Scholar]
- Światkowska-Stodulska, R.; Sworczak, K. Disorders of hemostasis in overt and subclinical hypercortisolism. Exp. Clin. Endocrinol. Diabetes 2013, 121, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Świątkowska-Stodulska, R.; Mital, A.; WiŚniewski, P.; Babińska, A.; Skibowska-Bielińska, A.; Sworczak, K. Assessment of platelet function in endogenous hypercortisolism. Endokrynol. Pol. 2015, 66, 207–213. [Google Scholar] [PubMed]
- Patrassi, G.; Dal Bo Zanon, R.; Boscaro, M.; Martinelli, S.; Girolami, A. Further studies on the hypercoagulable state of patients with Cushing’s syndrome. Thromb. Haemost. 1985, 54, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Giudice, E.; Giannetto, C.; Marafioti, S.; Piccione, G. Effects of hydrocortisone and aminophylline on the aggregation of equine platelets in vitro. J. Vet. Sci. 2011, 12, 215–219. [Google Scholar] [CrossRef]
- Kristo, C.; Ueland, T.; Godang, K.; Aukrust, P.; Bollerslev, J. Biochemical markers for cardiovascular risk following treatment in endogenous Cushing’s syndrome. J. Endocrinol. Investig. 2008, 31, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Touron, E.; de Flores, R.; Coulbault, L.; Palix, C.; Chocat, A.; Kuhn, E.; Landeau, B.; Mézenge, F.; Roquet, D.; Chauveau, L.; et al. Depressive symptoms in older adults are associated with changes in stress-related markers, functional connectivity and brain volume. Alzheimer’s Res. Ther. 2025, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. Nitric Oxide and the Vascular Endothelium. In The Vascular Endothelium I Handbook of Experimental Pharmacology; Moncada, S., Higgs, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 176/I, pp. 213–254. [Google Scholar]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, E14–E22. [Google Scholar] [CrossRef]
- Kossenjans, W.; Eis, A.; Sahay, R.; Brockman, D.; Myatt, L. Role of peroxynitrite in altered fetal-placental vascular reactivity in diabetes or preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1311–H1319. [Google Scholar] [CrossRef] [PubMed]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef]
- Zou, M.-H.; Shi, C.; Cohen, R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Investig. 2002, 109, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997, 411, 157–160. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Brock, T.A.; Chang, L.Y.; Crapo, J.; Briscoe, P.; Ku, D.; Bradley, W.A.; Gianturco, S.H.; Gore, J.; Freeman, B.A. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA 1994, 91, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Senderovic, A.; Galijasevic, S. The Role of Inducible Nitric Oxide Synthase in Assessing the Functional Level of Coronary Artery Lesions in Chronic Coronary Syndrome. Cardiol. Res. 2024, 15, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cork, L.C.; Griffin, J.W.; Cleveland, D.W. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell 1993, 73, 23–33. [Google Scholar] [CrossRef]
- Born, G.V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorello, M.G.; Leoncini, G. The Cortisol Effect on the NO/cGMP Pathway. Int. J. Mol. Sci. 2025, 26, 1421. https://doi.org/10.3390/ijms26041421
Signorello MG, Leoncini G. The Cortisol Effect on the NO/cGMP Pathway. International Journal of Molecular Sciences. 2025; 26(4):1421. https://doi.org/10.3390/ijms26041421
Chicago/Turabian StyleSignorello, Maria Grazia, and Giuliana Leoncini. 2025. "The Cortisol Effect on the NO/cGMP Pathway" International Journal of Molecular Sciences 26, no. 4: 1421. https://doi.org/10.3390/ijms26041421
APA StyleSignorello, M. G., & Leoncini, G. (2025). The Cortisol Effect on the NO/cGMP Pathway. International Journal of Molecular Sciences, 26(4), 1421. https://doi.org/10.3390/ijms26041421





