Short-Term Fasting Induces Hepatocytes’ Stress Response and Increases Their Resilience
Abstract
1. Introduction
2. Results
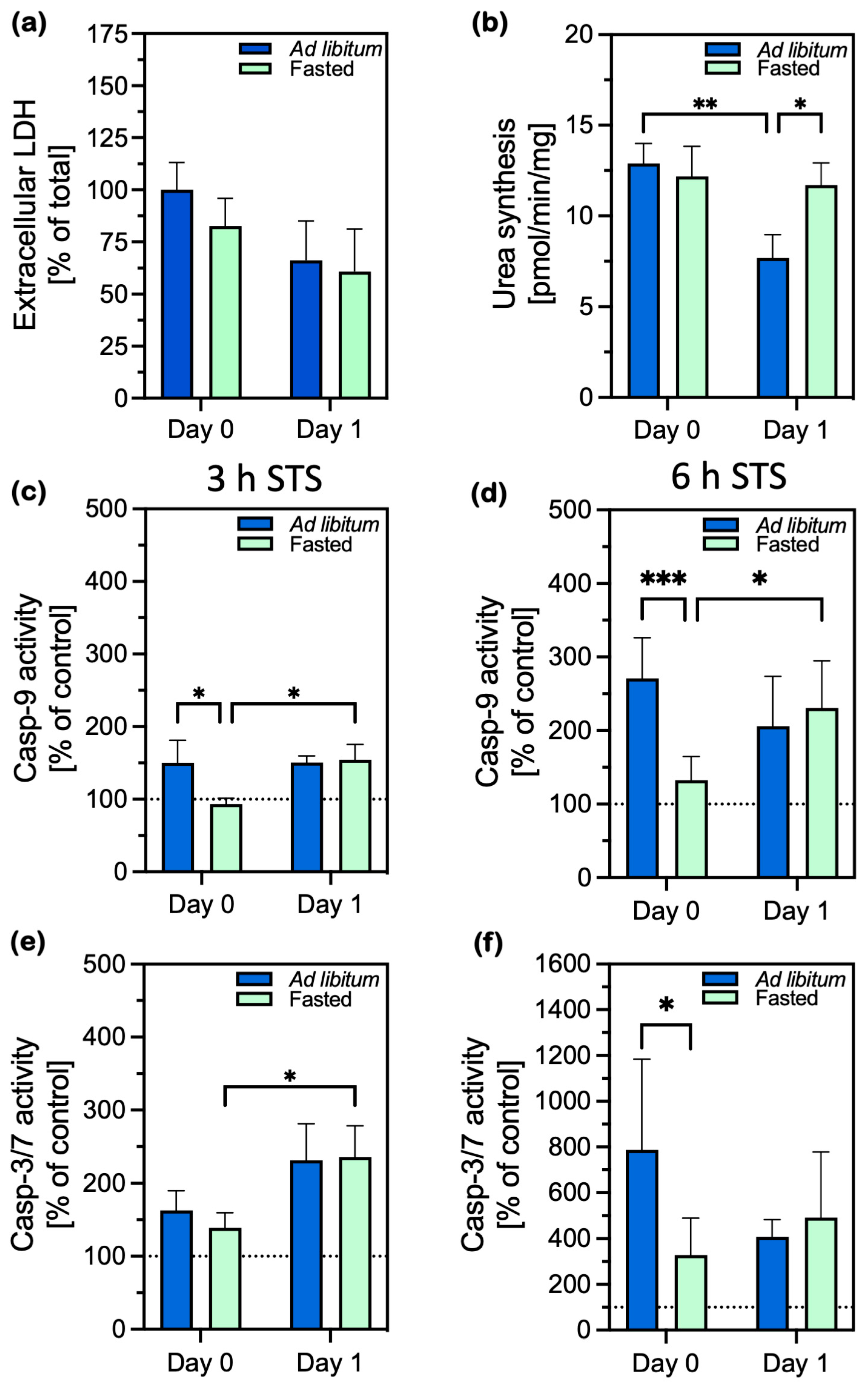
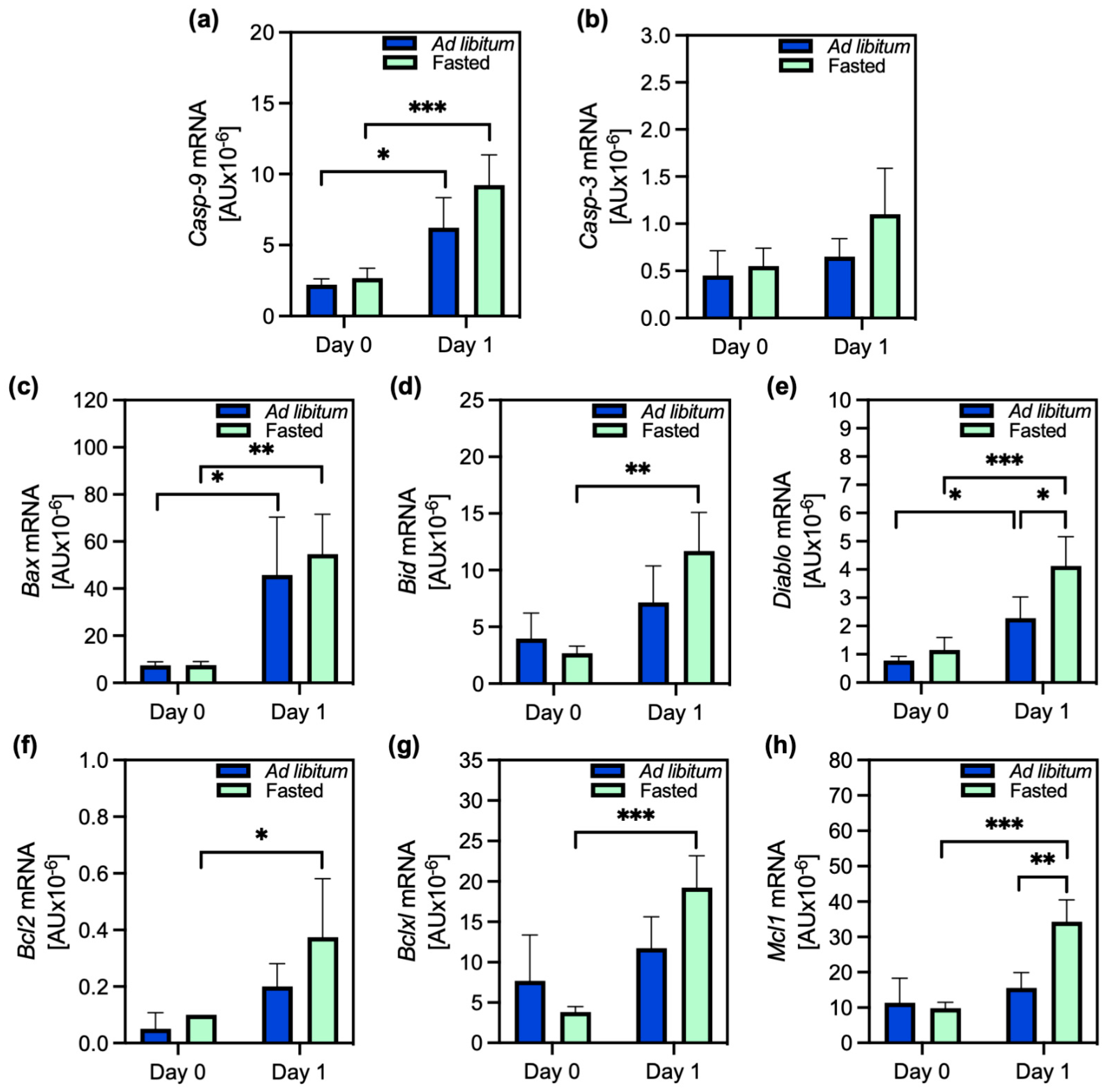
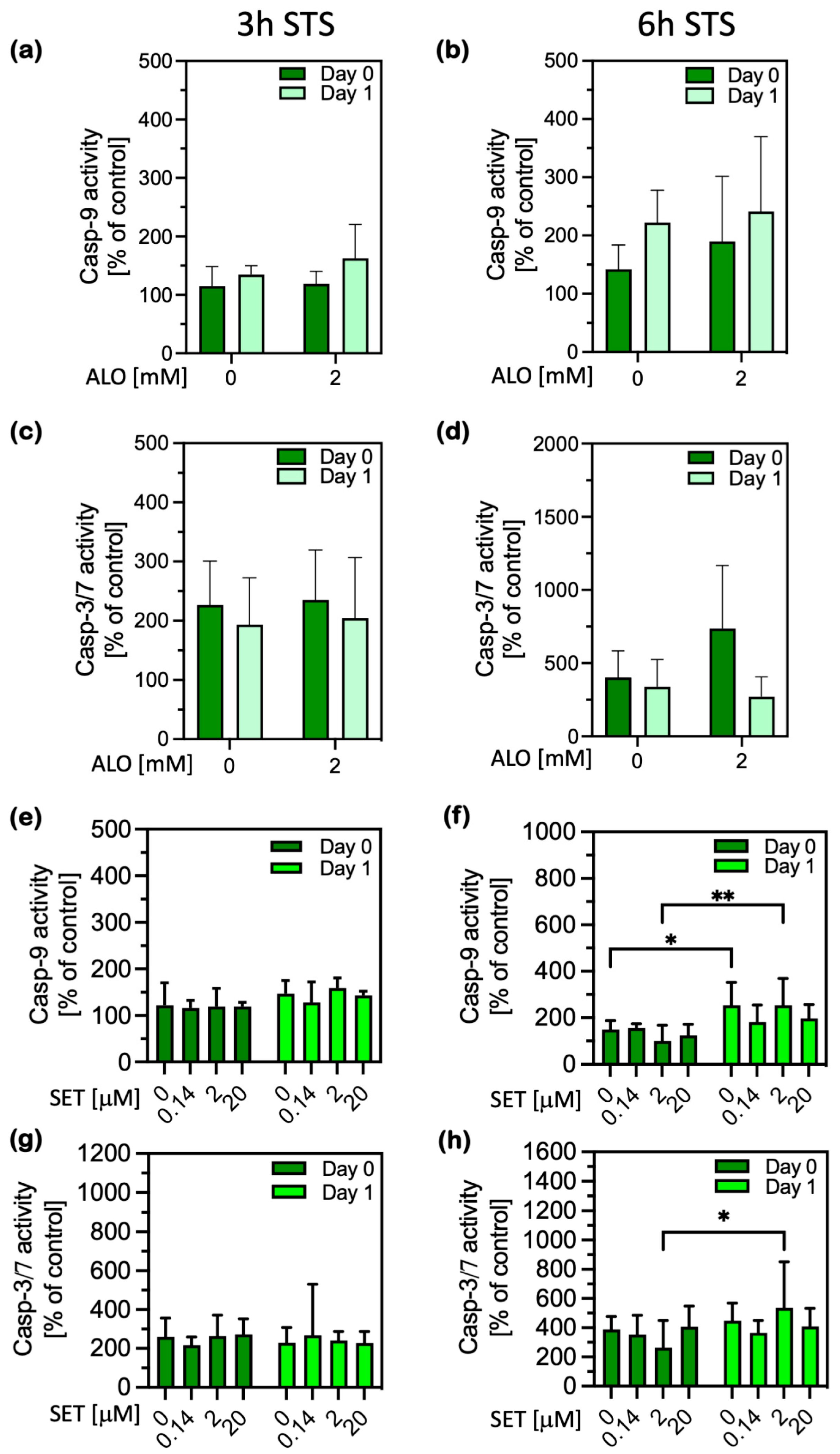
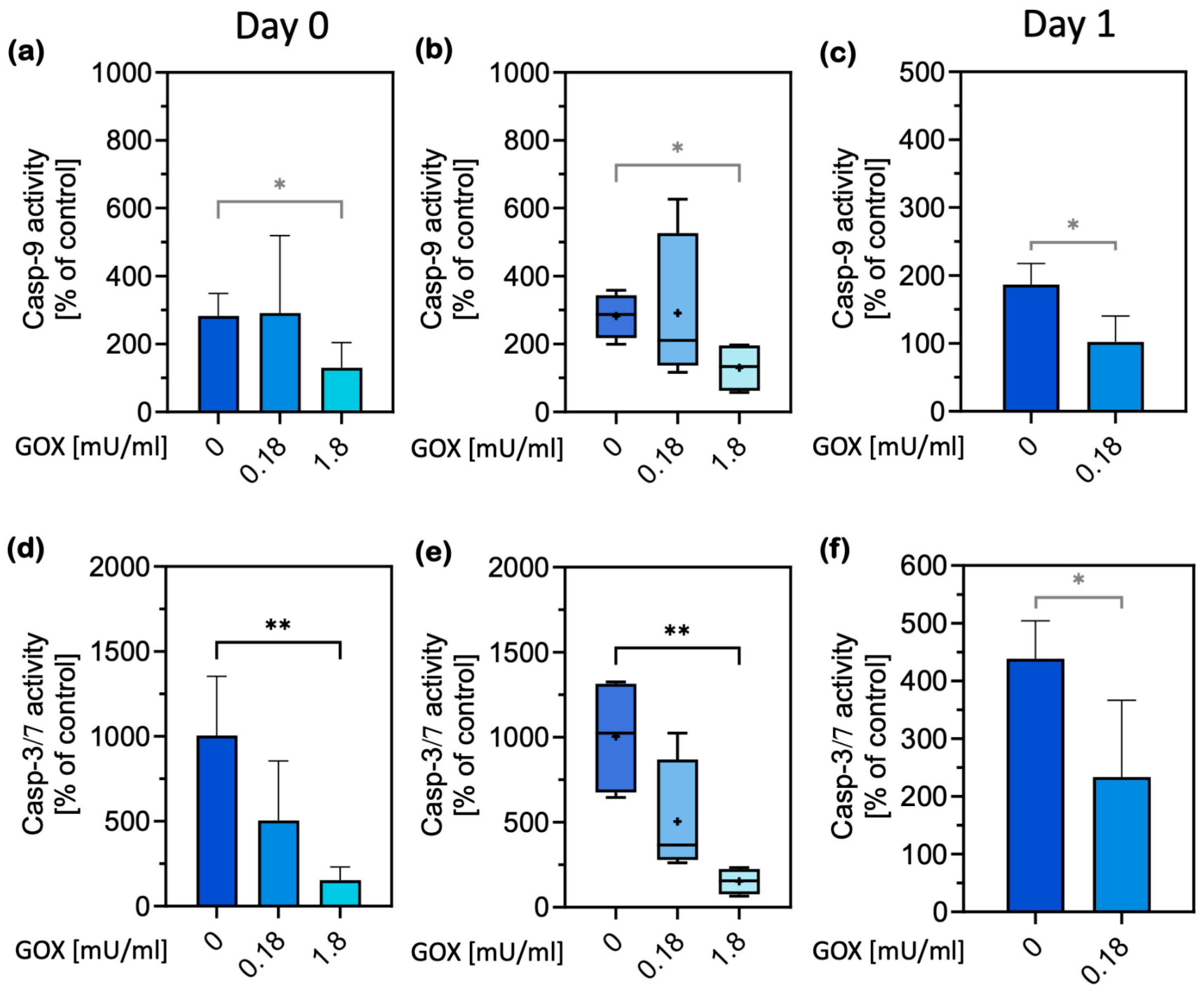
2.1. Cell Damage and Apoptosis
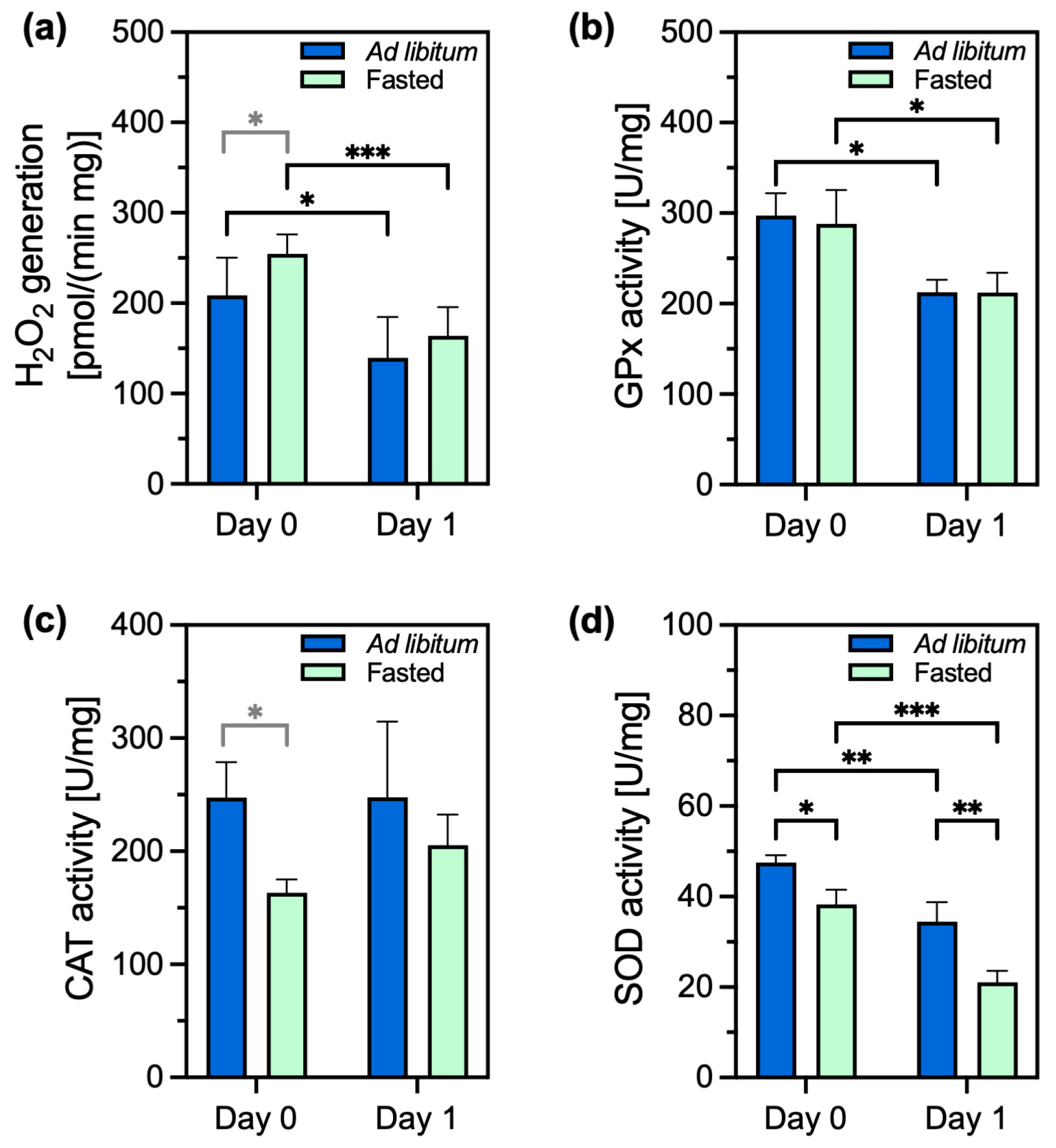
2.2. Oxidants and Antioxidants
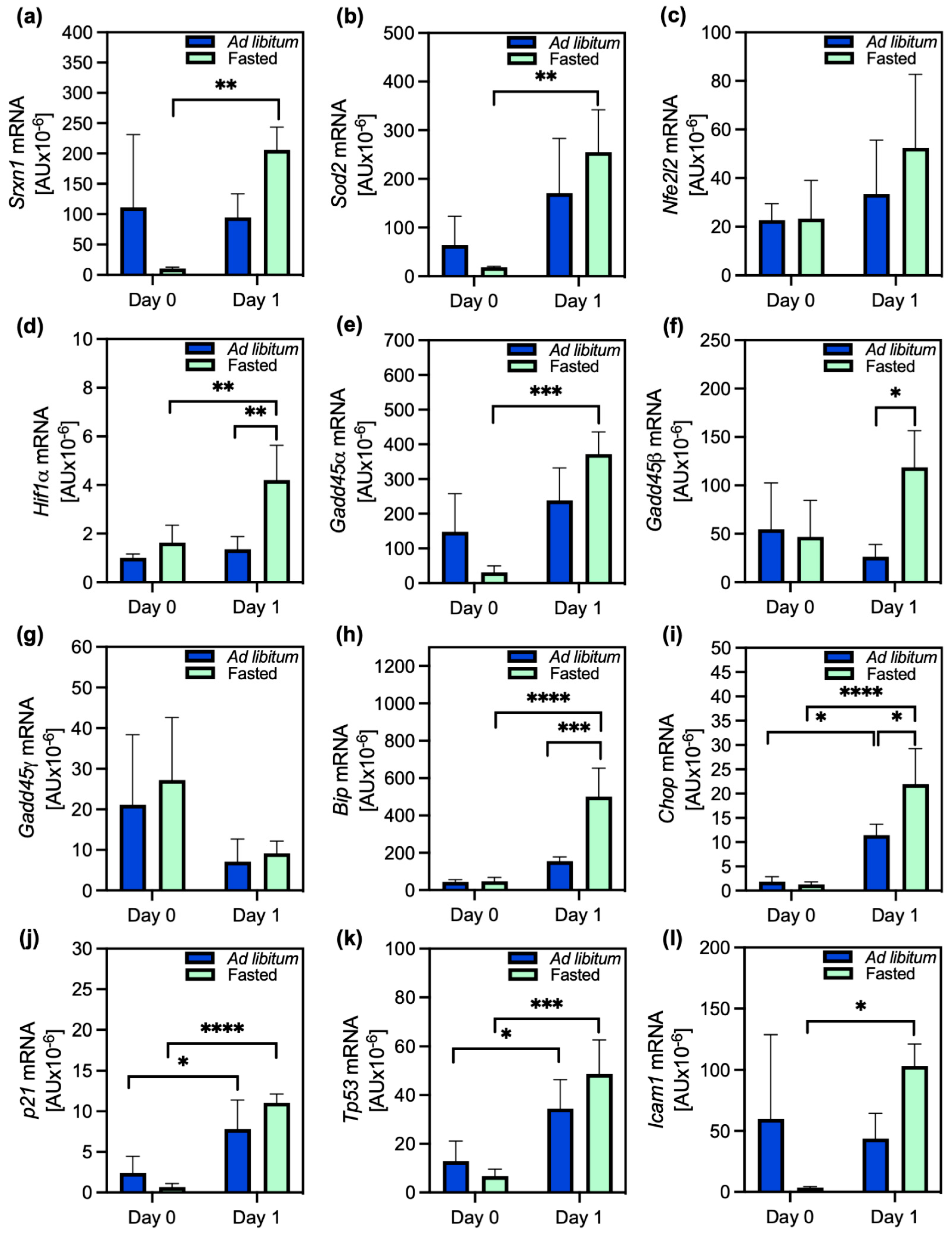
2.3. Stress Response and Modulation of PACOS
3. Discussion
4. Materials and Methods
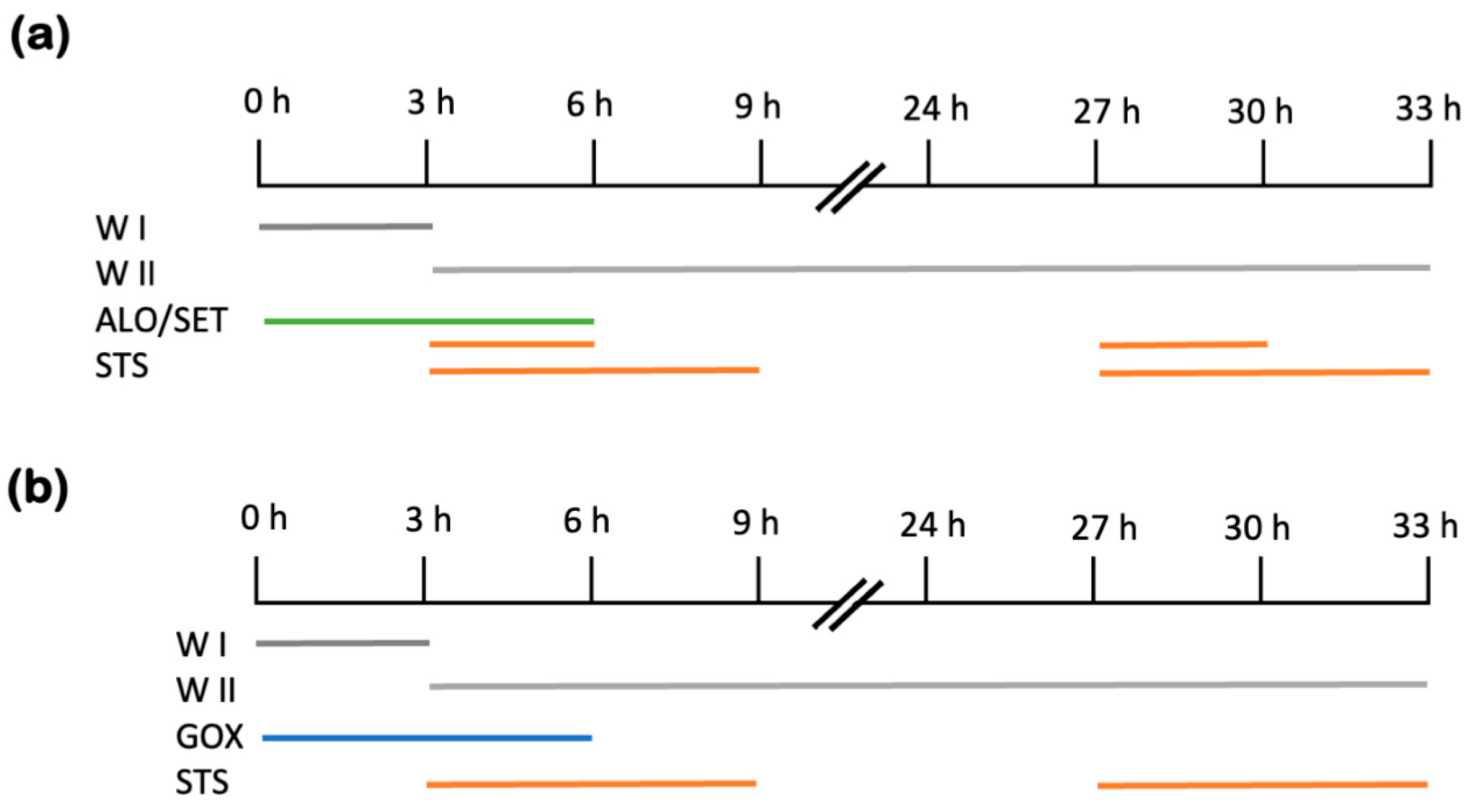
4.1. Cell Culture Treatments and Viability Assays
4.2. Protein, H2O2 and Urea Concentrations
4.3. Antioxidant Enzymes and Caspase Activity Measurements
4.4. RNA Isolation and Reverse-Transcription–Quantitative Polymerase Chain Reaction Analysis (RT-qPCR)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, L.; Luo, Y.; Yu, B.; Tian, G. Effects and possible mechanisms of intermittent fasting on health and disease: A narrative review. Nutr. Rev. 2023, 81, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef]
- Norecopa. Fasting in Rodents. 2009. Available online: https://norecopa.no/media/6351/food-deprivation.pdf (accessed on 19 July 2024).
- Shahrabani-Gargir, L.; Pandita, T.K.; Werner, H. Ataxia-telangiectasia mutated gene controls insulin-like growth factor I receptor gene expression in a deoxyribonucleic acid damage response pathway via mechanisms involving zinc-finger transcription factors Sp1 and WT1. Endocrinology 2004, 145, 5679–5687. [Google Scholar] [CrossRef]
- Liao, P.C.; Bergamini, C.; Fato, R.; Pon, L.A.; Pallotti, F. Isolation of mitochondria from cells and tissues. Methods Cell Biol. 2020, 155, 3–31. [Google Scholar] [CrossRef]
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Curr. Diabetes Rep. 2017, 17, 123. [Google Scholar] [CrossRef]
- Adrie, C.; Richter, C.; Bachelet, M.; Banzet, N.; Francois, D.; Dinh-Xuan, A.T.; Dhainaut, J.F.; Polla, B.S.; Richard, M.J. Contrasting effects of NO and peroxynitrites on HSP70 expression and apoptosis in human monocytes. Am. J. Physiol. Cell Physiol. 2000, 279, C452–C460. [Google Scholar] [CrossRef]
- Diab, R.; Dimachkie, L.; Zein, O.; Dakroub, A.; Eid, A.H. Intermittent Fasting Regulates Metabolic Homeostasis and Improves Cardiovascular Health. Cell Biochem. Biophys. 2024, 82, 1583–1597. [Google Scholar] [CrossRef]
- Shabkhizan, R.; Haiaty, S.; Moslehian, M.S.; Bazmani, A.; Sadeghsoltani, F.; Saghaei Bagheri, H.; Rahbarghazi, R.; Sakhinia, E. The Beneficial and Adverse Effects of Autophagic Response to Caloric Restriction and Fasting. Adv. Nutr. 2023, 14, 1211–1225. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxidative Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Zanieri, F.; Ceni, E.; Galli, A. Oxidative Stress in the Healthy and Wounded Hepatocyte: A Cellular Organelles Perspective. Oxidative Med. Cell. Longev. 2016, 2016, 8327410. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, G.; Horni, M.; Velecka, E.; Bousova, I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: A review. Arch. Toxicol. 2025, 99, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Quan, X.B.; Zeng, W.J.; Yang, X.O.; Wang, M.J. Mechanism of Hepatocyte Apoptosis. J. Cell Death 2016, 9, 19–29. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Samraj, A.K.; Keil, E.; Ueffing, N.; Schulze-Osthoff, K.; Schmitz, I. Loss of caspase-9 provides genetic evidence for the type I/II concept of CD95-mediated apoptosis. J. Biol. Chem. 2006, 281, 29652–29659. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Iksen; Witayateeraporn, W.; Hardianti, B.; Pongrakhananon, V. Comprehensive review of Bcl-2 family proteins in cancer apoptosis: Therapeutic strategies and promising updates of natural bioactive compounds and small molecules. Phytother. Res. 2024, 38, 2249–2275. [Google Scholar] [CrossRef]
- Milisav, I.; Poljsak, B.; Ribaric, S. Reduced risk of apoptosis: Mechanisms of stress responses. Apoptosis 2017, 22, 265–283. [Google Scholar] [CrossRef]
- Adrain, C.; Creagh, E.M.; Martin, S.J. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001, 20, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Milisav, I.; Poljsak, B.; Suput, D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int. J. Mol. Sci. 2012, 13, 10771–10806. [Google Scholar] [CrossRef] [PubMed]
- Banic, B.; Nipic, D.; Suput, D.; Milisav, I. DMSO modulates the pathway of apoptosis triggering. Cell. Mol. Biol. Lett. 2011, 16, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Nipic, D.; Pirc, A.; Banic, B.; Suput, D.; Milisav, I. Preapoptotic cell stress response of primary hepatocytes. Hepatology 2010, 51, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.P.; Pavlovic, I.; Poljsak, B.; Suput, D.; Milisav, I. Beneficial Role of ROS in Cell Survival: Moderate Increases in H2O2 Production Induced by Hepatocyte Isolation Mediate Stress Adaptation and Enhanced Survival. Antioxidants 2019, 8, 434. [Google Scholar] [CrossRef]
- Hikita, H.; Takehara, T.; Shimizu, S.; Kodama, T.; Li, W.; Miyagi, T.; Hosui, A.; Ishida, H.; Ohkawa, K.; Kanto, T.; et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology 2009, 50, 1217–1226. [Google Scholar] [CrossRef]
- Cazanave, S.C.; Gores, G.J. The liver’s dance with death: Two Bcl-2 guardian proteins from the abyss. Hepatology 2009, 50, 1009–1013. [Google Scholar] [CrossRef]
- Liebermann, D.A.; Hoffman, B. Gadd45 in stress signaling. J. Mol. Signal. 2008, 3, 15. [Google Scholar] [CrossRef]
- Fuhrmeister, J.; Zota, A.; Sijmonsma, T.P.; Seibert, O.; Cingir, S.; Schmidt, K.; Vallon, N.; de Guia, R.M.; Niopek, K.; Berriel Diaz, M.; et al. Fasting-induced liver GADD45beta restrains hepatic fatty acid uptake and improves metabolic health. EMBO Mol. Med. 2016, 8, 654–669. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, N.; Fan, L.; Yuan, L.; Wu, Q.; Gong, L.; Tao, Z.; Chen, J.; Ren, J. GADD45beta stabilized by direct interaction with HSP72 ameliorates insulin resistance and lipid accumulation. Pharmacol. Res. 2021, 173, 105879. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, X.; Zhou, B.; He, Z.; Zhai, Q. Gene expression profile change and associated physiological and pathological effects in mouse liver induced by fasting and refeeding. PLoS ONE 2011, 6, e27553. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Monsalve, M. ROS homeostasis, a key determinant in liver ischemic-preconditioning. Redox Biol. 2017, 12, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, R.H.; Curbishley, S.M.; Weston, C.J.; Adams, D.H.; Afford, S.C. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010, 16, 1303–1313. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Vazquez-Meza, H.; Vilchis-Landeros, M.M. NOX as a Therapeutic Target in Liver Disease. Antioxidants 2022, 11, 2038. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef]
- Gallage, S.; Ali, A.; Barragan Avila, J.E.; Seymen, N.; Ramadori, P.; Joerke, V.; Zizmare, L.; Aicher, D.; Gopalsamy, I.K.; Fong, W.; et al. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARalpha and PCK1. Cell Metab. 2024, 36, 1371–1393.e7. [Google Scholar] [CrossRef]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef]
- Milisav, I.; Nipic, D.; Suput, D. The riddle of mitochondrial caspase-3 from liver. Apoptosis 2009, 14, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Marotto, M.E.; Thurman, R.G.; Lemasters, J.J. Early midzonal cell death during low-flow hypoxia in the isolated, perfused rat liver: Protection by allopurinol. Hepatology 1988, 8, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Buck, T.; Hack, C.T.; Berg, D.; Berg, U.; Kunz, L.; Mayerhofer, A. The NADPH oxidase 4 is a major source of hydrogen peroxide in human granulosa-lutein and granulosa tumor cells. Sci. Rep. 2019, 9, 3585. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Millonig, G.; Waite, G.N. The GOX/CAT system: A novel enzymatic method to independently control hydrogen peroxide and hypoxia in cell culture. Adv. Med. Sci. 2009, 54, 121–135. [Google Scholar] [CrossRef]
- Bauer, J.A.; Zamocka, M.; Majtan, J.; Bauerova-Hlinkova, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef]
- Ibars, E.P.; Cortes, M.; Tolosa, L.; Gomez-Lechon, M.J.; Lopez, S.; Castell, J.V.; Mir, J. Hepatocyte transplantation program: Lessons learned and future strategies. World J. Gastroenterol. 2016, 22, 874–886. [Google Scholar] [CrossRef]
- Castell, J.V.; Gomez-Lechon, M.J. Liver cell culture techniques. Methods Mol. Biol. 2009, 481, 35–46. [Google Scholar] [CrossRef]
- Grunblatt, E.; Wu, N.; Zhang, H.; Liu, X.; Norton, J.P.; Ohol, Y.; Leger, P.; Hiatt, J.B.; Eastwood, E.C.; Thomas, R.; et al. MYCN drives chemoresistance in small cell lung cancer while USP7 inhibition can restore chemosensitivity. Genes Dev. 2020, 34, 1210–1226. [Google Scholar] [CrossRef]
- Beutler, E. Catalase, 3rd ed.; Grune and Stratton: New York, NY, USA, 1984. [Google Scholar]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Manns, J.; Daubrawa, M.; Driessen, S.; Paasch, F.; Hoffmann, N.; Loffler, A.; Lauber, K.; Dieterle, A.; Alers, S.; Iftner, T.; et al. Triggering of a novel intrinsic apoptosis pathway by the kinase inhibitor staurosporine: Activation of caspase-9 in the absence of Apaf-1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 3250–3261. [Google Scholar] [CrossRef]
- Wink, S.; Hiemstra, S.W.; Huppelschoten, S.; Klip, J.E.; van de Water, B. Dynamic imaging of adaptive stress response pathway activation for prediction of drug induced liver injury. Arch. Toxicol. 2018, 92, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Kramar, B.; Pirc Marolt, T.; Monsalve, M.; Suput, D.; Milisav, I. Antipsychotic Drug Aripiprazole Protects Liver Cells from Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 8292. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prša, P.; Miller, I.P.; Kramar, B.; Šuput, D.; Milisav, I. Short-Term Fasting Induces Hepatocytes’ Stress Response and Increases Their Resilience. Int. J. Mol. Sci. 2025, 26, 999. https://doi.org/10.3390/ijms26030999
Prša P, Miller IP, Kramar B, Šuput D, Milisav I. Short-Term Fasting Induces Hepatocytes’ Stress Response and Increases Their Resilience. International Journal of Molecular Sciences. 2025; 26(3):999. https://doi.org/10.3390/ijms26030999
Chicago/Turabian StylePrša, Patrik, Izak Patrik Miller, Barbara Kramar, Dušan Šuput, and Irina Milisav. 2025. "Short-Term Fasting Induces Hepatocytes’ Stress Response and Increases Their Resilience" International Journal of Molecular Sciences 26, no. 3: 999. https://doi.org/10.3390/ijms26030999
APA StylePrša, P., Miller, I. P., Kramar, B., Šuput, D., & Milisav, I. (2025). Short-Term Fasting Induces Hepatocytes’ Stress Response and Increases Their Resilience. International Journal of Molecular Sciences, 26(3), 999. https://doi.org/10.3390/ijms26030999





