PbrMYB14 Enhances Pear Resistance to Alternaria alternata by Regulating Genes in Lignin and Salicylic Acid Biosynthesis Pathways
Abstract
1. Introduction
2. Results
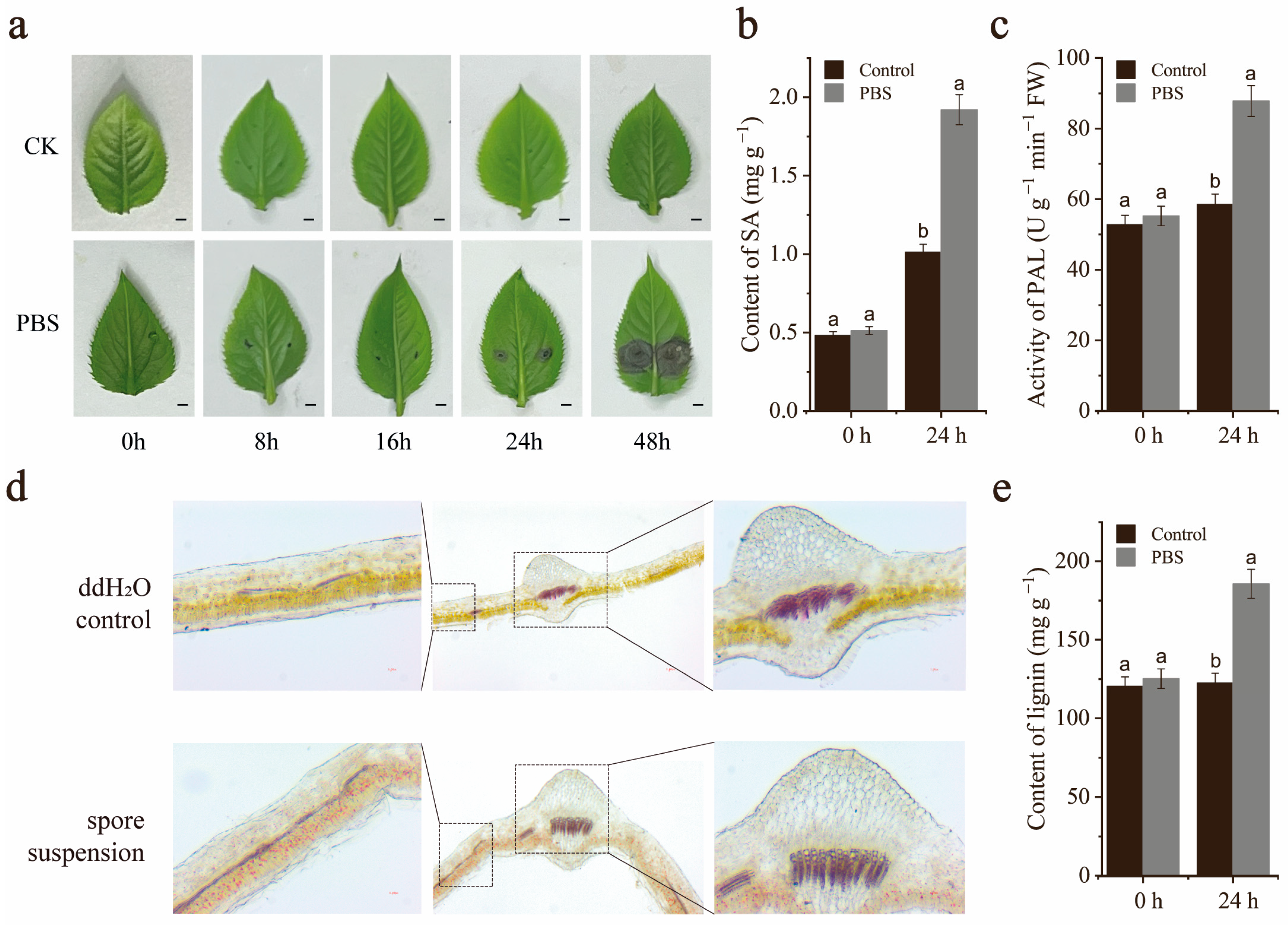
2.1. Effect of A. alternata on Pear Leaves
2.2. Cloning and Sequence Analysis of PbrMYB14
2.3. Analysis of the Subcellular Localization and Transcriptional Activity of PbrMYB14
2.4. PbrMYB14 Was Positively Induced by A. alternata
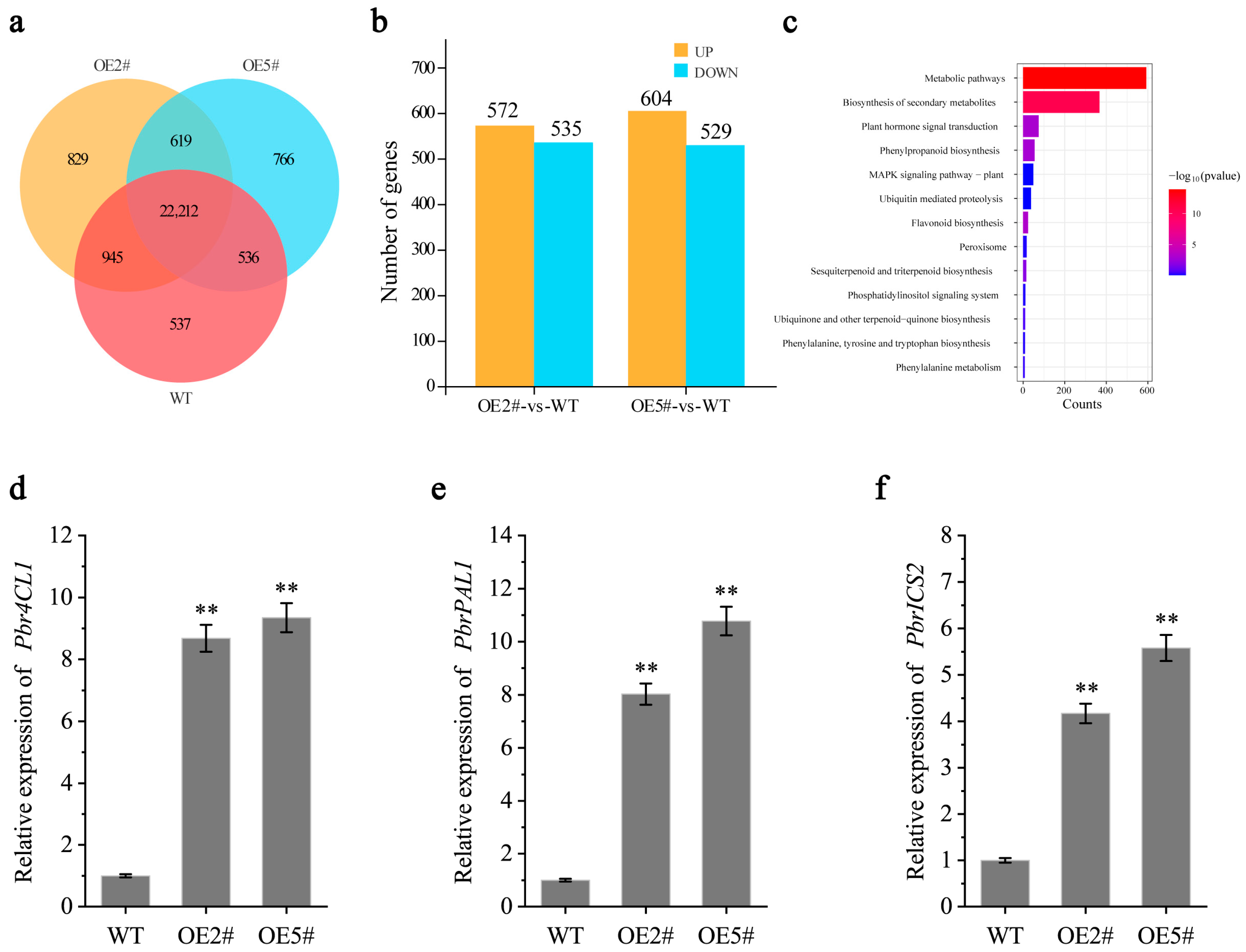
2.5. Overexpression of PbrMYB14 Increased the Transcript Abundance of Endogenous
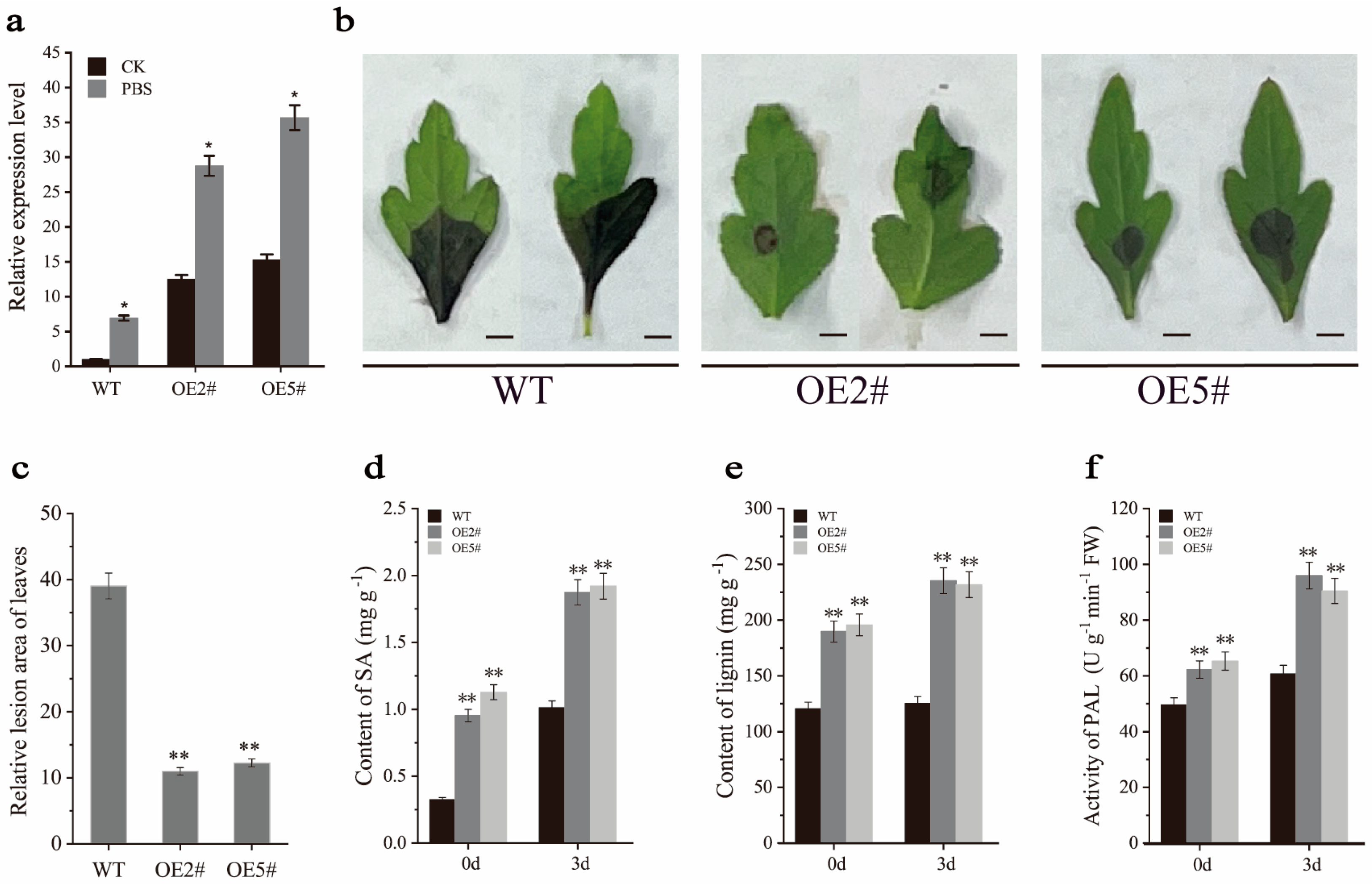
2.6. Overexpression of PbrMYB14 Affects SA and Lignin Content
2.7. PbrMYB14 Overexpression Hinders A. alternata Invade by Enhancing the Accumulation of Lignin and SA
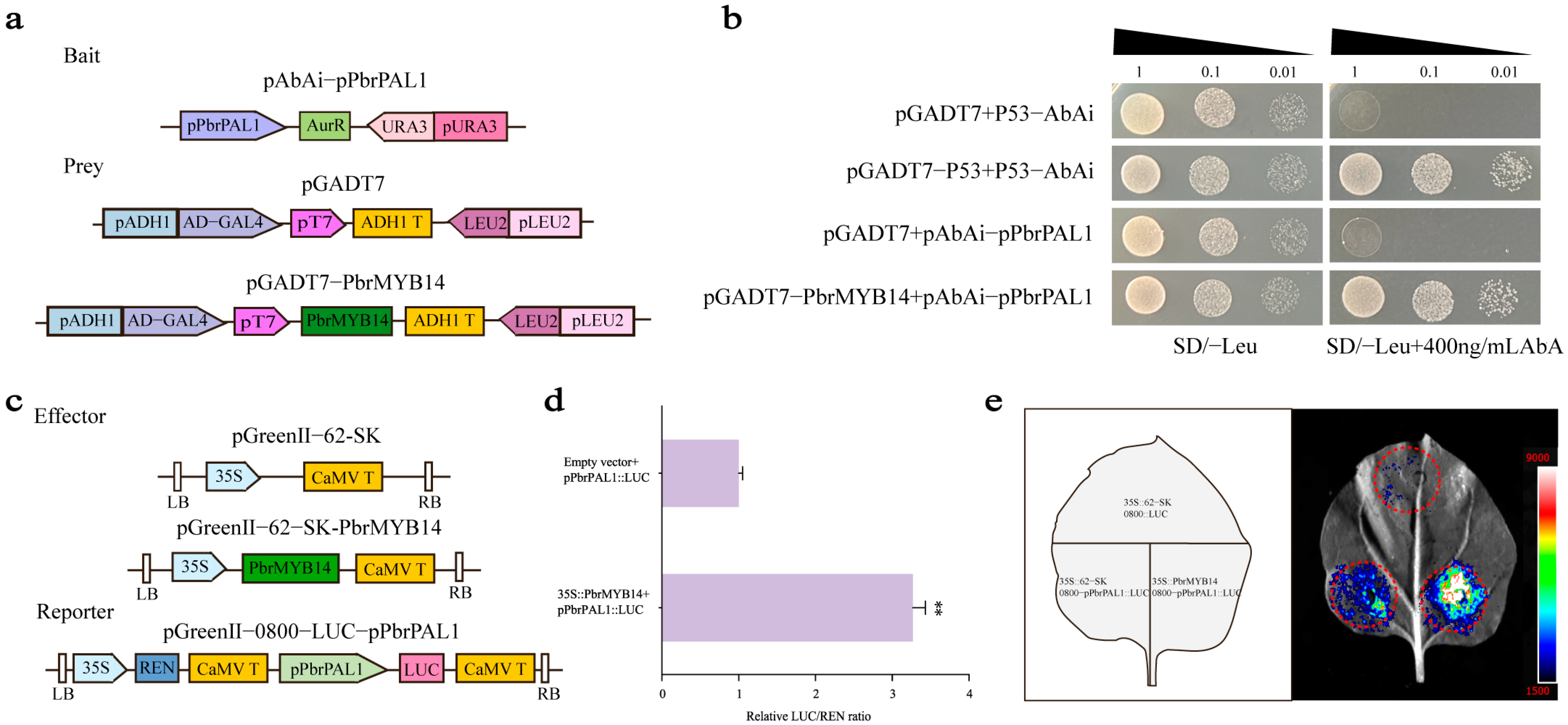
2.8. PbrMYB14 Specifically Binds to the Pbr4CL1 Promoter and Activates the Expression of Pbr4CL1 Genes
2.9. PbrMYB14 Specifically Binds to the PbrPAL1 Promoter and Activates the Expression of PbrPAL1 Genes
2.10. PbrMYB14 Improves Disease Resistance in Response to SA Signals
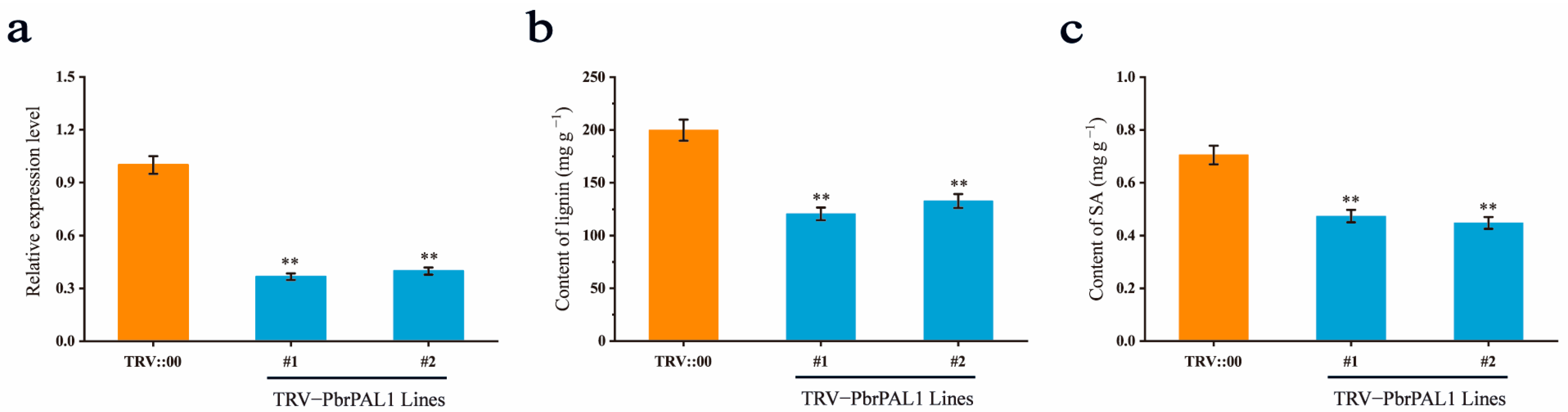
2.11. Influence of Decreasing PbrPAL1 Expression on Lignin and SA
3. Discussion
4. Materials and Methods
4.1. Plant Materials and A. alternata
4.2. Pear Leaf Inoculation
4.3. Histochemical Staining and Determination of Total Lignin Content
4.4. Analyses of SA Contents
4.5. Determination of Phenylalanine Ammonia Lyase Activity
4.6. Subcellular Localization Analysis
4.7. Transcriptional Activation Activity Assay
4.8. Plasmid Construction and Genetic Transformation of ‘Duli’ Seeds
4.9. RNA-Seq and Analysis
4.10. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.11. One-Hybrid Assays in Yeast
4.12. Dual-Luciferase Reporter Assay
4.13. Virus-Induced Gene Silencing
4.14. Quantification and Statistical Analysis
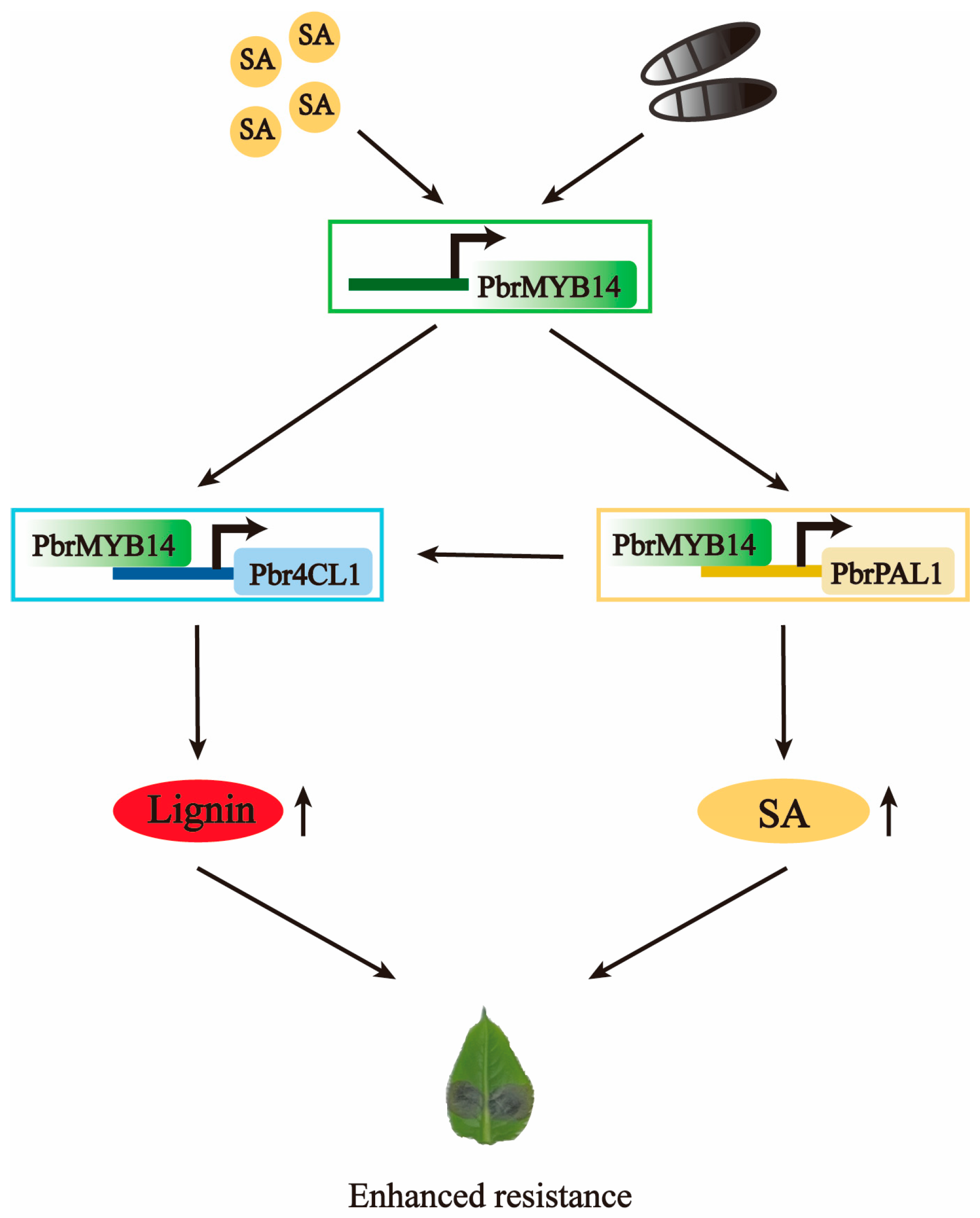
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdullah, M.; Cao, Y.P.; Cheng, X.; Meng, D.D.; Chen, Y.; Shakoor, A.; Gao, J.S.; Cai, Y.P. The Sucrose Synthase Gene Family in Chinese Pear (Pyrus bretschneideri Rehd.): Structure, Expression, and Evolution. Molecules 2018, 23, 1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Xue, Y.S.; Wang, R.Z.; Song, B.B.; Xue, C.; Shan, Y.F.; Xue, Z.L.; Wu, J. PbrMYB4, a R2R3-MYB protein, regulates pear stone cell lignification through activation of lignin biosynthesis genes. Hortic. Plant J. 2024, 11, 105–122. [Google Scholar] [CrossRef]
- Xiao, Y.X.; Zhang, S.C.; Liu, Y.; Chen, Y.; Zhai, R.; Yang, C.Q.; Wang, Z.G.; Ma, F.W.; Xu, L.F. Efficient Agrobacterium-mediated genetic transformation using cotyledons, hypocotyls and roots of ‘Duli’ (Pyrus betulifolia Bunge). Sci. Hortic. 2022, 296, 110906. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, S.H.; Wang, B.B.; Zang, Z.; Liu, F.; Ye, T.; Wang, K.M.; Hu, H.J.; Yang, X.P.; Fang, W. Biocontrol Potential of Streptomyces odonnellii SZF-179 toward Alternaria alternata to Control Pear Black Spot Disease. Int. J. Mol. Sci. 2023, 24, 17515. [Google Scholar] [CrossRef]
- Xing, C.H.; Chen, Q.M.; Qiao, Q.H.; Gu, S.N.; Cheng, X.Y.; Dong, H.Z.; Lin, L.K.; Zang, F.; Han, C.Y.; Zang, Z.; et al. PbrWRKY70 increases pear (Pyrus bretschneideri Rehd) black spot disease tolerance by negatively regulating ethylene synthesis via PbrERF1B-2. Plant Sci. 2023, 334, 111773. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qi, Y.J.; Al-Attala, M.N.; Gao, Z.H.; Yi, X.K.; Zhang, A.F.; Zang, H.Y.; Gu, C.Y.; Gao, T.C.; Chen, Y. Rapid Detection of Alternaria Species Involved in Pear Black Spot Using Loop-Mediated Isothermal Amplification. Plant Dis. 2019, 103, 3002–3008. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.C.; Bi, Y.; Wang, T.L.; Dong, Y.P.; Yang, Q.; Zhang, T.T. 2-Phenylethyl Isothiocyanate Exerts Antifungal Activity against Alternaria alternata by Affecting Membrane Integrity and Mycotoxin Production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kadiyala, V.; Nambrattil, S.; Mallavarapu, M. Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance? Sci. Total Environ. 2018, 654, 177–189. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.Q.; Yuan, D.Y.; Duan, M.J.; Liu, Y.L.; Shen, Z.J.; Yang, C.Y.; Qiu, Z.Y.; Liu, D.M.; Wen, P.Z.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.Q.; Wang, D.W.; Fu, Z.Q. Pandemonium Breaks Out: Disruption of Salicylic Acid-Mediated Defense by Plant Pathogens. Mol. Plant. 2018, 11, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Li, X.; Bonawitz, N.D.; Weng, J.K.; Chapple, C. The Growth Reduction Associated with Repressed Lignin Biosynthesis in Arabidopsis thaliana Is Independent of Flavonoids. Plant Cell 2010, 22, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.M.; Xie, J.; Zhao, Y.L.; Lu, H.; Liu, S.C.; Qu, L.; Li, J.M.; Gai, Y.; Jiang, X.N. Sense-, antisense- and RNAi-4CL1 regulate soluble phenolic acids, cell wall components and growth in transgenic Populus tomentosa Carr. Plant Physiol. Biochem. 2013, 65, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Gill, W.M.; Pinkard, E.A.; Mohammed, C.L. Anatomical and histochemical defence responses induced in juvenile leaves of Eucalyptus globulus and Eucalyptus nitens by Mycosphaerella infection. For. Pathol. 2010, 37, 361–373. [Google Scholar] [CrossRef]
- Martin, J.A.; Solla, A.; Woodward, S.; Gil, L. Detection of differential changes in lignin composition of elm xylem tissues inoculated with Ophiostoma novo-ulmi using Fourier transform-infrared spectroscopy. For. Pathol. 2010, 37, 187–191. [Google Scholar] [CrossRef]
- Zou, L. Salicylates/Jasmonates Regulate Lignin Biosynthesis in Rice. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2014. [Google Scholar] [CrossRef]
- Cao, Y.P.; Han, Y.H.; Li, D.H.; Lin, Y.; Cai, Y.P. MYB Transcription Factors in Chinese Pear (Pyrus bretschneideri Rehd.): Genome-Wide Identification, Classification, and Expression Profiling during Fruit Development. Front. Plant Sci. 2016, 7, 577. [Google Scholar] [CrossRef]
- Hao, S.X.; Lu, Y.F.; Peng, Z.; Wang, E.Y.; Chao, L.K.; Zhong, S.L.; Yao, Y.C. McMYB4 improves temperature adaptation by regulating phenylpropanoid metabolism and hormone signaling in apple. Hortic. Res. 2021, 8, 182. [Google Scholar] [CrossRef]
- Li, H.L.; Guo, D.; Peng, S.Q. Molecular characterization of the Jatropha curcas JcR1MYB1 gene encoding a putative R1-MYB transcription factor. Genet Mol Biol. 2014, 37, 549–555. [Google Scholar] [CrossRef]
- Chezem, W.R.; Memon, A.; Li, F.S.; Weng, J.K.; Clay, N.K. SG2-Type R2R3-MYB Transcription Factor MYB15 Controls Defense-Induced Lignification and Basal Immunity in Arabidopsis. Plant Cell. 2017, 29, 1907–1926. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Wang, K.; Chern, M.; Liu, Y.C.; Zhu, Z.W.; Liu, J.; Zhu, X.B.; Yin, J.J.; Ran, L.; Xiong, J.; et al. Sclerenchyma cell thickening through enhanced lignification induced by OsMYB30 prevents fungal penetration of rice leaves. New Phytol. 2020, 226, 1850–1863. [Google Scholar] [CrossRef]
- Hu, Z.W.; Zhong, X.; Zhang, H.R.; Luo, X.C.; Wang, Y.X.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, X.P.; An, H.L.; et al. GhMYB18 confers Aphis gossypii Glover resistance through regulating the synthesis of salicylic acid and flavonoids in cotton plants. Plant Cell Rep. 2023, 42, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.D.; Zhang, Q.Y.; Yu, J.Q.; Wang, J.H.; Zhang, F.J.; Wang, C.K.; Zhao, Y.W.; Sun, C.H.; You, C.X.; Hu, D.G.; et al. R2R3-MYB Transcription Factor MdMYB73 Confers Increased Resistance to the Fungal Pathogen Botryosphaeria dothidea in Apples via the Salicylic Acid Pathway. J. Agric. Food Chem. 2021, 69, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Park, C.M. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol. 2010, 186, 471–483. [Google Scholar] [CrossRef]
- Wang, L.J.; Ran, L.Y.; Hou, Y.S.; Tian, Q.Y.; Li, C.F.; Liu, R.; Fan, D.; Luo, K.M. The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytol. 2017, 251, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Wang, D.; Zhang, J.; Xu, Y.; Zhang, C.H.; Zhang, J.X.; Wang, X.P.; Wang, Y.J. VqMYB154 promotes polygene expression and enhances resistance to pathogens in Chinese wild grapevine. Hortic. Res. 2021, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Kishi-Kaboshi, M.; Seo, S.; Takahashi, A.; Hirochika, H. The MAMP-Responsive MYB Transcription Factors MYB30, MYB55 and MYB110 Activate the HCAA Synthesis Pathway and Enhance Immunity in Rice. Plant Cell Physiol. 2018, 59, 903–915. [Google Scholar] [CrossRef]
- Takaoka, S.; Kurata, M.; Harimoto, Y.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014, 202, 1297–1309. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, Q.H.; Zhao, K.K.; Zhai, W.H.; Zhang, F.; Dong, H.Z.; Lin, L.K.; Xing, C.H.; Su, Z.Y.; Pan, Z.J.; et al. PbWRKY18 promotes resistance against black spot disease by activation of the chalcone synthase gene PbCHS3 in pear. Plant Sci. 2024, 341, 112015. [Google Scholar] [CrossRef]
- Lincoln, J.E.; Richael, C.; Overduin, B.; Smith, K.; Bostock, R.; Gilchrist, D.G. Expression of the antiapoptotic baculovirus p35 gene in tomato blocks programmed cell death and provides broad-spectrum resistance to disease. Proc. Natl. Acad. Sci. USA 2002, 99, 15217–15221. [Google Scholar] [CrossRef]
- Praveen, B.; Kumar, M.K.P.; Devanna, P.; Palanna, K.B.; Buella, P.P.; Ramesh, G.V.; Reddy, N.K.; Nagaraja, A. First Report of Alternaria alternata Causing Leaf Spot on Oat (Avena sativa) in India. Plant Dis. 2021, 105, 3301. [Google Scholar] [CrossRef] [PubMed]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef]
- Long, Q.; Zhang, L.H.; Zhu, T.X.; Zhao, S.Y.; Zou, C.Y.; Xu, L.Z.; He, Y.R.; Chen, S.C.; Zou, X.P. Competitive control of CsNCED1-1 by CsLOB1 and CsbZIP40 triggers susceptibility to citrus canker. Plant J. 2024, 120, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Y.; Liu, X.B.; Francis, F.; Fan, J.; Liu, H.; Wang, Q.; Sun, Y.; Zhang, Y.M.; Chen, J.L. SmCSP4 from aphid saliva stimulates salicylic acid-mediated defence responses in wheat by interacting with transcription factor TaWKRY76. Plant Biotechnol. J. 2023, 21, 2389–2407. [Google Scholar] [CrossRef] [PubMed]
- Butselaar, T.V.; Ackerveken, G.V.D. Salicylic Acid Steers the Growth-Immunity Tradeoff. Trends Plant Sci. 2020, 25, 566–576. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y.L. Plant Immunity: Danger Perception and Signaling. Cell. 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Q.; Zhu, L.F.; Xu, L.; Yuan, D.J.; Min, L.; Zhang, X.L. Cotton cytochrome P450 CYP82D regulates systemic cell death by modulating the octadecanoid pathway. Nat. Commun. 2014, 5, 5372. [Google Scholar] [CrossRef]
- Rogers, L.A.; Campbell, M.M. The genetic control of lignin deposition during plant growth and development. New Phytol. 2004, 164, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.H.; Ming, Y.Q.; Hu, Q.; Ye, Z.X.; Si, H.; Liu, S.M.; Zhang, X.J.; Wang, W.R.; Yu, Y.; Kong, J.; et al. GhWRKY41 forms a positive feedback regulation loop and increases cotton defence response against Verticillium dahliae by regulating phenylpropanoid metabolism. Plant Biotechnol. J. 2023, 21, 961–978. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Peng, Z.; Khan, S.; Qayyum, A.; Rehman, A.; Du, X.M. Unveiling the power of MYB transcription factors: Master regulators of multi-stress responses and development in cotton. Int. J. Biol. Macromol. 2024, 276, 133885. [Google Scholar] [CrossRef]
- Yang, L.J.; Chen, Y.; Xu, L.; Wang, J.X.; Qi, H.Y.; Guo, J.Z.; Zhang, L.; Shen, J.; Wang, H.Y.; Zhang, F.; et al. The OsFTIP6-OsHB22-OsMYBR57 module regulates drought response in rice. Mol. Plant. 2022, 15, 1227–1242. [Google Scholar] [CrossRef]
- Jing, W.K.; Gong, F.F.; Liu, G.Q.; Deng, Y.L.; Liu, J.Q.; Yang, W.J.; Sun, X.M.; Li, Y.H.; Gao, J.P.; Zhou, X.F.; et al. Petal size is controlled by the MYB73/TPL/HDA19-miR159-CKX6 module regulating cytokinin catabolism in Rosa hybrida. Nat. Commun. 2023, 14, 7106. [Google Scholar] [CrossRef]
- Wang, H.Z.; Hou, J.B.; Ye, P.; Hu, L.; Huang, J.S.; Dai, Z.K.; Zhang, B.; Dai, S.; Que, J.M.; Min, H.X.; et al. A teosinte-derived allele of a MYB transcription repressor confers multiple disease resistance in maize. Mol. Plant. 2021, 14, 1846–1863. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Chen, T.Z.; Kan, J.L.; Yao, Y.; Guo, D.S.; Yang, Y.W.; Ling, X.T.; Wang, J.Y.; Zhang, B.L. The GhMYB36 transcription factor confers resistance to biotic and abiotic stress by enhancing PR1 gene expression in plants. Plant Biotechnol. J. 2021, 20, 722–735. [Google Scholar] [CrossRef]
- Xiao, S.H.; Ming, Y.Q.; Zhou, S.L.; Dong, X.M.; Liu, S.M.; Zhang, X.J.; Zhang, X.L.; Hu, Q.; Zhu, L.F. A GhLac1-centered transcriptional regulatory cascade mediates cotton resistance to Verticillium dahliae through the lignin biosynthesis pathway. J. Biol. Macromol. 2024, 279, 135042. [Google Scholar] [CrossRef]
- Gui, J.S.; Luo, L.F.; Zhong, Y.; Sun, J.Y.; Umezawa, T.; Li, L.G. Phosphorylation of LTF1, an MYB Transcription Factor in Populus, Acts as a Sensory Switch Regulating Lignin Biosynthesis in Wood Cells. Mol. Plant. 2019, 12, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, M.Y.; Chen, Y.; Nomura, K.; Hui, S.G.; Gui, J.S.; Zhang, X.W.; Wu, Y.; Liu, J.Y.; Li, Q.; et al. An MKP-MAPK protein phosphorylation cascade controls vascular immunity in plants. Sci. Adv. 2022, 8, 8723. [Google Scholar] [CrossRef]
- Song, Y.G.; Tang, H.Y.; Zhang, Z.R.; Sun, X.Y.; Ding, X.R.; Guo, X.Y.; Wang, Q.; Chen, J.F.; Dong, W. A Novel MsEOBI-MsPAL1 Module Enhances Salinity Stress Tolerance, Floral Scent Emission and Seed Yield in Alfalfa. Plant Cell Environ. 2025, 48, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sahu, P.P.; Prasad, A.; Muthamilarasan, M.; Waseem, M.; Khan, Y.; Thakur, J.K.; Chakraborty, S.; Prasad, M. The Sw5a gene confers resistance to ToLCNDV and triggers an HR response after direct AC4 effector recognition. Proc. Natl. Acad. Sci. USA 2021, 118, e2101833118. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Tsai, C.J.; Unsicker, S.B.; Xue, L.J.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic acid activates poplar defense against the biotrophic rust fungus Melampsora larici-populina via increased biosynthesis of catechin and proanthocyanidins. New Phytol. 2018, 221, 960–975. [Google Scholar] [CrossRef]
- Chen, H.C.; Song, J.; Williams, C.M.; Shuford, C.M.; Liu, J.; Wang, J.P.; Li, Q.; Shi, R.; Gokce, E.; Ducoste, J.; et al. Monolignol Pathway 4-Coumaric Acid: Coenzyme A Ligases in Populus trichocarpa: Novel Specificity, Metabolic Regulation, and Simulation of Coenzyme A Ligation Fluxes1. Plant Physiol. 2013, 161, 1501–1516. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.L.; Jia, Y.J.; Chen, Z.D.; Kang, D.X.; Zhang, L.; Pan, C.P. Nano-selenium enhances melon resistance to Podosphaera xanthii by enhancing the antioxidant capacity and promoting alterations in the polyamine, phenylpropanoid and hormone signaling pathways. J. Nanobiotechnology 2023, 21, 377. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.L.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.L.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant. 2019, 12, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, W.T.; Zhao, Q. Salicylic acid biosynthesis is not from phenylalanine in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Adams-Phillips, L.; Briggs, A.G.; Bent, A.F. Disruption of Poly (ADP-ribosyl) ation Mechanisms Alters Responses of Arabidopsis to Biotic Stress. Plant Physiol. 2010, 152, 267–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.S.; Zhang, S.L. Extraction and Determination of Endogenous Salicylic Acid from Kiwifruit and Its Application to Postharvest Research. J. Chin. Inst. Food Sci. Technol. 2004, 4, 6–9. [Google Scholar] [CrossRef]
- Sun, F.A.; Yu, H.Q.; Qu, J.T.; Cao, Y.; Ding, L.; Feng, W.Q.; Muhammad, H.B.K.; Li, W.C.; Fu, F.L. Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 996. [Google Scholar] [CrossRef]
- Long, Q.; Du, M.X.; Long, J.H.; Xie, Y.; Zhang, J.Y.; Xu, L.Z.; He, Y.R.; Li, Q.; Chen, S.C.; Zou, X.P. Transcription factor WRKY22 regulates canker susceptibility in sweet orange (Citrus sinensis Osbeck) by enhancing cell enlargement and CsLOB1 expression. Hortic. Res. 2021, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.G.; Yan, Q.; Zhao, S.L.; He, F.; Xu, J.F.; Qi, B.X.; Zhang, Y.X. Knockout of the S-acyltransferase Gene, PbPAT14, Confers the Dwarf Yellowing Phenotype in First Generation Pear by ABA Accumulation. Int. J. Mol. Sci. 2019, 20, 6347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.J.; Guo, J.; Qiao, Q.; Guo, X.F.; Ma, Y. The WRKY transcription factor PlWRKY65 enhances the resistance of Paeonia lactiflora (herbaceous peony) to Alternaria tenuissima. Hortic. Res. 2020, 7, 57. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Chen, W.; Zhang, H.; Liu, P.; Zhang, Y. PbrMYB14 Enhances Pear Resistance to Alternaria alternata by Regulating Genes in Lignin and Salicylic Acid Biosynthesis Pathways. Int. J. Mol. Sci. 2025, 26, 972. https://doi.org/10.3390/ijms26030972
Yan Q, Chen W, Zhang H, Liu P, Zhang Y. PbrMYB14 Enhances Pear Resistance to Alternaria alternata by Regulating Genes in Lignin and Salicylic Acid Biosynthesis Pathways. International Journal of Molecular Sciences. 2025; 26(3):972. https://doi.org/10.3390/ijms26030972
Chicago/Turabian StyleYan, Qi, Weiyi Chen, Hui Zhang, Peng Liu, and Yuxing Zhang. 2025. "PbrMYB14 Enhances Pear Resistance to Alternaria alternata by Regulating Genes in Lignin and Salicylic Acid Biosynthesis Pathways" International Journal of Molecular Sciences 26, no. 3: 972. https://doi.org/10.3390/ijms26030972
APA StyleYan, Q., Chen, W., Zhang, H., Liu, P., & Zhang, Y. (2025). PbrMYB14 Enhances Pear Resistance to Alternaria alternata by Regulating Genes in Lignin and Salicylic Acid Biosynthesis Pathways. International Journal of Molecular Sciences, 26(3), 972. https://doi.org/10.3390/ijms26030972




