Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives
Abstract
1. Introduction
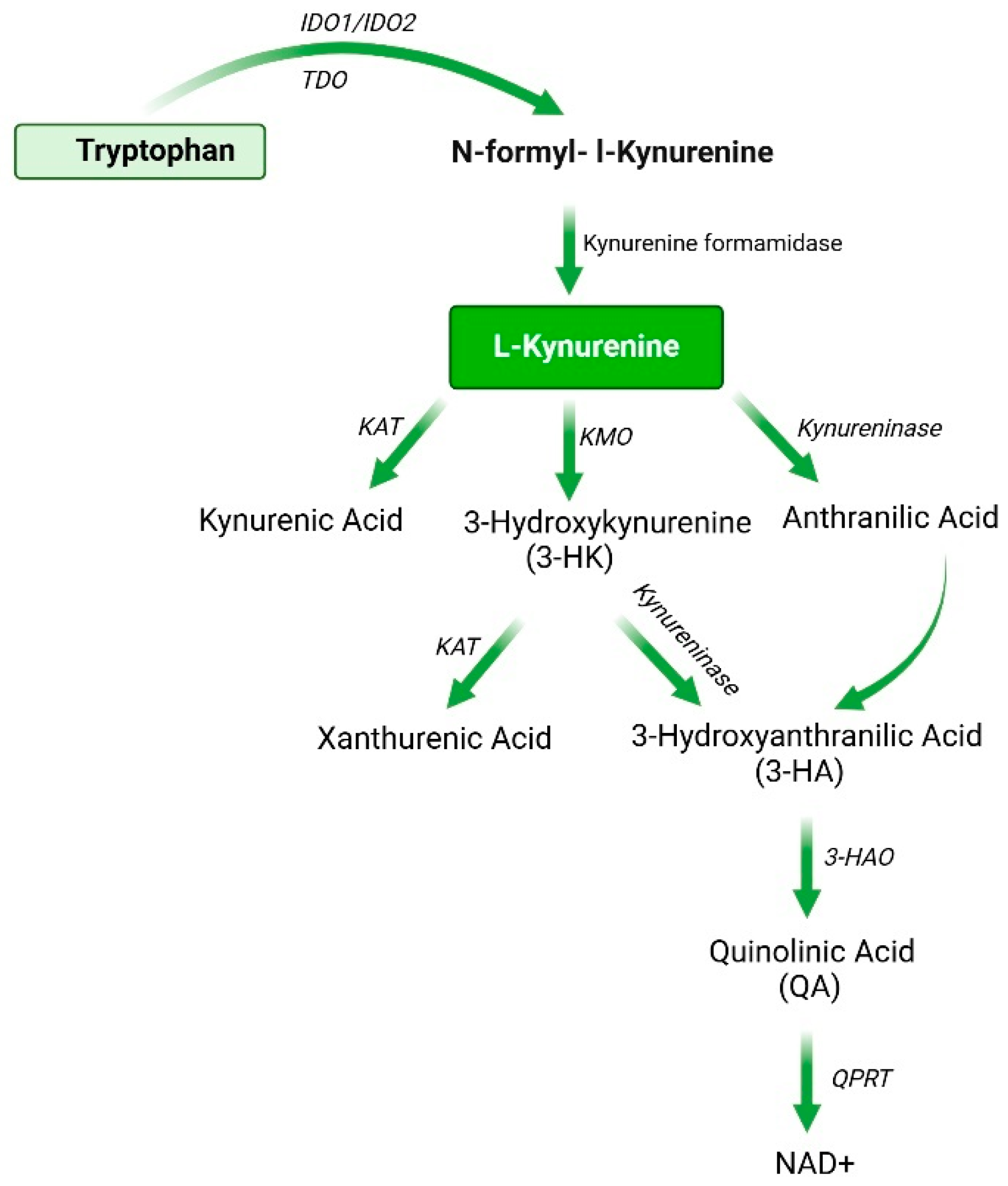
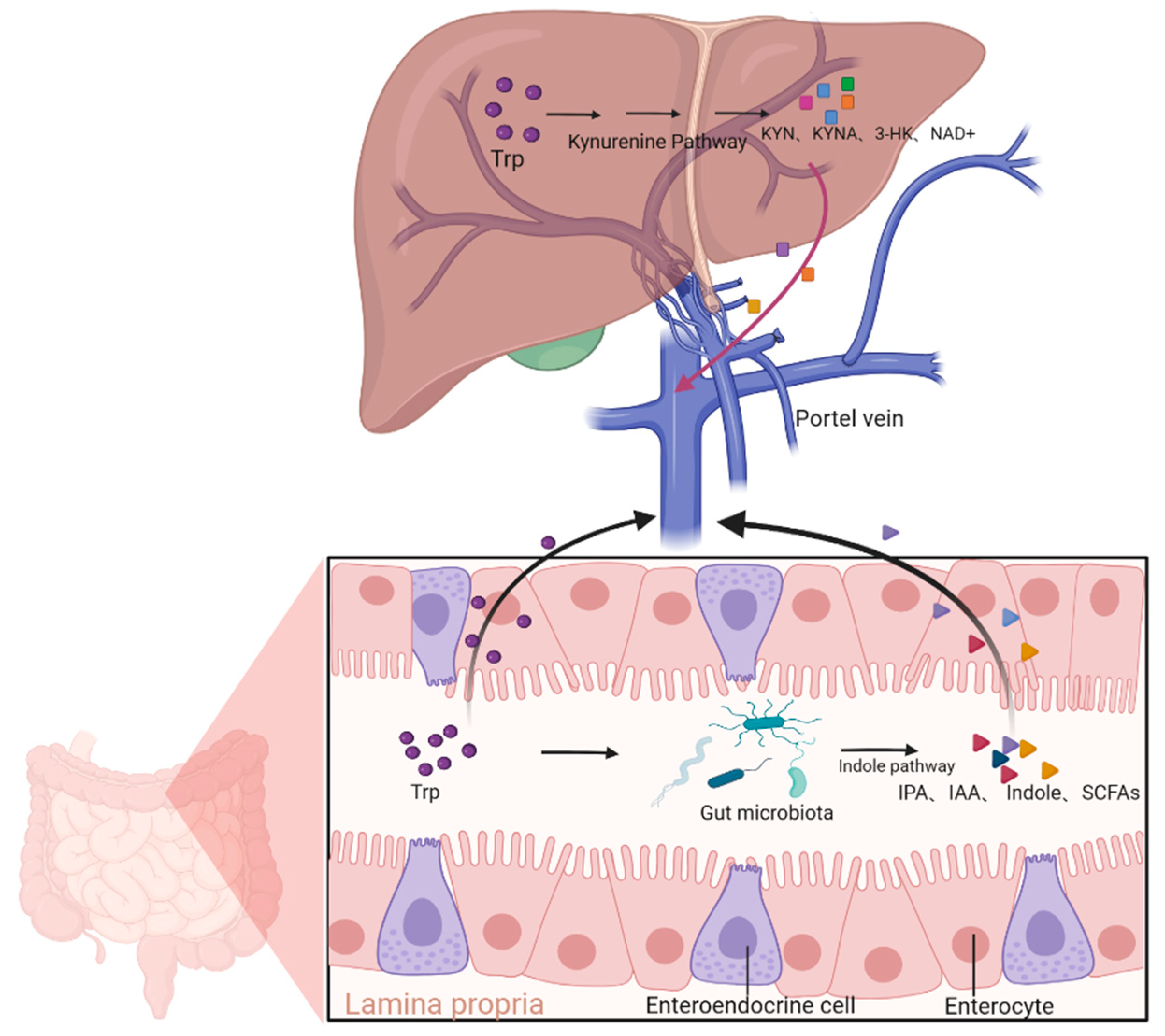
2. Synthesis, Metabolism, and Biological Functions of Kynurenine
2.1. Synthesis and Metabolism of Kynurenine
2.2. Biological Activity of the Kynurenine Pathway and Its Derivatives
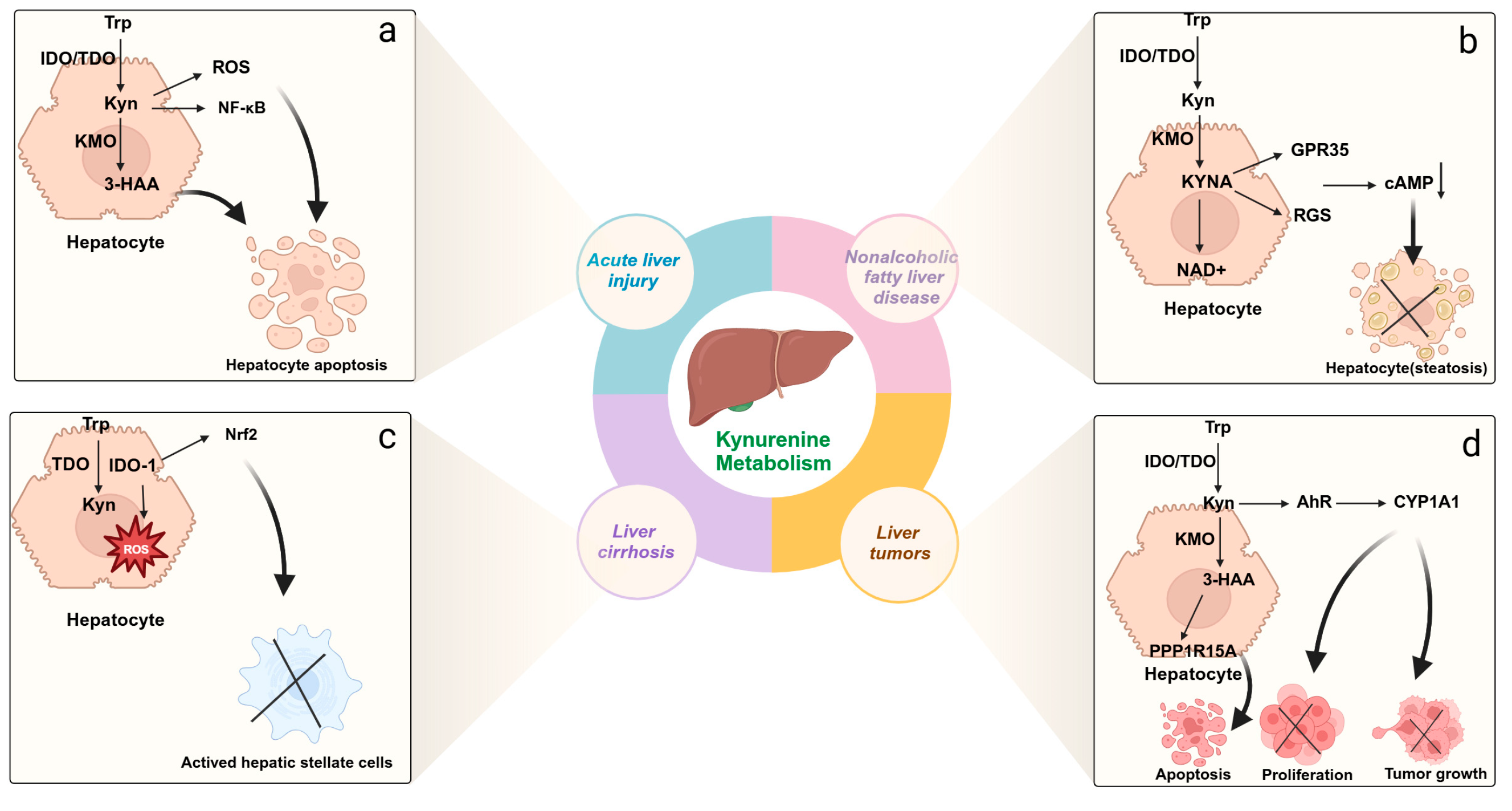
3. The Role of Kynurenine and Its Derivatives in Liver Diseases
3.1. Acute Liver Injury
3.2. Metabolic Dysfunction-Associated Steatotic Liver Disease
3.3. Chronic Liver Injury and Liver Cirrhosis
3.4. Hepatocellular Carcinoma
4. Clinical Applications of the Kynurenine Pathway
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NAD+ | Nicotinamide adenine dinucleotide |
| IDO | Indoleamine 2,3-dioxygenase |
| TDO | Tryptophan 2,3-dioxygenase |
| KMO | Kynurenine 3-monooxygenase |
| KAT | Kynurenine aminotransferases |
| KMO | Kynurenine 3-monooxygenase |
| 3-HAO | 3-Hydroxyanthranilate 3,4-dioxygenase |
| KYN | Kynurenine |
| KYNA | Kynurenic acid |
| 3-HK | 3-Hydrokynurenine |
| Trp | Tryptophan |
| IPA | Indole-3-propionic acid |
| IAA | Indole-3-acetic acid |
| SCFAs | Short chain fatty acids |
| 3-HA | 3-Hydroxyanthranilic acid |
| QA | Quinolinic acid |
| 3-HAA | 3-Hydroxyanthranilic acid |
| QPRT | Quinolinic acid phosphoribosyl transferase |
| BBB | Blood–brain barrier |
| KP | Kynurenine pathway |
| AhR | Aryl hydrocarbon receptor |
| ROS | Reactive oxygen species |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-alpha |
| ALI | Acute liver injury |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| HCC | Hepatocellular carcinoma |
| RGS | Regulator of G protein |
| GPR35 | G protein-coupled receptor 35 |
| CYP1A1 | Cytochrome P450, family 1, subfamily A, polypeptide 1 |
| NMDAR | N-methyl-D-aspartate receptor |
| ORR | Objective response rate |
| CRR | Complete response rate |
References
- He, Y.; Hwang, S.; Ahmed, Y.A.; Feng, D.; Li, N.; Ribeiro, M.; Lafdil, F.; Kisseleva, T.; Szabo, G.; Gao, B. Immunopathobiology and Therapeutic Targets Related to Cytokines in Liver Diseases. Cell Mol. Immunol. 2021, 18, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Gimson, A.E. Fulminant and Late Onset Hepatic Failure. Br. J. Anaesth. 1996, 77, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. Nf-Κb in the Liver--Linking Injury, Fibrosis and Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Terai, M.; Londin, E.; Rochani, A.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Fallarino, F.; Puccetti, P. Tolerance, Dcs and Tryptophan: Much Ado About Ido. Trends Immunol. 2003, 24, 242–248. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Dey, M.; Chang, A.; Lesniak, M.S. Targeting Tregs in Malignant Brain Cancer: Overcoming Ido. Front. Immunol. 2013, 4, 116. [Google Scholar] [CrossRef]
- Ogiso, H.; Ito, H.; Ando, T.; Arioka, Y.; Kanbe, A.; Ando, K.; Ishikawa, T.; Saito, K.; Hara, A.; Moriwaki, H.; et al. The Deficiency of Indoleamine 2,3-Dioxygenase Aggravates the Ccl4-Induced Liver Fibrosis in Mice. PLoS ONE 2016, 11, e0162183. [Google Scholar] [CrossRef]
- van Baren, N.; Van den Eynde, B.J. Tumoral Immune Resistance Mediated by Enzymes That Degrade Tryptophan. Cancer Immunol. Res. 2015, 3, 978–985. [Google Scholar] [CrossRef]
- Xue, P.; Fu, J.; Zhou, Y. The Aryl Hydrocarbon Receptor and Tumor Immunity. Front. Immunol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Ohtaki, H.; Ito, H.; Ando, K.; Ishikawa, T.; Hoshi, M.; Ando, T.; Takamatsu, M.; Hara, A.; Moriwaki, H.; Saito, K.; et al. Kynurenine Production Mediated by Indoleamine 2,3-Dioxygenase Aggravates Liver Injury in Hbv-Specific Ctl-Induced Fulminant Hepatitis. Biochim. Biophys. Acta 2014, 1842, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, S.; Guillemin, G.J.; Rashidian, A.; Faghir-Ghanesefat, H.; Mani, A.R.; Tavangar, S.M.; Dehpour, A.R. 1-Methyl Tryptophan, an Indoleamine 2,3-Dioxygenase Inhibitor, Attenuates Cardiac and Hepatic Dysfunction in Rats with Biliary Cirrhosis. Eur. J. Pharmacol. 2021, 908, 174309. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, Y.; Wang, X.; Fan, F.; Yang, Z.; Luo, D. Exploring Tryptophan Metabolism: The Transition from Disturbed Balance to Diagnostic and Therapeutic Potential in Metabolic Diseases. Biochem. Pharmacol. 2024, 230 Pt 1, 116554. [Google Scholar] [CrossRef]
- Xue, C.; Gu, X.; Zheng, Q.; Shi, Q.; Yuan, X.; Chu, Q.; Jia, J.; Su, Y.; Bao, Z.; Lu, J.; et al. Effects of 3-Haa on Hcc by Regulating the Heterogeneous Macrophages-a Scrna-Seq Analysis. Adv. Sci. 2023, 10, e2207074. [Google Scholar] [CrossRef]
- Lepkovsky, S.; Roboz, E.; Haagen-Smit, A.J. Xanthurenic Acid and Its Role Ix the Tryptophane Metabolism Ok Pyridoxine-Deficient Rats. Nutr. Rev. 1974, 32, 338–339. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis During Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Mitaka, T.; Sato, F.; Mizuguchi, T.; Yokono, T.; Mochizuki, Y. Reconstruction of Hepatic Organoid by Rat Small Hepatocytes and Hepatic Nonparenchymal Cells. Hepatology 1999, 29, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.R.; Sadik, A.; Sharma, S.; Poschet, G.; Gegner, H.M.; Lanz, T.V.; Lucarelli, P.; Klingmüller, U.; Platten, M.; Heiland, I.; et al. Hypoxia Routes Tryptophan Homeostasis Towards Increased Tryptamine Production. Front. Immunol. 2021, 12, 590532. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Litzenburger, U.M.; Rauschenbach, K.J.; Bunse, L.; Ochs, K.; Sahm, F.; Pusch, S.; Opitz, C.A.; Blaes, J.; von Deimling, A.; et al. Suppression of Tdo-Mediated Tryptophan Catabolism in Glioblastoma Cells by a Steroid-Responsive Fkbp52-Dependent Pathway. Glia 2015, 63, 78–90. [Google Scholar] [CrossRef]
- Zheng, R.; Wu, X.; Li, S.; Chen, X.; Yan, D.; He, J. Mechanism Exploration on the Immunoregulation of Allogeneic Heart Transplantation Rejection in Rats with Exosome Mirna and Proteins from Overexpressed Ido1 Bmscs. Cell Transplant. 2024, 33, 9636897241245796. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; Nagamura, F. Umbilical Cord Blood and Cord Tissue Banking as Somatic Stem Cell Resources to Support Medical Cell Modalities. Inflamm. Regen. 2023, 43, 59. [Google Scholar] [CrossRef]
- Van der Leek, A.P.; Yanishevsky, Y.; Kozyrskyj, A.L. The Kynurenine Pathway as a Novel Link between Allergy and the Gut Microbiome. Front. Immunol. 2017, 8, 1374. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Owens, T.C.; Melancon, S.; Workeneh, I.; Cao, H.S.T.; Vauthey, J.N.; Huang, S.Y. Current Perspectives and Progress in Preoperative Portal Vein Embolization with Stem Cell Augmentation (Pvesa). Stem Cell Rev. Rep. 2024, 20, 1236–1251. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-Dioxygenase Pathways of Pathogenic Inflammation and Immune Escape in Cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar]
- Li, J.; Yan, Y.; Fu, Y.; Chen, Z.; Yang, Y.; Li, Y.; Pan, J.; Li, F.; Zha, C.; Miao, K.; et al. Ace2 Mediates Tryptophan Alleviation on Diarrhea by Repairing Intestine Barrier Involved Mtor Pathway. Cell. Mol. Biol. Lett. 2024, 29, 90. [Google Scholar]
- Launay, J.M.; Delorme, R.; Pagan, C.; Callebert, J.; Leboyer, M.; Vodovar, N. Impact of Ido Activation and Alterations in the Kynurenine Pathway on Hyperserotonemia, Nad(+) Production, and Ahr Activation in Autism Spectrum Disorder. Transl. Psychiatry 2023, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal Serotonin and Fluoxetine Exposure Modulate Bacterial Colonization in the Gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Wu, J.; Quan, W.; Zhou, Y.; Hong, H.; Niu, G.Y.; Li, T.; Huang, S.B.; Qiao, C.M.; Zhao, W.J.; et al. Dss-Induced Colitis Activates the Kynurenine Pathway in Serum and Brain by Affecting Ido-1 and Gut Microbiota. Front. Immunol. 2022, 13, 1089200. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xiao, S.; Cao, Y.; Zhang, Y.; Yang, J.; Zheng, L.; Zhao, F.; Liu, X.; Liu, D.; Zhou, Z.; et al. Organophosphate Insecticide Malathion Induces Alzheimer’s Disease-Like Cognitive Impairment in Mice: Evidence of the Microbiota-Gut-Brain Axis. Environ. Sci. Technol. 2024, 58, 21966–21977. [Google Scholar] [CrossRef]
- Paydaş Hataysal, E.; Körez, M.K.; Guler, E.M.; Vatansev, H.; Bozalı, K.; Basaranoglu, M.; Vatansev, H. Impaired Kynurenine Pathway in Inflammatory Bowel Disease. J. Clin. Med. 2024, 13, 6147. [Google Scholar] [CrossRef]
- Salminen, A. Activation of Aryl Hydrocarbon Receptor (Ahr) in Alzheimer’s Disease: Role of Tryptophan Metabolites Generated by Gut Host-Microbiota. J. Mol. Med. 2023, 101, 201–222. [Google Scholar] [CrossRef]
- Schwarcz, R.; Du, F.; Schmidt, W.; Turski, W.A.; Gramsbergen, J.B.; Okuno, E.; Roberts, R.C. Kynurenic Acid: A Potential Pathogen in Brain Disorders. Ann. N. Y Acad. Sci. 1992, 648, 140–153. [Google Scholar] [CrossRef]
- Kuc, D.; Rahnama, M.; Tomaszewski, T.; Rzeski, W.; Wejksza, K.; Urbanik-Sypniewska, T.; Parada-Turska, J.; Wielosz, M.; Turski, W.A. Kynurenic Acid in Human Saliva—Does It Influence Oral Microflora? Pharmacol. Rep. 2006, 58, 393–398. [Google Scholar]
- Dolecka, J.; Urbanik-Sypniewska, T.; Skrzydło-Radomańska, B.; Parada-Turska, J. Effect of Kynurenic Acid on the Viability of Probiotics in Vitro. Pharmacol. Rep. 2011, 63, 548–551. [Google Scholar] [CrossRef]
- Schwarcz, R.; Foo, A.; Sathyasaikumar, K.V.; Notarangelo, F.M. The Probiotic Lactobacillus reuteri Preferentially Synthesizes Kynurenic Acid from Kynurenine. Int. J. Mol. Sci. 2024, 25, 3679. [Google Scholar] [CrossRef]
- Qi, H.; Li, Y.; Yun, H.; Zhang, T.; Huang, Y.; Zhou, J.; Yan, H.; Wei, J.; Liu, Y.; Zhang, Z.; et al. Lactobacillus Maintains Healthy Gut Mucosa by Producing L-Ornithine. Commun. Biol. 2019, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, R.; Aftabi, Y.; Doaei, S.; Gholamalizadeh, M. Synergistic Effect of Endurance Training and Nettle Leaf Extract on the Ido1-Kyn-Ahr Pathway Homeostasis and Inhibiting of Liver Toxicity in Rats with Stz-Induced Diabetes. Front. Endocrinol. 2023, 14, 1071424. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.G.; Schnabl, B. From Intestinal Dysbiosis to Alcohol-Associated Liver Disease. Clin. Mol. Hepatol. 2020, 26, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl Hydrocarbon Receptor and Intestinal Immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, X.; Chen, Z.M.; He, L.; Sonnenberg, G.F.; Artis, D.; Fu, Y.X.; Zhou, L. Group 3 Innate Lymphoid Cells Inhibit T-Cell-Mediated Intestinal Inflammation through Aryl Hydrocarbon Receptor Signaling and Regulation of Microflora. Immunity 2013, 39, 386–399. [Google Scholar] [CrossRef]
- Bock, K.W. Modulation of Aryl Hydrocarbon Receptor (Ahr) and the Nad(+)-Consuming Enzyme Cd38: Searches of Therapeutic Options for Nonalcoholic Fatty Liver Disease (Nafld). Biochem. Pharmacol. 2020, 175, 113905. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Gillan, N.; Neil, J.; Seidler, K. The Role of the Aryl Hydrocarbon Receptor (Ahr) in Modulating Intestinal Ilc3s to Optimise Gut Pathogen Resistance in Lupus and Benefits of Nutritional Ahr Ligands. Clin. Nutr. 2024, 43, 1199–1215. [Google Scholar] [CrossRef]
- Bolatimi, O.E.; Hua, Y.; Ekuban, F.A.; Gripshover, T.C.; Ekuban, A.; Luulay, B.; Watson, W.H.; Hardesty, J.E.; Wahlang, B. Low Dose Exposure to Dioxins Alters Hepatic Energy Metabolism and Steatotic Liver Disease Development in a Sex-Specific Manner. Environ. Int. 2024, 194, 109152. [Google Scholar] [CrossRef]
- Vogel, C.F.; Sciullo, E.; Matsumura, F. Involvement of Relb in Aryl Hydrocarbon Receptor-Mediated Induction of Chemokines. Biochem. Biophys. Res. Commun. 2007, 363, 722–726. [Google Scholar] [CrossRef]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a Natural Flavone, Alleviates Inflammatory Bowel Disease by the Inhibition of Nlrp3 Inflammasome Activation Via an Ahr/Nrf2/Nqo1 Pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef]
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Munn, D.; Blazeck, J. Immunosuppressive Metabolites in Tumoral Immune Evasion: Redundancies, Clinical Efforts, and Pathways Forward. J. Immunother. Cancer 2021, 9, e003013. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Cunningham, D.M.; Meyer, K.; Toth, K.; Ray, R.B.; Heczey, A.; Ray, R. Targeting Lin28 Axis Enhances Glypican-3-Car T cell Efficacy against Hepatic Tumor Initiating Cell Population. Mol. Ther. 2023, 31, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Ovalle Rodríguez, P.; Ortega, D.R.; Ayala, T.B.; Roldán, G.R.; de la Cruz, G.P.; Esquivel, D.F.G.; Gómez-Manzo, S.; Chapul, L.S.; Salazar, A.; Pineda, B.; et al. Modulation of Kynurenic Acid Production by N-Acetylcysteine Prevents Cognitive Impairment in Adulthood Induced by Lead Exposure During Lactation in Mice. Antioxidants 2023, 12, 2035. [Google Scholar] [CrossRef]
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A Glycine Site Associated with N-Methyl-D-Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic Acid as a Ligand for Orphan G Protein-Coupled Receptor Gpr35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand That Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Amori, L.; Guidetti, P.; Muchowski, P.J.; Schwarcz, R. Dysfunctional Kynurenine Pathway Metabolism in the R6/2 Mouse Model of Huntington’s Disease. J. Neurochem. 2010, 113, 1416–1425. [Google Scholar] [CrossRef]
- Hogan-Cann, A.D.; Anderson, C.M. Physiological Roles of Non-Neuronal Nmda Receptors. Trends Pharmacol. Sci. 2016, 37, 750–767. [Google Scholar] [CrossRef]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Galvis, M.L.E.; Brundin, P.; Huang, X.; et al. Tryptophan Metabolites Are Associated with Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 2020, 35, 2028–2037. [Google Scholar] [CrossRef]
- Pawlowski, T.; Malyszczak, K.; Inglot, M.; Zalewska, M.; Radkowski, M.; Laskus, T.; Pawlak, D. Alterations in the Metabolism of Tryptophan in Patients with Chronic Hepatitis C Six Months after Pegylated Interferon-A 2a Treatment. Psychoneuroendocrinology 2018, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, C.M.; Kegel, M.E.; Bergen, S.E.; Ekman, C.J.; Olsson, S.; Larsson, M.; Vawter, M.P.; Backlund, L.; Sullivan, P.F.; Sklar, P.; et al. A Genome-Wide Association Study of Kynurenic Acid in Cerebrospinal Fluid: Implications for Psychosis and Cognitive Impairment in Bipolar Disorder. Mol. Psychiatry 2016, 21, 1342–1350. [Google Scholar] [CrossRef]
- Mandal, G.; Kirkpatrick, M.; Alboni, S.; Mariani, N.; Pariante, C.M.; Borsini, A. Ketamine Prevents Inflammation-Induced Reduction of Human Hippocampal Neurogenesis Via Inhibiting the Production of Neurotoxic Metabolites of the Kynurenine Pathway. Int. J. Neuropsychopharmacol. 2024, 27, pyae041. [Google Scholar] [CrossRef] [PubMed]
- Antenucci, N.; D’Errico, G.; Fazio, F.; Nicoletti, F.; Bruno, V.; Battaglia, G. Changes in Kynurenine Metabolites in the Gray and White Matter of the Dorsolateral Prefrontal Cortex of Individuals Affected by Schizophrenia. Schizophrenia 2024, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T Cell Apoptosis by Tryptophan Catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Siska, P.J.; Jiao, J.; Matos, C.; Singer, K.; Berger, R.S.; Dettmer, K.; Oefner, P.J.; Cully, M.D.; Wang, Z.; Quinn, I.W.; et al. Kynurenine Induces T Cell Fat Catabolism and Has Limited Suppressive Effects in Vivo. EBioMedicine 2021, 74, 103734. [Google Scholar] [CrossRef]
- Morita, T.; Saito, K.; Takemura, M.; Maekawa, N.; Fujigaki, S.; Fujii, H.; Wada, H.; Takeuchi, S.; Noma, A.; Seishima, M. 3-Hydroxyanthranilic Acid, an L-Tryptophan Metabolite, Induces Apoptosis in Monocyte-Derived Cells Stimulated by Interferon-Gamma. Ann. Clin. Biochem. 2001, 38 Pt. 3, 242–251. [Google Scholar] [CrossRef]
- Bishnupuri, K.S.; Alvarado, D.M.; Khouri, A.N.; Shabsovich, M.; Chen, B.; Dieckgraefe, B.K.; Ciorba, M.A. Ido1 and Kynurenine Pathway Metabolites Activate Pi3k-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019, 79, 1138–1150. [Google Scholar] [CrossRef]
- Clària, J.; Moreau, R.; Fenaille, F.; Amorós, A.; Junot, C.; Gronbaek, H.; Coenraad, M.J.; Pruvost, A.; Ghettas, A.; Chu-Van, E.; et al. Orchestration of Tryptophan-Kynurenine Pathway, Acute Decompensation, and Acute-on-Chronic Liver Failure in Cirrhosis. Hepatology 2019, 69, 1686–1701. [Google Scholar] [CrossRef]
- Hoshi, M.; Osawa, Y.; Nakamoto, K.; Morita, N.; Yamamoto, Y.; Ando, T.; Tashita, C.; Nabeshima, T.; Saito, K. Kynurenine Produced by Indoleamine 2,3-Dioxygenase 2 Exacerbates Acute Liver Injury by Carbon Tetrachloride in Mice. Toxicology 2020, 438, 152458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, Y.; Xie, Q.; Gong, W.; Li, J.; Chen, T.; Xu, Y.; Xu, N.; Chen, S.; Chen, M.; et al. The Metabolites of De Novo Nad(+) Synthesis Are a Valuable Predictor of Acute Kidney Injury. Clin. Kidney J. 2023, 16, 711–721. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, P.; Tang, X.; Wang, S.; Shen, J.; Zheng, Y.; Gao, C.; Mi, P.; Zhang, C.; Qu, H.; et al. Metabolic Rewiring of Kynurenine Pathway During Hepatic Ischemia-Reperfusion Injury Exacerbates Liver Damage by Impairing Nad Homeostasis. Adv. Sci. 2022, 9, e2204697. [Google Scholar] [CrossRef]
- Ma, S.; Li, J.; Ye, H.; Wu, C.; Zhang, J.; Xu, S.; Song, Y.; Gu, Y.; Gao, L. Indoleamine 2, 3-Dioxygenase 1 Activation in Macrophage Exacerbates Hepatic Ischemia-Reperfusion Injury by Triggering Hepatocyte Ferroptosis. Int. Immunopharmacol. 2024, 130, 111692. [Google Scholar] [CrossRef]
- Berge, R.K.; Cacabelos, D.; Señarís, R.; Nordrehaug, J.E.; Nygård, O.; Skorve, J.; Bjørndal, B. Hepatic Steatosis Induced in C57bl/6 Mice by a Non-ß Oxidizable Fatty Acid Analogue Is Associated with Reduced Plasma Kynurenine Metabolites and a Modified Hepatic Nad(+)/Nadh Ratio. Lipids Health Dis. 2020, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Dorochow, E.; Kraus, N.; Chenaux-Repond, N.; Pierre, S.; Kolbinger, A.; Geisslinger, G.; Ortiz, C.; Welsch, C.; Trebicka, J.; Gurke, R.; et al. Differential Lipidomics, Metabolomics and Immunological Analysis of Alcoholic and Non-Alcoholic Steatohepatitis in Mice. Int. J. Mol. Sci. 2023, 24, 10351. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e5. [Google Scholar] [CrossRef] [PubMed]
- Pyun, D.H.; Kim, T.J.; Kim, M.J.; Hong, S.A.; El-Aty, A.M.A.; Jeong, J.H.; Jung, T.W. Endogenous Metabolite, Kynurenic Acid, Attenuates Nonalcoholic Fatty Liver Disease Via Ampk/Autophagy- and Ampk/Orp150-Mediated Signaling. J. Cell. Physiol. 2021, 236, 4902–4912. [Google Scholar] [CrossRef]
- Mo, C.; Xie, S.; Zhong, W.; Zeng, T.; Huang, S.; Lai, Y.; Deng, G.; Zhou, C.; Yan, W.; Chen, Y.; et al. Mutual Antagonism between Indoleamine 2,3-Dioxygenase 1 and Nuclear Factor E2-Related Factor 2 Regulates the Maturation Status of Dcs in Liver Fibrosis. Free Radic. Biol. Med. 2020, 160, 178–190. [Google Scholar] [CrossRef]
- Lercher, A.; Popa, A.M.; Viczenczova, C.; Kosack, L.; Klavins, K.; Agerer, B.; Opitz, C.A.; Lanz, T.V.; Platten, M.; Bergthaler, A. Hepatocyte-Intrinsic Type I Interferon Signaling Reprograms Metabolism and Reveals a Novel Compensatory Mechanism of the Tryptophan-Kynurenine Pathway in Viral Hepatitis. PLoS Pathog. 2020, 16, e1008973. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Kong, W.; Wu, C.; Zeng, T.; Xie, S.; Chen, Q.; Kuang, S.; Zheng, R.; Wang, F.; et al. Corilagin Alleviates Liver Fibrosis in Zebrafish and Mice by Repressing Ido1-Mediated M2 Macrophage Repolarization. Phytomedicine 2023, 119, 155016. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Uemura, M.; Saito, K.; Sasaki, E.; Ishiguro, I. Relationship between the Level of Serum L-Tryptophan and Its Hepatic Uptake and Metabolism in Rats with Carbon Tetrachloride-Induced Liver Cirrhosis. Amino Acids 1996, 10, 369–378. [Google Scholar] [CrossRef]
- Gan, G.; Shi, Z.; Shangguan, C.; Zhang, J.; Yuan, Y.; Chen, L.; Liu, W.; Li, B.; Meng, S.; Xiong, W.; et al. The Kynurenine Derivative 3-Haa Sensitizes Hepatocellular Carcinoma to Sorafenib by Upregulating Phosphatases. Theranostics 2021, 11, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Wang, X.; Chen, F.; Gou, S. Novel Conjugates with Dual Suppression of Glutathione S-Transferases and Tryptophan-2,3-Dioxygenase Activities for Improving Hepatocellular Carcinoma Therapy. Bioorganic Chem. 2019, 92, 103191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hua, S.; Wang, X.; Chen, F.; Gou, S. The Introduction of Immunosuppressor (Tdo Inhibitor) Significantly Improved the Efficacy of Irinotecan in Treating Hepatocellular Carcinoma. Cancer Immunol. Immunother. 2021, 70, 497–508. [Google Scholar] [CrossRef]
- Cecchi, M.; Anceschi, C.; Silvano, A.; Coniglio, M.L.; Chinnici, A.; Magnelli, L.; Lapucci, A.; Laurenzana, A.; Parenti, A. Unveiling the Role of Tryptophan 2,3-Dioxygenase in the Angiogenic Process. Pharmaceuticals 2024, 17, 558. [Google Scholar] [CrossRef]
- Xu, J.B.; Gao, G.C.; Yuan, M.J.; Huang, X.; Zhou, H.Y.; Zhang, Y.; Zheng, Y.X.; Wu, Z.; Feng, J.M.; Wu, J.M. Lignans from Schisandra Chinensis Ameliorate Alcohol and Ccl(4)-Induced Long-Term Liver Injury and Reduce Hepatocellular Degeneration Via Blocking Etbr. J. Ethnopharmacol. 2020, 258, 112813. [Google Scholar] [CrossRef]
- Hu, J.; Dai, J.; Sheng, N. Kynurenic Acid Plays a Protective Role in Hepatotoxicity Induced by Hfpo-Da in Male Mice. Environ. Sci. Technol. 2024, 58, 1842–1853. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine Is an Endothelium-Derived Relaxing Factor Produced During Inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef]
- Jung, I.D.; Lee, M.G.; Chang, J.H.; Lee, J.S.; Jeong, Y.I.; Lee, C.M.; Park, W.S.; Han, J.; Seo, S.K.; Lee, S.Y.; et al. Blockade of Indoleamine 2,3-Dioxygenase Protects Mice against Lipopolysaccharide-Induced Endotoxin Shock. J. Immunol. 2009, 182, 3146–3154. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, C.; Bjørndal, B.; Lund, A.; Slettom, G.; Skorve, J.; Nygård, O.; Svardal, A.; Berge, R.K. Increased Fatty Acid Oxidation and Mitochondrial Proliferation in Liver Are Associated with Increased Plasma Kynurenine Metabolites and Nicotinamide Levels in Normolipidemic and Carnitine-Depleted Rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158543. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle Pgc-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef]
- Aggarwal, S.; Yadav, V.; Maiwall, R.; Rastogi, A.; Pamecha, V.; Bedi, O.; Maras, J.S.; Trehanpati, N.; Ramakrishna, G. Metabolomic Analysis Shows Dysregulation in Amino Acid and Nad+ Metabolism in Palmitate Treated Hepatocytes and Plasma of Non-Alcoholic Fatty Liver Disease Spectrum. Biochem. Biophys. Res. Commun. 2023, 643, 129–138. [Google Scholar] [CrossRef]
- Arto, C.; Rusu, E.C.; Clavero-Mestres, H.; Barrientos-Riosalido, A.; Bertran, L.; Mahmoudian, R.; Aguilar, C.; Riesco, D.; Chicote, J.U.; Parada, D.; et al. Metabolic Profiling of Tryptophan Pathways: Implications for Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease. Eur. J. Clin. Investig. 2024, 54, e14279. [Google Scholar] [CrossRef]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage Activation and Polarization as an Adaptive Component of Innate Immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar]
- Lee, K.D.; Kuo, T.K.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K. In Vitro Hepatic Differentiation of Human Mesenchymal Stem Cells. Hepatology 2004, 40, 1275–1284. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Su, J.; Chen, X.; Huang, Y.; Li, W.; Li, J.; Cao, K.; Cao, G.; Zhang, L.; Li, F.; Roberts, A.I.; et al. Phylogenetic Distinction of Inos and Ido Function in Mesenchymal Stem Cell-Mediated Immunosuppression in Mammalian Species. Cell Death Differ. 2014, 21, 388–396. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, L.; Zhou, Z.; Lin, H.; Chen, C.; Huang, P.; Huang, W.; Zhou, C.; Huang, S.; Nie, L.; et al. Indoleamine 2,3-Dioxygenase 1 Deficiency Attenuates Ccl4-Induced Fibrosis through Th17 Cells Down-Regulation and Tryptophan 2,3-Dioxygenase Compensation. Oncotarget 2017, 8, 40486–40500. [Google Scholar] [CrossRef]
- Trézéguet, V.; Fatrouni, H.; Merched, A.J. Immuno-Metabolic Modulation of Liver Oncogenesis by the Tryptophan Metabolism. Cells 2021, 10, 3469. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Rao, D.; Zhu, H.; Liu, Q.; Huang, W.; Zhang, L.; Liang, H.; Song, J.; Ding, Z. Tdo2 Was Downregulated in Hepatocellular Carcinoma and Inhibited Cell Proliferation by Upregulating the Expression of P21 and P27. BioMed Res. Int. 2021, 2021, 4708439. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Chung, S.; Sakai, H.; Ohata, H.; Obata, Y.; Shiokawa, D.; Mizoguchi, Y.; Kubo, T.; Ichikawa, H.; Taniguchi, H.; et al. Stemness and Immune Evasion Conferred by the Tdo2-Ahr Pathway Are Associated with Liver Metastasis of Colon Cancer. Cancer Sci. 2022, 113, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Bekki, S.; Hashimoto, S.; Yamasaki, K.; Komori, A.; Abiru, S.; Nagaoka, S.; Saeki, A.; Suehiro, T.; Kugiyama, Y.; Beppu, A.; et al. Serum Kynurenine Levels Are a Novel Biomarker to Predict the Prognosis of Patients with Hepatocellular Carcinoma. PLoS ONE 2020, 15, e0241002. [Google Scholar] [CrossRef]
- Naing, A.; Algazi, A.P.; Falchook, G.S.; Creelan, B.C.; Powderly, J.; Rosen, S.; Barve, M.; Mettu, N.B.; Triozzi, P.L.; Hamm, J.; et al. Phase 1/2 Study of Epacadostat in Combination with Durvalumab in Patients with Metastatic Solid Tumors. Cancer 2023, 129, 71–81. [Google Scholar] [CrossRef]
- Blocking Ido1 Helps Shrink Bladder, Cervical Tumors. Cancer Discov. 2018, 8, Of3. [CrossRef]
- Zakharia; McWilliams, R.R.; Rixe, O.; Drabick, J.; Shaheen, M.F.; Grossmann, K.F.; Kolhe, R.; Pacholczyk, R.; Sadek, R.; Tennant, L.L.; et al. Phase Ii Trial of the Ido Pathway Inhibitor Indoximod Plus Pembrolizumab for the Treatment of Patients with Advanced Melanoma. J. Immunother. Cancer 2021, 9, e002057. [Google Scholar]
- Reardon, D.A.; Desjardins, A.; Rixe, O.; Cloughesy, T.; Alekar, S.; Williams, J.H.; Li, R.; Taylor, C.T.; Lassman, A.B. A Phase 1 Study of Pf-06840003, an Oral Indoleamine 2,3-Dioxygenase 1 (Ido1) Inhibitor in Patients with Recurrent Malignant Glioma. Investig. New Drugs 2020, 38, 1784–1795. [Google Scholar] [CrossRef]
- Chuang, T.D.; Ton, N.; Rysling, S.; Quintanilla, D.; Boos, D.; Khorram, O. Therapeutic Effects of In vivo Administration of an Inhibitor of Tryptophan 2,3-Dioxygenase (680c91) for the Treatment of Fibroids: A Preclinical Study. Fertil. Steril. 2024, 121, 669–678. [Google Scholar] [CrossRef]
- Hu, S.; Lu, H.; Xie, W.; Wang, D.; Shan, Z.; Xing, X.; Wang, X.M.; Fang, J.; Dong, W.; Dai, W.; et al. Tdo2+ Myofibroblasts Mediate Immune Suppression in Malignant Transformation of Squamous Cell Carcinoma. J. Clin. Investig. 2022, 132, e157649. [Google Scholar] [CrossRef]
- Naing, A.; Eder, J.P.; Piha-Paul, S.A.; Gimmi, C.; Hussey, E.; Zhang, S.; Hildebrand, V.; Hosagrahara, V.; Habermehl, C.; Moisan, J.; et al. Preclinical Investigations and a First-in-Human Phase I Trial of M4112, the First Dual Inhibitor of Indoleamine 2,3-Dioxygenase 1 and Tryptophan 2,3-Dioxygenase 2, in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2020, 8, e000870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Z.; Zhang, J.; Ren, C.; Wang, Y. Dual-Target Inhibitors of Indoleamine 2, 3 Dioxygenase 1 (Ido1): A Promising Direction in Cancer Immunotherapy. Eur. J. Med. Chem. 2022, 238, 114524. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-Monooxygenase Inhibition in Blood Ameliorates Neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Jochems, C.; Fantini, M.; Fernando, R.I.; Kwilas, A.R.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Kim, Y.S.; Brechbiel, M.W.; Gulley, J.L.; et al. The Ido1 Selective Inhibitor Epacadostat Enhances Dendritic Cell Immunogenicity and Lytic Ability of Tumor Antigen-Specific T Cells. Oncotarget 2016, 7, 37762–37772. [Google Scholar] [CrossRef]
- Sharma, M.D.; Hou, D.Y.; Baban, B.; Koni, P.A.; He, Y.; Chandler, P.R.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Reprogrammed Foxp3(+) Regulatory T Cells Provide Essential Help to Support Cross-Presentation and Cd8(+) T Cell Priming in Naive Mice. Immunity 2010, 33, 942–954. [Google Scholar] [CrossRef]
- Yan, J.; Chen, D.; Ye, Z.; Zhu, X.; Li, X.; Jiao, H.; Duan, M.; Zhang, C.; Cheng, J.; Xu, L.; et al. Molecular Mechanisms and Therapeutic Significance of Tryptophan Metabolism and Signaling in Cancer. Mol. Cancer 2024, 23, 241. [Google Scholar] [CrossRef]
- Erhardt, S.; Pocivavsek, A.; Repici, M.; Liu, X.C.; Imbeault, S.; Maddison, D.C.; Thomas, M.A.R.; Smalley, J.L.; Larsson, M.K.; Muchowski, P.J.; et al. Adaptive and Behavioral Changes in Kynurenine 3-Monooxygenase Knockout Mice: Relevance to Psychotic Disorders. Biol. Psychiatry 2017, 82, 756–765. [Google Scholar] [CrossRef]
- Beaumont, V.; Mrzljak, L.; Dijkman, U.; Freije, R.; Heins, M.; Rassoulpour, A.; Tombaugh, G.; Gelman, S.; Bradaia, A.; Steidl, E.; et al. The Novel Kmo Inhibitor Chdi-340246 Leads to a Restoration of Electrophysiological Alterations in Mouse Models of Huntington’s Disease. Exp. Neurol. 2016, 282, 99–118. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, Y.; Chen, C.; Wu, F.; Chen, Z. A Narrative Review of the Roles of Indoleamine 2,3-Dioxygenase and Tryptophan-2,3-Dioxygenase in Liver Diseases. Ann. Transl. Med. 2021, 9, 174. [Google Scholar] [CrossRef]
- Zou, T.; Huang, Y.; Zhou, Z.; He, S.; Liu, J.; Chen, Y.; Liu, H.; Luo, Z.; Liu, M.; Wei, H.; et al. A Minimalist Multifunctional Nano-Prodrug for Drug Resistance Reverse and Integration with Pd-L1 Mab for Enhanced Immunotherapy of Hepatocellular Carcinoma. J. Nanobiotechnol. 2024, 22, 750. [Google Scholar] [CrossRef]
| Liver Diseases | Levels of Kynureine Metabolites | Roles of Kynureine Metabolites |
| Acute liver injury | Increased KYN and KYNA [71] Decreased 3-HAA and QA [72] | 1. Modulate endothelial cells to reduce liver injury [70] 2. Reduce oxidative stress in liver cells [73] 3. Regulate immune cells, such as monocytes/macrophages, T cells, dendritic cells, NK cells, etc. [74] |
| Metabolic dysfunction-associated steatotic liver disease | Increased NAD+ [75] Decreased KYN [75] | 1. Inhibit immune cell activity [76] 2. Promoting the activity and proliferation of regulatory T cells [76] 3. Alleviating oxidative damage in the liver [76] 4. Regulating fatty acid metabolism and lipid transport [77] 5. Promoting hepatocyte repair and regeneration [78] |
| Liver fibrosis and cirrhosis | Increased KYN [79] | 1. Reduce cellular oxidative damage and inflammation [79,80] 2. Reducing immune–inflammatory responses [79] 3. Eliminating free radicals to reduce oxidative injury [79] 4. Suppressing the activation of hepatic stellate cell [81] 5. Correcting metabolic disturbances in the liver [82] |
| Liver tumors | Increased KYN and 3-HA [83] | 1. Inhibit immune cell function [84] 2. Inhibit proliferation and metastasis of liver cancer cells [84] 3. Alleviating tumor-associated inflammation [84] 4. Inhibiting the tumor microenvironment [85] 5. Suppressing tumor angiogenesis [86] |
| Drug Name | Formulation and Dose | Mechanism of Action | Disease | Clinical Results |
|---|---|---|---|---|
| Epacadostat | Oral, 100 mg twice daily | IDO1 inhibitor | Melanoma | In a phase 1/2 study, ORR was 80% in melanoma patients (n = 5), with 40% of the patients achieving complete response [105]. |
| Bladder cancer | In a phase 1/2 study, ORR was 15.8% in bladder cancer patients (n = 19), and CRR was 5.3% [105]. | |||
| BMS-986205 | Oral, 100 mg once daily | IDO1 inhibitor | Bladder cancer | In a clinical study, ORR in bladder cancer patients (n = 25) was 32% [106]. |
| Cervical cancer | In a clinical study, ORR in cervical cancer patients (n = 22) was 14% [106]. | |||
| Indoximod | Oral, 600 mg twice daily | IDO1 inhibitor | Melanoma | In a phase II trial, ORR was 51% in melanoma patients (n = 89), with 20% of the patients achieving complete response [107]. |
| PF-06840003 | Oral, 500 mg twice daily | IDO1 inhibitor | Recurrent malignant glioma | A phase I study showed 47% of patients (n = 8) had disease control, indicating sustained clinical efficacy [108]. |
| 680C91 | Intraperitoneal injection, 100 mL per day | TDO inhibitor | Uterine fibroids | A preclinical study showed a 30% reduction in the weight of uterine fibroid xenografts in immunodeficient mice [109]. |
| LM10 | Oral gavage, 160 mg/kg per day | TDO inhibitor | Squamous cell carcinoma | Preclinical studies in mouse models showed T cell anti-tumor responses and prevented the malignant progression of squamous cell carcinoma [110]. |
| M4112 | Oral, 600 mg twice daily | Dual IDO1–TDO inhibitor | Solid tumors | A phase I study showed 60% of patients (n = 15) had disease control [111]. |
| RY103 | Intraperitoneal injection, 12 mg/kg every 36 h | Dual IDO1–TDO inhibitor | Pancreatic cancer | In pancreatic cancer mouse model, RY103 inhibited tumor growth, metastasis, and improved immune suppression [112]. |
| JM6 | Oral gavage, 100 mg/kg per day | KMO inhibitor | Alzheimer’s disease | In transgenic Alzheimer’s mouse models, JM6 prevented spatial memory deficits, anxiety behaviors, synapse loss, and extended survival [113]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Q.; Deng, S.; Xiong, L. Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives. Int. J. Mol. Sci. 2025, 26, 968. https://doi.org/10.3390/ijms26030968
Tan Q, Deng S, Xiong L. Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives. International Journal of Molecular Sciences. 2025; 26(3):968. https://doi.org/10.3390/ijms26030968
Chicago/Turabian StyleTan, Qiwen, Shenghe Deng, and Lijuan Xiong. 2025. "Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives" International Journal of Molecular Sciences 26, no. 3: 968. https://doi.org/10.3390/ijms26030968
APA StyleTan, Q., Deng, S., & Xiong, L. (2025). Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives. International Journal of Molecular Sciences, 26(3), 968. https://doi.org/10.3390/ijms26030968





