Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity
Abstract
1. Introduction
2. Results
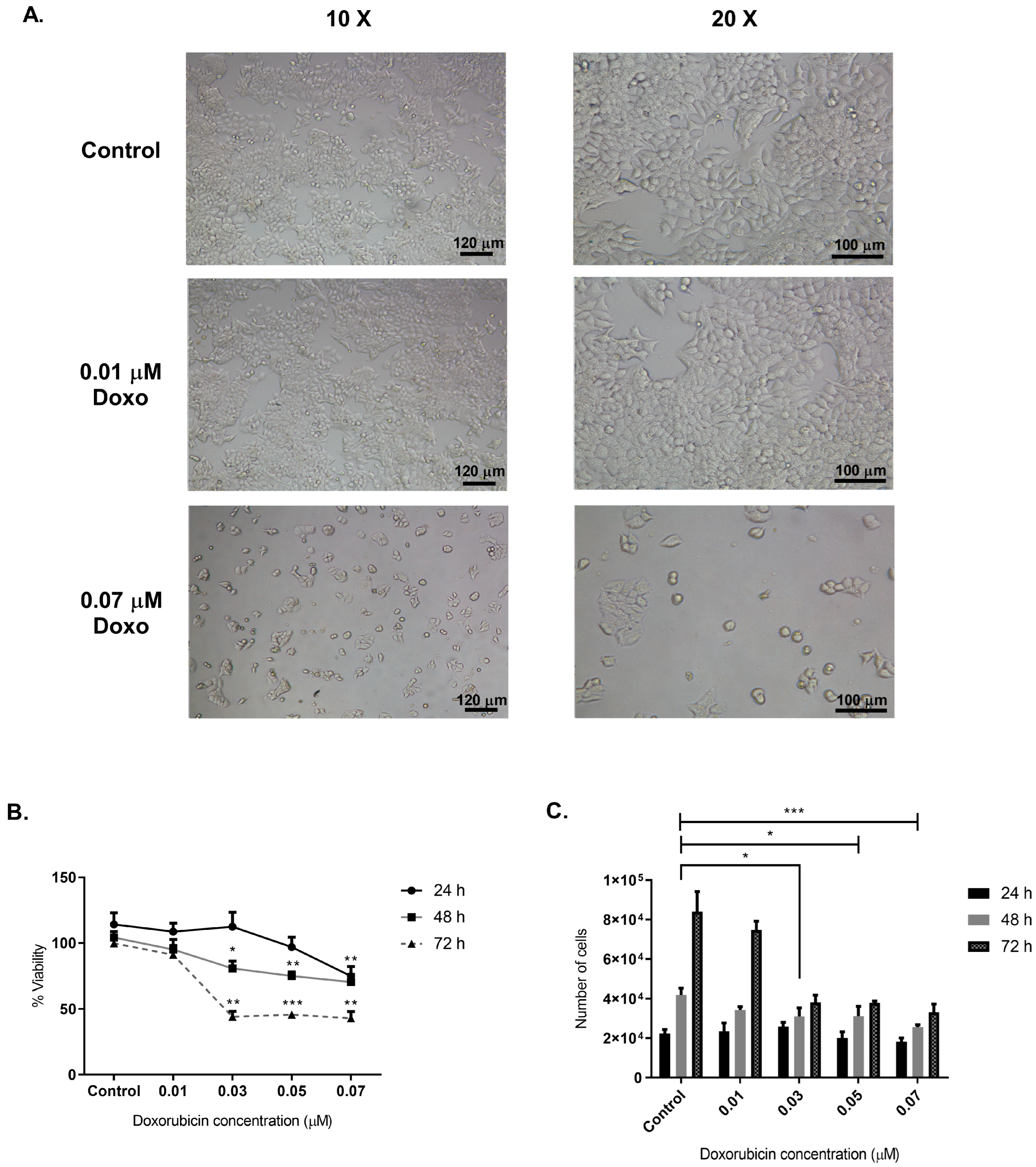
2.1. Optimal Doxorubicin Concentration for sEVs Isolation from MCF-7 Cancer Cells
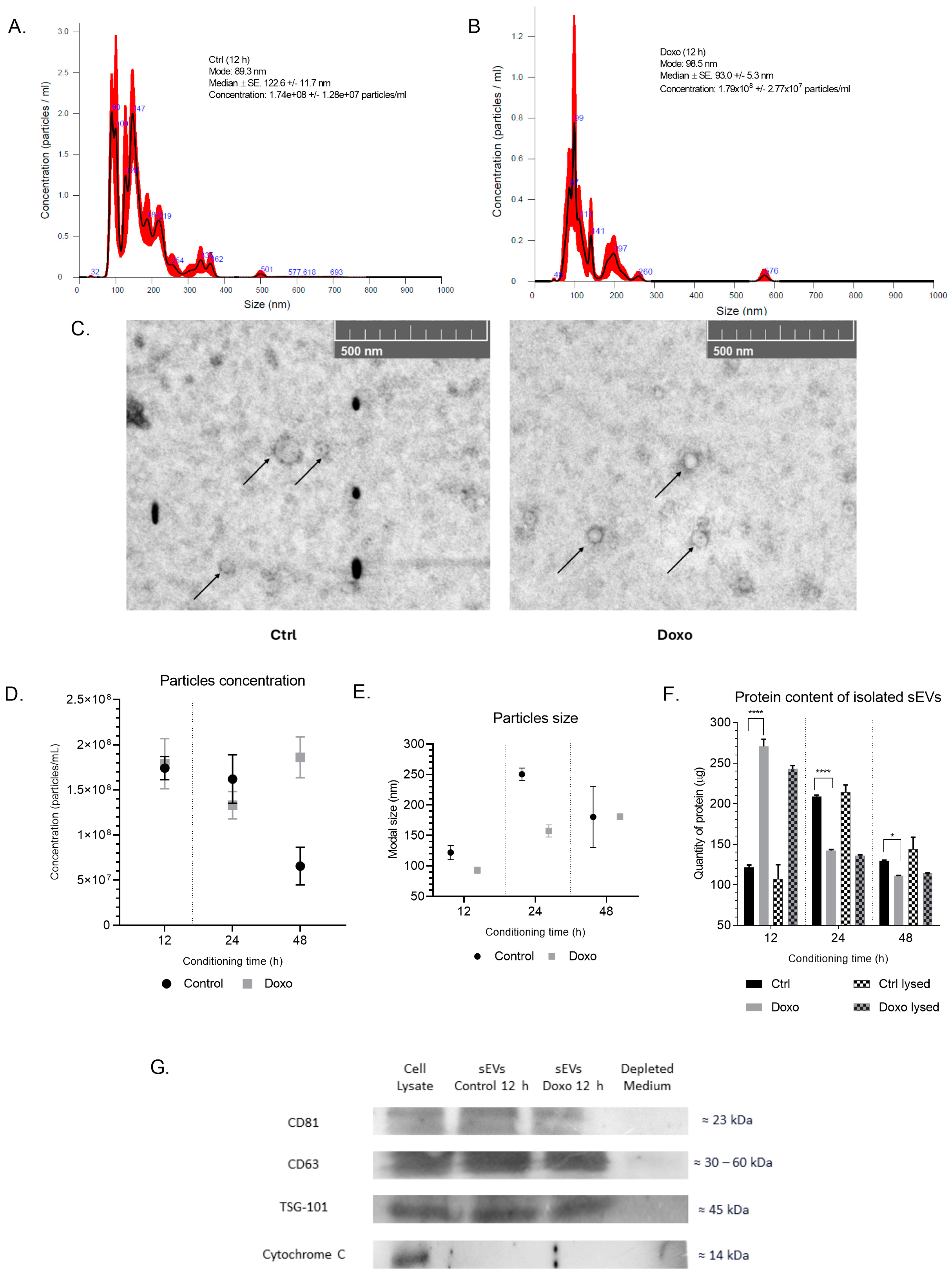
2.2. Isolation and Characterization of sEVs from Conditioned Media of Doxo-Treated MCF-7 Cells
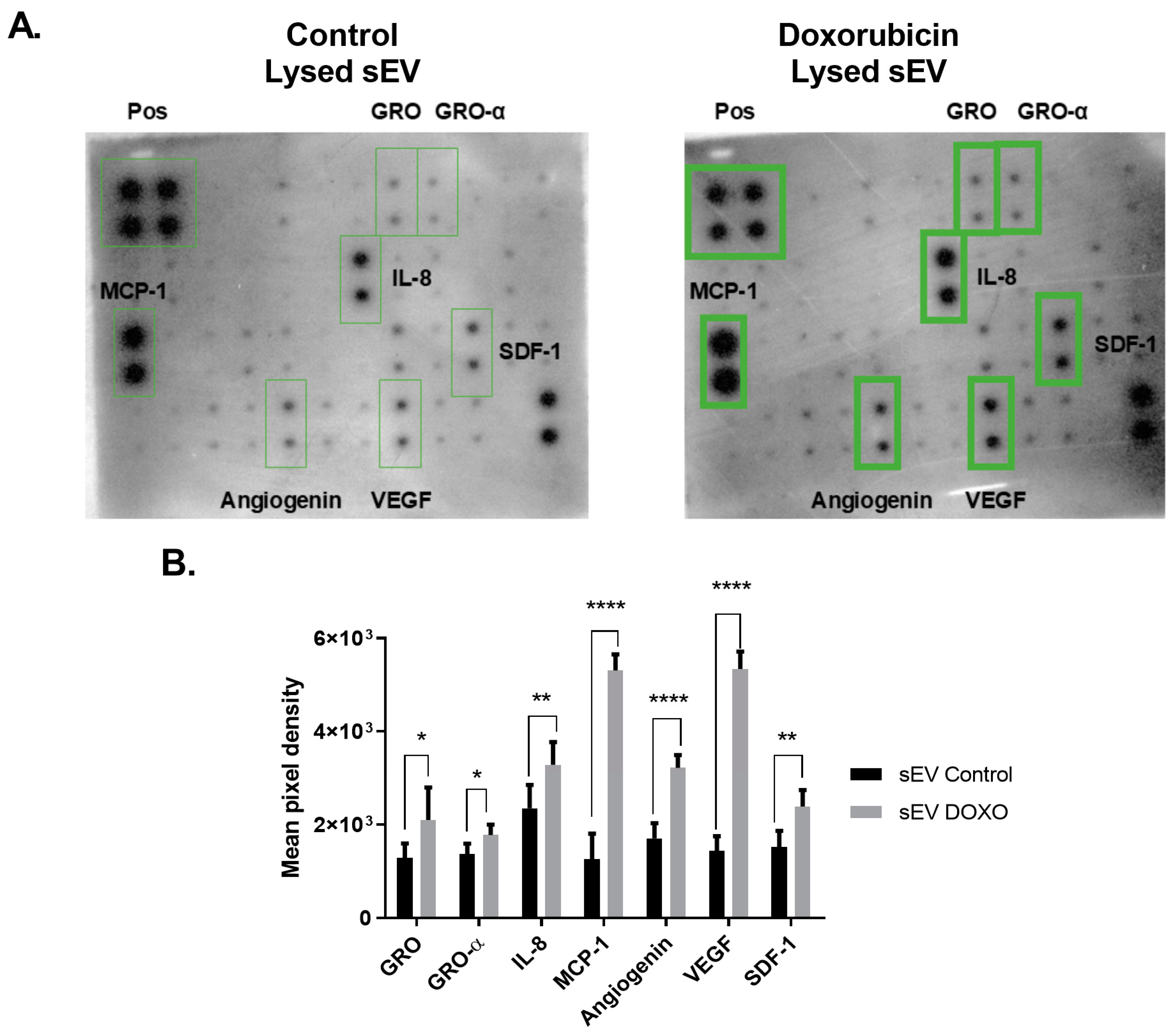
2.3. Differential Cytokine Profile in Doxorubicin-Exposed MCF-7 sEVs
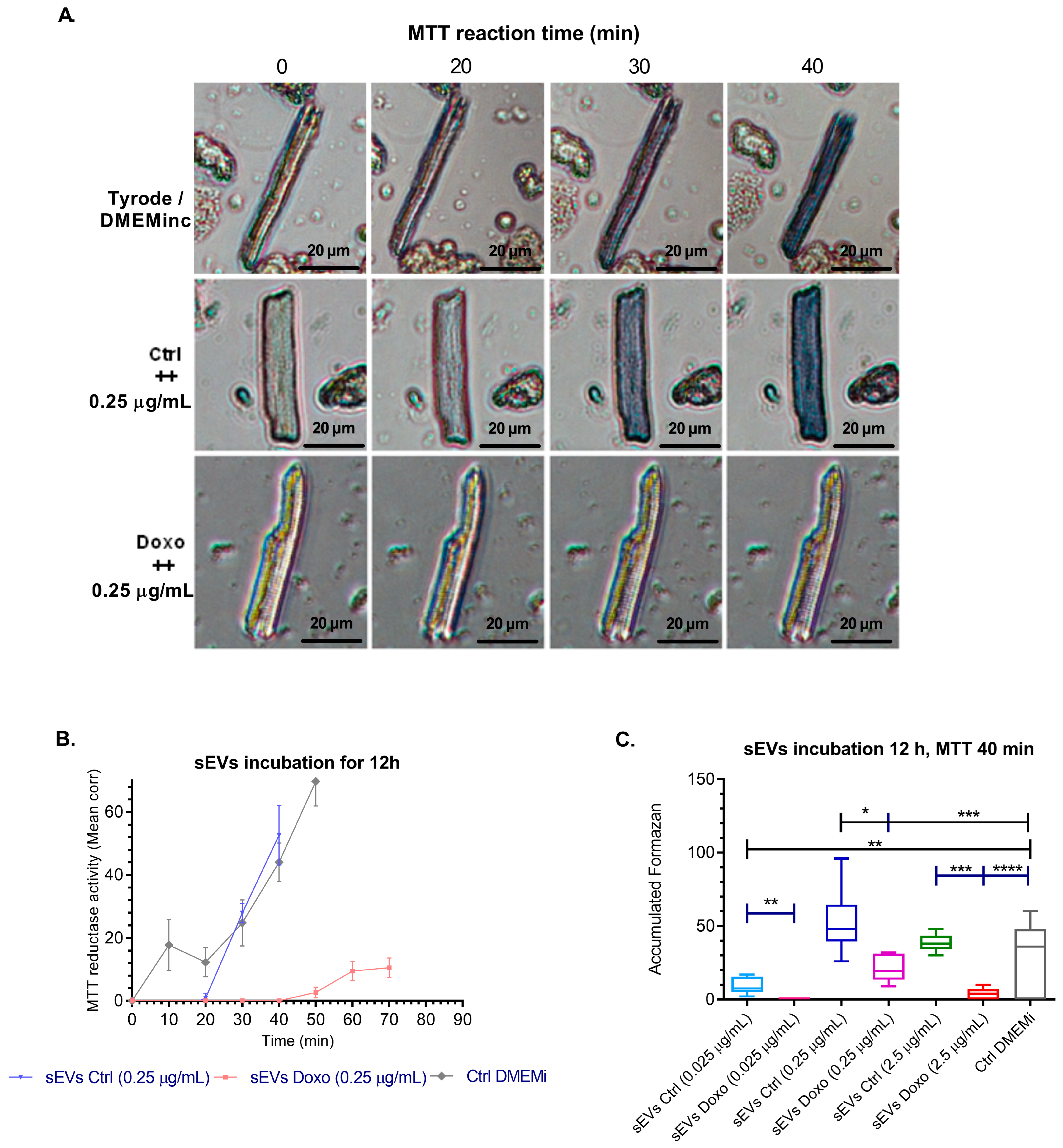
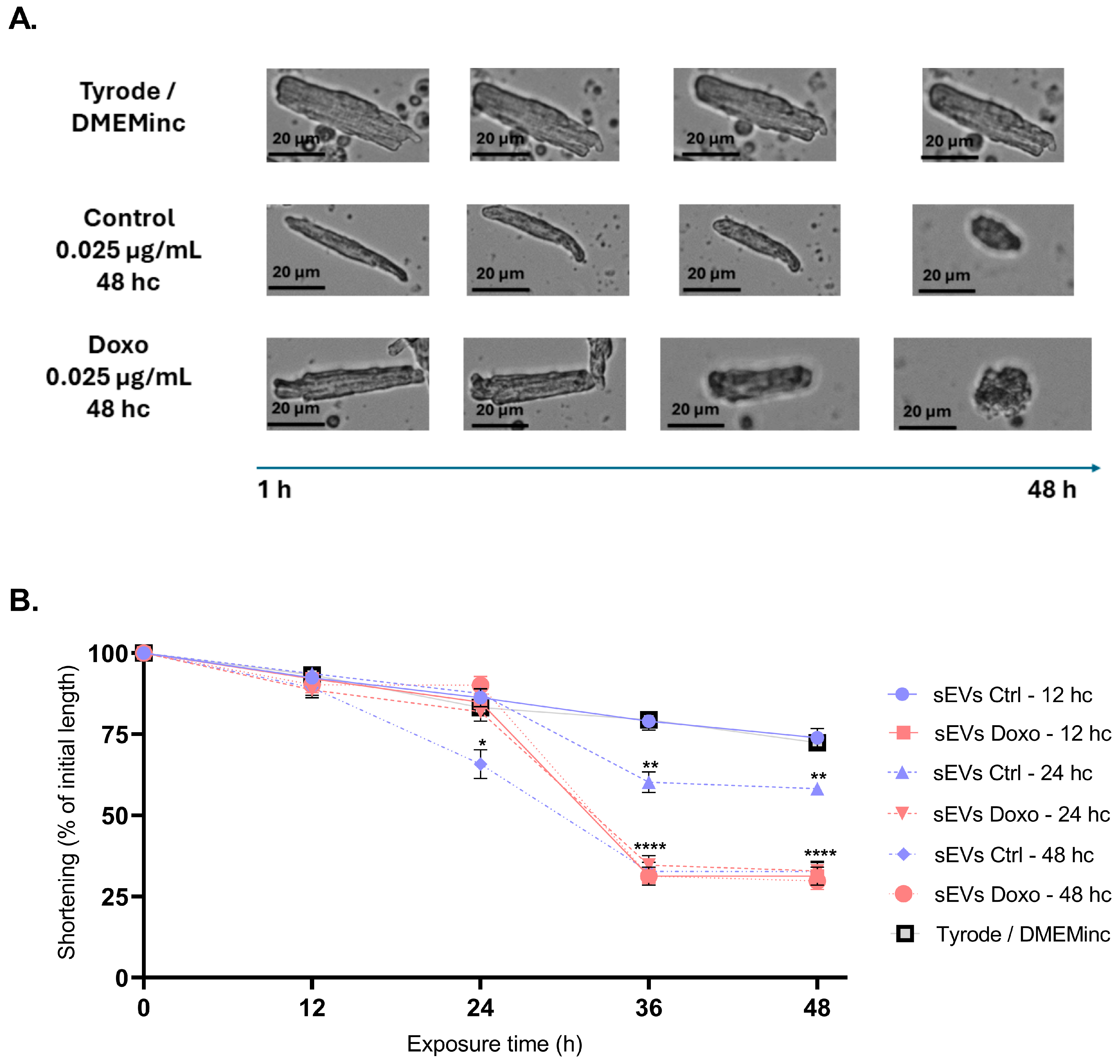
2.4. Effect of MCF-7-Derived sEVs on Cardiomyocyte Viability, Cellular Morphology, Oxidative Stress, Intracellular Calcium Levels, and Mitochondrial Membrane Potential in Isolated Guinea Pig Cardiomyocytes
3. Discussion
3.1. Optimizing Doxorubicin Concentration to Induce Cytotoxic Stress in MCF-7 Cells for sEV Isolation and Functional Analysis
3.2. Effective sEVs Isolation and Characterization from MCF-7 Cells
3.3. Cytokine Profile of sEVs from Doxorubicin-Treated MCF-7 Cells
3.4. Cardiotoxic Effects of sEVs from MCF-7 Cells on Isolated Cardiomyocytes
- Study limitations, challenges/future focus
- Clinical perspectives
4. Materials and Methods
4.1. Cell Culture and Doxorubicin Treatment
4.2. Isolation of Extracellular Vesicles and Characterization Using Nanoparticle Tracking Analysis (NTA) and Western Blot
4.3. Scanning Transmission Electron Microscopy (STEM)
4.4. sEVs Lysis and Cytokine Profiling
4.5. Cardiomyocyte Isolation, Vesicle Treatment, and Imaging Analysis
4.6. ROS Levels Assessment in Cardiomyocytes with DHE Dye
4.7. Mitochondrial Membrane Potential Assessment in Cardiomyocytes with JC-1 Dye
4.8. Evaluation of Intracellular Calcium Levels by Fluo-4 AM Reagent
4.9. Statistical Analysis
- Interpretation of Cohen’s d:
- Small effect: 0.2 ≤ d < 0.5;
- Medium effect: 0.5 ≤ d < 0.8;
- Large effect: d ≥ 0.8.
- Interpretation of η2:
- Small effect: 0.01 ≤ η2 < 0.060;
- Medium effect: 0.06 ≤ η2 < 0.140;
- Large effect: η2 ≥ 0.14.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Heart Federation. World Heart Report 2023 Confronting The World’ S Number; World Heart Federation: Geneva, Switzerland, 2023. [Google Scholar]
- Stoltzfus, K.C.; Zhang, Y.; Sturgeon, K.; Sinoway, L.I.; Trifiletti, D.M.; Chinchilli, V.M.; Zaorsky, N.G. Fatal Heart Disease among Cancer Patients. Nat. Commun. 2020, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.V.; Panageas, K.S.; DeAngelis, L.M. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol. 2017, 70, 926. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Okwuosa, T.M.; Anzevino, S.; Rao, R. Cardiovascular Disease in Cancer Survivors. Postgrad. Med. J. 2017, 93, 82–90. [Google Scholar] [CrossRef]
- Maillet, A.; Tan, K.; Chai, X.; Sadananda, S.N.; Mehta, A.; Ooi, J.; Hayden, M.R.; Pouladi, M.A.; Ghosh, S.; Shim, W.; et al. Modeling Doxorubicin-Induced Cardiotoxicity in Human Pluripotent Stem Cell Derived-Cardiomyocytes. Sci. Rep. 2016, 6, 25333. [Google Scholar] [CrossRef]
- Syukri, A.; Budu; Hatta, M.; Amir, M.; Rohman, M.S.; Mappangara, I.; Kaelan, C.; Wahyuni, S.; Bukhari, A.; Junita, A.R.; et al. Doxorubicin Induced Immune Abnormalities and Inflammatory Responses via HMGB1, HIF1-α and VEGF Pathway in Progressive of Cardiovascular Damage. Ann. Med. Surg. 2022, 76, 103501. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Alfrad, M.; Bhuiyan, N.; Panchatcharam, M.; Pattillo, C.B. Doxorubicin-Induced Cardiomyopathy Associated with Inhibition of Autophagic Degradation Process and Defects in Mitochondrial Respiration. Sci. Rep. 2019, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Wang, Z.V.; Ding, G.; Tan, W.; Luo, X.; Criollo, A.; Xie, M.; Jiang, N.; May, H.; Kyrychenko, V.; et al. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation 2016, 133, 1668–1687. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, B. Doxorubicin Induces Cardiotoxicity through Upregulation of Death Receptors Mediated Apoptosis in Cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-Induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin Resistance in Breast Cancer Cells Is Mediated by Extracellular Matrix Proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes Released from Breast Cancer Carcinomas Stimulate Cell Movement. PLoS ONE 2015, 10, e0117495. [Google Scholar] [CrossRef]
- Badila, E.; Japie, C.; Vrabie, A.-M.; Badila, A.; Georgescu, A.; Badila, E.; Japie, C.; Vrabie, A.-M.; Badila, A.; Georgescu, A. Cardiovascular Disease as a Consequence or a Cause of Cancer: Potential Role of Extracellular Vesicles. Biomolecules 2023, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, X.H.; Wang, D.; Luo, C.L.; Chen, L.X. Bladder Cancer Cell-Derived Exosomes Inhibit Tumor Cell Apoptosis and Induce Cell Proliferation in Vitro. Mol. Med. Rep. 2013, 8, 1272–1278. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Aikawa, E. Extracellular Vesicles in Cardiovascular Homeostasis and Disease. Curr. Opin. Cardiol. 2018, 33, 290–297. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Lynch, C.; Panagopoulou, M.; Gregory, C.D. Extracellular Vesicles Arising from Apoptotic Cells in Tumors: Roles in Cancer Pathogenesis and Potential Clinical Applications. Front. Immunol. 2017, 8, 1174. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-Specific Regulation of Extracellular Vesicle Biogenesis and Cargo Selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454. [Google Scholar] [CrossRef]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac Fibroblast–Derived MicroRNA Passenger Strand-Enriched Exosomes Mediate Cardiomyocyte Hypertrophy. J. Clin. Investig. 2014, 124, 2136. [Google Scholar] [CrossRef] [PubMed]
- Fandl, H.K.; Garcia, V.P.; Treuth, J.W.; Brewster, L.M.; Greiner, J.J.; Davy, K.P.; Stauffer, B.L.; Desouza, C.A. Endothelial-Derived Extracellular Vesicles from Obese/Hypertensive Adults Increase Factors Associated with Hypertrophy and Fibrosis in Cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 2023, 324, H675–H685. [Google Scholar] [CrossRef] [PubMed]
- James, V.; Nizamudeen, Z.A.; Lea, D.; Dottorini, T.; Holmes, T.L.; Johnson, B.B.; Arkill, K.P.; Denning, C.; Smith, J.G.W. Transcriptomic Analysis of Cardiomyocyte Extracellular Vesicles in Hypertrophic Cardiomyopathy Reveals Differential SnoRNA Cargo. Stem Cells Dev. 2021, 30, 1215–1227. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Qiao, S.; Chen, S.; Zheng, H.; Wei, X.; Li, Q.; Xu, B.; Huang, W. The Effects of Extracellular Vesicles Derived from Krüppel-Like Factor 2 Overexpressing Endothelial Cells on the Regulation of Cardiac Inflammation in the Dilated Cardiomyopathy. J. Nanobiotechnol. 2022, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A Critical Role of Cardiac Fibroblast-Derived Exosomes in Activating Renin Angiotensin System in Cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Hu, G.; Gao, L.; Hackfort, B.T.; Zucker, I.H. Extracellular Vesicular MicroRNA-27a* Contributes to Cardiac Hypertrophy in Chronic Heart Failure. J. Mol. Cell. Cardiol. 2020, 143, 120–131. [Google Scholar] [CrossRef]
- Santoso, M.R.; Ikeda, G.; Tada, Y.; Jung, J.H.; Vaskova, E.; Sierra, R.G.; Gati, C.; Goldstone, A.B.; von Bornstaedt, D.; Shukla, P.; et al. Exosomes From Induced Pluripotent Stem Cell–Derived Cardiomyocytes Promote Autophagy for Myocardial Repair. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2020, 9, e014345. [Google Scholar] [CrossRef]
- Yu, H.; Qin, L.; Peng, Y.; Bai, W.; Wang, Z. Exosomes Derived From Hypertrophic Cardiomyocytes Induce Inflammation in Macrophages via MiR-155 Mediated MAPK Pathway. Front. Immunol. 2021, 11, 606045. [Google Scholar] [CrossRef]
- Li, X.; Raisinghani, N.; Gallinat, A.; Zhang, S.; Phan, A.; Yoon, S.; Salvia, S.L.; Sachs, D.; Dogra, N.; Erdbruegger, U.; et al. Abstract 4148020: Circulating Extracellular Vesicles-Mediated Cardiotoxicity: A Novel Mechanism Exacerbating Heart Failure in Chronic Kidney Disease. Circulation 2024, 150, A4148020. [Google Scholar] [CrossRef]
- Buoncervello, M.; Maccari, S.; Ascione, B.; Gambardella, L.; Marconi, M.; Spada, M.; Macchia, D.; Stati, T.; Patrizio, M.; Malorni, W.; et al. Inflammatory Cytokines Associated with Cancer Growth Induce Mitochondria and Cytoskeleton Alterations in Cardiomyocytes. J. Cell. Physiol. 2019, 234, 20453–20468. [Google Scholar] [CrossRef] [PubMed]
- Khandagale, A.; Lindahl, B.; Lind, S.B.; Shevchenko, G.; Siegbahn, A.; Christersson, C. Plasma-Derived Extracellular Vesicles from Myocardial Infarction Patients Inhibits Tumor Necrosis Factor-Alpha Induced Cardiac Cell Death. Curr. Res. Transl. Med. 2022, 70, 103323. [Google Scholar] [CrossRef]
- Hanelova, K.; Raudenska, M.; Kratochvilova, M.; Navratil, J.; Vicar, T.; Bugajova, M.; Gumulec, J.; Masarik, M.; Balvan, J. Autophagy Modulators Influence the Content of Important Signalling Molecules in PS-Positive Extracellular Vesicles. Cell Commun. Signal. 2023, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Kovalchuk, S.I.; Popkov, V.A.; Chernikov, V.P.; Zharikova, A.A.; Khutornenko, A.A.; Zorov, S.D.; Plokhikh, K.S.; Zinovkin, R.A.; Evtushenko, E.A.; et al. Do Extracellular Vesicles Derived from Mesenchymal Stem Cells Contain Functional Mitochondria? Int. J. Mol. Sci. 2022, 23, 7408. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Biemmi, V.; Lazzarini, E.; Balbi, C.; Ciullo, A.; Bolis, S.; Ameri, P.; Di Silvestre, D.; Mauri, P.; Barile, L.; et al. Intravenous Administration of Cardiac Progenitor Cell-Derived Exosomes Protects against Doxorubicin/Trastuzumab-Induced Cardiac Toxicity. Cardiovasc. Res. 2020, 116, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Song, M.; Hoang, D.H.; Tran, Q.H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Melatonin Prevents Doxorubicin-Induced Cardiotoxicity through Suppression of AMPKα2-Dependent Mitochondrial Damage. Exp. Mol. Med. 2020, 52, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Yang, Y.; Bai, B.; Wang, S.; Liu, M.; Sun, R.C.; Yu, T.; Chu, X.M. Potential of Exosomes as Diagnostic Biomarkers and Therapeutic Carriers for Doxorubicin-Induced Cardiotoxicity. Int. J. Biol. Sci. 2021, 17, 1328. [Google Scholar] [CrossRef]
- Im, K.; Baek, J.; Kwon, W.S.; Rha, S.Y.; Hwang, K.W.; Kim, U.; Min, H. The Comparison of Exosome and Exosomal Cytokines between Young and Old Individuals with or without Gastric Cancer. Int. J. Gerontol. 2018, 12, 233–238. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes Released from Macrophages Infected with Intracellular Pathogens Stimulate a Proinflammatory Response in Vitro and in Vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef]
- Biemmi, V.; Milano, G.; Ciullo, A.; Cervio, E.; Burrello, J.; Cas, M.D.; Paroni, R.; Tallone, T.; Moccetti, T.; Pedrazzini, G.; et al. Inflammatory Extracellular Vesicles Prompt Heart Dysfunction via TRL4-Dependent NF-ΚB Activation. Theranostics 2020, 10, 2773–2790. [Google Scholar] [CrossRef]
- Gomez, L.A.; Alekseev, A.E.; Aleksandrova, L.A.; Brady, P.A.; Terzic, A. Use of the MTT Assay in Adult Ventricular Cardiomyocytes to Assess Viability: Effects of Adenosine and Potassium on Cellular Survival. J. Mol. Cell. Cardiol. 1997, 29, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Grosso, L.A. Preacondicionamiento isquémico en cardiomiocitos ventriculares aislados. Identificación y expresión de algunos micrornas asociados. Rev. Acad. Colomb. Ciencias Exactas Físicas Nat. 2013, 37, 433–447. [Google Scholar] [CrossRef]
- Yang, F.; Kemp, C.J.; Henikoff, S. Doxorubicin Enhances Nucleosome Turnover around Promoters. Curr. Biol. 2013, 23, 782–787. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA Torsion, and Chromatin Dynamics. Biochim. Biophys. Acta-Rev. Cancer 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J. 2020, 288, 6095. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Hill, T.A.; Lim, J.; Fairlie, D.P. Protease-Activated Receptor 2 Attenuates Doxorubicin-Induced Apoptosis in Colon Cancer Cells. J. Cell Commun. Signal. 2023, 17, 1293. [Google Scholar] [CrossRef]
- Tran, Q.H.; Hoang, D.H.; Song, M.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Melatonin and Doxorubicin Synergistically Enhance Apoptosis via Autophagy-Dependent Reduction of AMPKα1 Transcription in Human Breast Cancer Cells. Exp. Mol. Med. 2021, 53, 1413. [Google Scholar] [CrossRef]
- Lin, R.W.; Ho, C.J.; Chen, H.W.; Pao, Y.H.; Chen, L.E.; Yang, M.C.; Huang, S.B.; Wang, S.; Chen, C.H.; Wang, C. P53 Enhances Apoptosis Induced by Doxorubicin Only under Conditions of Severe DNA Damage. Cell Cycle 2018, 17, 2175. [Google Scholar] [CrossRef] [PubMed]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive Release of Extracellular Vesicles from Cancer Cells after Photodynamic Treatment or Chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, 12404. [Google Scholar] [CrossRef]
- Wang, F.; Cerione, R.A.; Antonyak, M.A. Isolation and Characterization of Extracellular Vesicles Produced by Cell Lines. STAR Protoc. 2021, 2, 100295. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Platzgummer, A.; Cunha, R.; Morini, S.; Carvalho, M.; Moreno-Cid, J.; García, C.; Cabral, J.M.S.; da Silva, C.L. Optimized Operation of a Controlled Stirred Tank Reactor System for the Production of Mesenchymal Stromal Cells and Their Extracellular Vesicles. Biotechnol. Bioeng. 2023, 120, 2742–2755. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Mebarek, S.; Buchet, R.; Pikula, S.; Strzelecka-Kiliszek, A.; Brizuela, L.; Corti, G.; Collacchi, F.; Anghieri, G.; Magrini, A.; Ciancaglini, P.; et al. Do Media Extracellular Vesicles and Extracellular Vesicles Bound to the Extracellular Matrix Represent Distinct Types of Vesicles? Biomolecules 2023, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A Brief History of Nearly EV-Erything—The Rise and Rise of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Morishita, M.; Charoenviriyakul, C.; Saji, H.; Takakura, Y. Accelerated Growth of B16BL6 Tumor in Mice through Efficient Uptake of Their Own Exosomes by B16BL6 Cells. Cancer Sci. 2017, 108, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Zhang, F.; Zhao, Q.; Zhong, H. Increased Anti-Tumour Activity by Exosomes Derived from Doxorubicin-Treated Tumour Cells via Heat Stress. Int. J. Hyperth. 2015, 31, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Satlof, L.; Stoffels, G.; Kothapalli, U.; Ziluck, N.; Lema, M.; Poretsky, L.; Avtanski, D. Cytokine Secretion in Breast Cancer Cells—MILLIPLEX Assay Data. Data Br. 2020, 28, 104798. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef]
- Mirabdollahi, M.; Javanmard, S.H.; Sadeghi-Aliabadi, H. In Vitro Assessment of Cytokine Expression Profile of MCF-7 Cells in Response to HWJ-MSCs Secretome. Adv. Pharm. Bull. 2019, 9, 649–654. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes Secreted under Hypoxia Enhance Invasiveness and Stemness of Prostate Cancer Cells by Targeting Adherens Junction Molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef]
- Hamza, A.A.; Ahmed, M.M.; Elwey, H.M.; Amin, A. Melissa Officinalis Protects against Doxorubicin-Induced Cardiotoxicity in Rats and Potentiates Its Anticancer Activity on MCF-7 Cells. PLoS ONE 2016, 11, e0167049. [Google Scholar] [CrossRef]
- Yin, J.; Guo, J.; Zhang, Q.; Cui, L.; Zhang, L.; Zhang, T.; Zhao, J.; Li, J.; Middleton, A.; Carmichael, P.L.; et al. Doxorubicin-Induced Mitophagy and Mitochondrial Damage Is Associated with Dysregulation of the PINK1/Parkin Pathway. Toxicol. Vitr. 2018, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Takefuji, M.; Sakaguchi, T.; Ishihama, S.; Mori, Y.; Tsuda, T.; Takikawa, T.; Yoshida, T.; Ohashi, K.; Shimizu, Y.; et al. Cardiomyocytes Capture Stem Cell-Derived, Anti-Apoptotic MicroRNA-214 via Clathrin-Mediated Endocytosis in Acute Myocardial Infarction. J. Biol. Chem. 2019, 294, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Nunes, J.P.S.; Andrieux, P.; Brochet, P.; Almeida, R.R.; Kitano, E.; Honda, A.K.; Iwai, L.K.; Andrade-Silva, D.; Goudenège, D.; Alcântara Silva, K.D.; et al. Co-Exposure of Cardiomyocytes to IFN-γ and TNF-α Induces Mitochondrial Dysfunction and Nitro-Oxidative Stress: Implications for the Pathogenesis of Chronic Chagas Disease Cardiomyopathy. Front. Immunol. 2021, 12, 755862. [Google Scholar] [CrossRef]
- Lebedeva, A.; Fitzgerald, W.; Molodtsov, I.; Shpektor, A.; Vasilieva, E.; Margolis, L. Differential Clusterization of Soluble and Extracellular Vesicle-Associated Cytokines in Myocardial Infarction. Sci. Rep. 2020, 10, 21114. [Google Scholar] [CrossRef] [PubMed]
- Shibakura, M.; Niiya, K.; Kiguchi, T.; Kitajima, I.; Niiya, M.; Asaumi, N.; Huh, N.-h.; Nakata, Y.; Harada, M.; Tanimoto, M. Induction of IL-8 and Monocyte Chemoattractant Protein-1 by Doxorubicin in Human Small Cell Lung Carcinoma Cells. Int. J. Cancer 2003, 103, 380–386. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, T.; Chen, L.; Luo, W.; Chao, J. MCP-1 Mediates Ischemia-Reperfusion-Induced Cardiomyocyte Apoptosis via MCPIP1 and CaSR. Am. J. Physiol.-Heart Circ. Physiol. 2020, 318, H59–H71. [Google Scholar] [CrossRef]
- Strnadová, K.; Pfeiferová, L.; Přikryl, P.; Dvořánková, B.; Vlčák, E.; Frýdlová, J.; Vokurka, M.; Novotný, J.; Šáchová, J.; Hradilová, M.; et al. Exosomes Produced by Melanoma Cells Significantly Influence the Biological Properties of Normal and Cancer-Associated Fibroblasts. Histochem. Cell Biol. 2022, 157, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.P.; Gao, L. Proinflammatory Cytokines Increase Vascular Endothelial Growth Factor Expression in Alveolar Epithelial Cells. Mediators Inflamm. 2015, 2015, 387842. [Google Scholar] [CrossRef] [PubMed]
- Loftus, A.; Cappariello, A.; George, C.; Ucci, A.; Shefferd, K.; Green, A.; Paone, R.; Ponzetti, M.; Delle Monache, S.; Muraca, M.; et al. Extracellular Vesicles From Osteotropic Breast Cancer Cells Affect Bone Resident Cells. J. Bone Miner. Res. 2020, 35, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-h.; Luo, B.; Lv, Y.-x.; Zheng, F.; Wang, L.; Wei, M.-x.; Li, X.-y.; Zhang, L.; Wang, J.-n.; Chen, S.-y.; et al. Dual Effects of VEGF-B on Activating Cardiomyocytes and Cardiac Stem Cells to Protect the Heart against Short- and Long-Term Ischemia-Reperfusion Injury. J. Transl. Med. 2016, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Park, H.; Lim, E.H.; Kim, K.H.; Choi, J.S.; Lee, J.H.; Shin, J.W.; Lee, K.W. Exosomes from Ovarian Cancer Cells Induce Adipose Tissue-Derived Mesenchymal Stem Cells to Acquire the Physical and Functional Characteristics of Tumor-Supporting Myofibroblasts. Gynecol. Oncol. 2011, 123, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ascione, R.; Rowlinson, J.; Avolio, E.; Katare, R.; Meloni, M.; Spencer, H.L.; Mangialardi, G.; Norris, C.; Kränkel, N.; Spinetti, G.; et al. Migration towards SDF-1 Selects Angiogenin-Expressing Bone Marrow Monocytes Endowed with Cardiac Reparative Activity in Patients with Previous Myocardial Infarction. Stem Cell Res. Ther. 2015, 6, 53. [Google Scholar] [CrossRef][Green Version]
- Tan, Y.; Luo, X.; Lv, W.; Hu, W.; Zhao, C.; Xiong, M.; Yi, Y.; Wang, D.; Wang, Y.; Wang, H.; et al. Tumor-Derived Exosomal Components: The Multifaceted Roles and Mechanisms in Breast Cancer Metastasis. Cell Death Dis. 2021, 12, 547. [Google Scholar] [CrossRef]
- Chiba, M.; Kubota, S.; Sato, K.; Monzen, S. Exosomes Released from Pancreatic Cancer Cells Enhance Angiogenic Activities via Dynamin-Dependent Endocytosis in Endothelial Cells in Vitro. Sci. Rep. 2018, 8, 11972. [Google Scholar] [CrossRef]
- Hafez, A.A.; Jamali, Z.; Samiei, S.; Khezri, S.; Salimi, A. Reduction of Doxorubicin-Induced Cytotoxicity and Mitochondrial Damage by Betanin in Rat Isolated Cardiomyocytes and Mitochondria. Hum. Exp. Toxicol. 2021, 40, 2123–2134. [Google Scholar] [CrossRef]
- Miller, V.M.; Rocca, W.A.; Faubion, S.S. Sex Differences Research, Precision Medicine, and the Future of Women’s Health. J. Women’s Health 2015, 24, 969. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, N.S.; Mazure, C.M. Precision Medicine Requires Understanding How Both Sex and Gender Influence Health. Cell 2022, 185, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Schumacher Dimech, A.; Ferretti, M.T.; Sandset, E.C.; Santuccione Chadha, A. The Role of Sex and Gender Differences in Precision Medicine: The Work of the Women’s Brain Project. Eur. Heart J. 2021, 42, 3215–3217. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A Review of the Pathophysiological Mechanisms of Doxorubicin-Induced Cardiotoxicity and Aging. NPJ Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Brandão, S.R.; Reis-Mendes, A.; Domingues, P.; Duarte, J.A.; Bastos, M.L.; Carvalho, F.; Ferreira, R.; Costa, V.M. Exploring the Aging Effect of the Anticancer Drugs Doxorubicin and Mitoxantrone on Cardiac Mitochondrial Proteome Using a Murine Model. Toxicology 2021, 459, 152852. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gwilt, P.R. The Effect of Age on the Early Disposition of Doxorubicin. Cancer Chemother. Pharmacol. 2003, 51, 395–402. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, G.; Sun, K.; Chen, A.F. Aging-Associated ALKBH5-M6A Modification Exacerbates Doxorubicin-Induced Cardiomyocyte Apoptosis Via AT-Rich Interaction Domain 2. J. Am. Heart Assoc. 2024, 13, 31353. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Sinclair, D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac Changes Associated with Vascular Aging. Clin. Cardiol. 2020, 43, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Rattanasopa, C.; Kirk, J.A.; Bupha-Intr, T.; Papadaki, M.; De Tombe, P.P.; Wattanapermpool, J. Estrogen but Not Testosterone Preserves Myofilament Function from Doxorubicin-Induced Cardiotoxicity by Reducing Oxidative Modifications. Am. J. Physiol.-Heart Circ. Physiol. 2018, 316, H360. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Cao, X.-Q.; Zhang, C.-S.; Zhao, Z. 17β-Estradiol Protects against Doxorubicin-Induced Cardiotoxicity in Male Sprague-Dawley Rats by Regulating NADPH Oxidase and Apoptosis Genes. Mol. Med. Rep. 2017, 15, 2695–2702. [Google Scholar] [CrossRef][Green Version]
- Chu, T. Gender Differences in Pharmacokinetics. U.S. Pharm. 2014, 39, 40–43. [Google Scholar]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Prendergast, B.J. Sex Differences in Pharmacokinetics Predict Adverse Drug Reactions in Women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Oi Yan Chan, J.; Moullet, M.; Williamson, B.; Arends, R.H.; Pilla Reddy, V. Harnessing Clinical Trial and Real-World Data Towards an Understanding of Sex Effects on Drug Pharmacokinetics, Pharmacodynamics and Efficacy. Front. Pharmacol. 2022, 13, 874606. [Google Scholar] [CrossRef] [PubMed]
- Dorostkar, H.; Haghiralsadat, B.F.; Hemati, M.; Safari, F.; Hassanpour, A.; Naghib, S.M.; Roozbahani, M.H.; Mozafari, M.R.; Moradi, A. Reduction of Doxorubicin-Induced Cardiotoxicity by Co-Administration of Smart Liposomal Doxorubicin and Free Quercetin: In Vitro and In Vivo Studies. Pharmaceutics 2023, 15, 1920. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into Doxorubicin-Induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol. Pharmacol. 2019, 96, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.K.; Shah, G. Exploring in Vivo and in Vitro Models for Heart Failure with Biomarker Insights: A Review. Egypt. Heart J. 2024, 76, 141. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L. In Vitro and In Vivo Cardioprotective Effects of Curcumin against Doxorubicin-Induced Cardiotoxicity: A Systematic Review. J. Oncol. 2022, 2022, 7277562. [Google Scholar] [CrossRef]
- Płoska, A.; Wozniak, M.; Hedhli, J.; Konopka, C.J.; Skondras, A.; Matatov, S.; Stawarz, A.; Schuh, S.; Czerwinski, A.; Dobrucki, L.W.; et al. In Vitro and In Vivo Imaging-Based Evaluation of Doxorubicin Anticancer Treatment in Combination with the Herbal Medicine Black Cohosh. Int. J. Mol. Sci. 2023, 24, 17506. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Li, Y.F.; Matsa, E.; Wu, H.; Ong, S.G.; Sharma, A.; Holmström, A.; Chang, A.C.; Coronado, M.J.; Ebert, A.D.; et al. Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes Recapitulate the Predilection of Breast Cancer Patients to Doxorubicin–Induced Cardiotoxicity. Nat. Med. 2016, 22, 547. [Google Scholar] [CrossRef]
- Haupt, L.P.; Rebs, S.; Maurer, W.; Hübscher, D.; Tiburcy, M.; Pabel, S.; Maus, A.; Köhne, S.; Tappu, R.; Haas, J.; et al. Doxorubicin Induces Cardiotoxicity in a Pluripotent Stem Cell Model of Aggressive B Cell Lymphoma Cancer Patients. Basic Res. Cardiol. 2022, 117, 13. [Google Scholar] [CrossRef]
- Arzt, M.; Gao, B.; Mozneb, M.; Pohlman, S.; Cejas, R.B.; Liu, Q.; Huang, F.; Yu, C.; Zhang, Y.; Fan, X.; et al. Protein-Encapsulated Doxorubicin Reduces Cardiotoxicity in HiPSC-Cardiomyocytes and Cardiac Spheroids While Maintaining Anticancer Efficacy. Stem Cell Rep. 2023, 18, 1913–1924. [Google Scholar] [CrossRef]
- Allan-Rahill, N.H.; Lamont, M.R.E.; Chilian, W.M.; Nishimura, N.; Small, D.M. Intravital Microscopy of the Beating Murine Heart to Understand Cardiac Leukocyte Dynamics. Front. Immunol. 2020, 11, 506616. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Yoon, J.; Kim, P. Intravital Imaging of Cardiac Tissue Utilizing Tissue-Stabilized Heart Window Chamber in Live Animal Model. Eur. Heart J.-Imaging Methods Pract. 2024, 2, qyae062. [Google Scholar] [CrossRef]
- Cooper, D.; Deniset, J.; Philip, D.; Kavanagh, J.; Kalia, N. Live Intravital Imaging of Cellular Trafficking in the Cardiac Microvasculature—Beating the Odds. Front. Immunol. 2019, 10, 2782. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, R.; Lan, M.; Wen, C.; Wang, H.; Guo, J.; Zhao, X.; Xu, H.; Deng, P.; Pi, H.; et al. Integration of Transcriptomics, Metabolomics, and Lipidomics Reveals the Mechanisms of Doxorubicin-Induced Inflammatory Responses and Myocardial Dysfunction in Mice. Biomed. Pharmacother. 2023, 162, 114733. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, S.; Shu, L.; Huang, W.; Xie, L.; Bi, C.; Yu, H.; Wang, Y.; Li, Y. Exploration the Mechanism of Doxorubicin-Induced Heart Failure in Rats by Integration of Proteomics and Metabolomics Data. Front. Pharmacol. 2020, 11, 600561. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Yang, D.; Xu, W.; Qian, H. Extracellular Vesicles: Emerging Roles, Biomarkers and Therapeutic Strategies in Fibrotic Diseases. J. Nanobiotechnol. 2023, 21, 164. [Google Scholar] [CrossRef]
- Beetler, D.J.; Di Florio, D.N.; Bruno, K.A.; Ikezu, T.; March, K.L.; Cooper, L.T.; Wolfram, J.; Fairweather, D.L. Extracellular Vesicles as Personalized Medicine. Mol. Aspects Med. 2023, 91, 101155. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Nicolás-Boluda, A.; Mulens-Arias, V.; Richard, S.; Rahmi, G.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Extracellular Vesicles for Personalized Medicine: The Input of Physically Triggered Production, Loading and Theranostic Properties. Adv. Drug Deliv. Rev. 2019, 138, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Al Marzooqi, S.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Zhou, E.; Li, Y.; Wu, F.; Guo, M.; Xu, J.; Wang, S.; Tan, Q.; Ma, P.; Song, S.; Jin, Y. Circulating Extracellular Vesicles Are Effective Biomarkers for Predicting Response to Cancer Therapy. EBioMedicine 2021, 67, 103365. [Google Scholar] [CrossRef] [PubMed]
- Silver, B.B.; Kreutz, A.; Weick, M.; Gerrish, K.; Tokar, E.J. Biomarkers of Chemotherapy-Induced Cardiotoxicity: Toward Precision Prevention Using Extracellular Vesicles. Front. Oncol. 2024, 14, 1393930. [Google Scholar] [CrossRef]
- Sourani, A.; Saghaei, S.; Sabouri, M.; Soleimani, M.; Dehghani, L. A Systematic Review of Extracellular Vesicles as Non-Invasive Biomarkers in Glioma Diagnosis, Prognosis, and Treatment Response Monitoring. Mol. Biol. Rep. 2021, 48, 6971–6985. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Burrello, J.; Fedrigo, M.; Burrello, A.; Bolis, S.; Di Silvestre, D.; Tona, F.; Bottio, T.; Biemmi, V.; Toscano, G.; et al. Circulating Extracellular Vesicles as Non-Invasive Biomarker of Rejection in Heart Transplant. J. Heart Lung Transplant. 2020, 39, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1. [Google Scholar] [CrossRef]
- Mazzeo, C.; Cañas, J.A.; Zafra, M.P.; Rojas Marco, A.; Fernández-Nieto, M.; Sanz, V.; Mittelbrunn, M.; Izquierdo, M.; Baixaulli, F.; Sastre, J.; et al. Exosome Secretion by Eosinophils: A Possible Role in Asthma Pathogenesis. J. Allergy Clin. Immunol. 2015, 135, 1603–1613. [Google Scholar] [CrossRef]
- Kowkabany, G.; Bao, Y. Nanoparticle Tracking Analysis: An Effective Tool to Characterize Extracellular Vesicles. Molecules 2024, 29, 4672. [Google Scholar] [CrossRef] [PubMed]
- ParticleMetrix GmbH Introduction to Nanoparticles Tracking Analysis (NTA). Available online: https://analytik.co.uk/wp-content/uploads/2016/12/whitepaper-nanoparticle-tracking-analysis.pdf/ (accessed on 15 November 2024).
- Limited, M.I. Nanoscale Material Characterization: A Review of the Use of Nanoparticle Tracking Analysis (NTA). Available online: https://www.chem.uci.edu/~dmitryf/manuals/Fundamentals/Review%20of%20Nanoparticle%20Tracking%20Analysis.pdf (accessed on 15 November 2024).
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 2018, 56482. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, H.; Chen, Q.; Yang, G. A Method for Extraction of Exosomes from Breast Tumour Cells and Characterisation by Transmission Electron Microscopy. J. Microsc. 2023, 292, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, H.; Ruan, H.; Chen, Z.; Chen, S.; Wang, B.; Xie, Y. Isolation and Identification of Exosomes from Feline Plasma, Urine and Adipose-Derived Mesenchymal Stem Cells. BMC Vet. Res. 2021, 17, 272. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, W.; Basyal, M.; Chang, K.H.; Ostermann, L.; Burks, J.K.; Ly, C.; Mu-Mosley, H.; Zhang, Q.; Han, X.; et al. FLT3 Inhibitors Upregulate CXCR4 and E-Selectin Ligands via ERK Suppression in AML Cells and CXCR4/E-Selectin Inhibition Enhances Anti-Leukemia Efficacy of FLT3-Targeted Therapy in AML. Leukemia 2023, 37, 1379–1383. [Google Scholar] [CrossRef]
- Kelly, R.; Aviles, D.; Krisulevicz, C.; Hunter, K.; Krill, L.; Warshal, D.; Ostrovsky, O. The Effects of Natural Epigenetic Therapies in 3D Ovarian Cancer and Patient-Derived Tumor Explants: New Avenues in Regulating the Cancer Secretome. Biomolecules 2023, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Batalha, S.; Gomes, C.M.; Brito, C. Immune Microenvironment Dynamics of HER2 Overexpressing Breast Cancer under Dual Anti-HER2 Blockade. Front. Immunol. 2023, 14, 1267621. [Google Scholar] [CrossRef]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea Pig Models for Translation of the Developmental Origins of Health and Disease Hypothesis into the Clinic. J. Physiol. 2018, 596, 5535. [Google Scholar] [CrossRef] [PubMed]
- Haq, K.T.; McLean, K.; Salameh, S.; Swift, L.; Posnack, N.G. Electroanatomical Adaptations in the Guinea Pig Heart from Neonatal to Adulthood. bioRxiv Prepr. Serv. Biol. 2024, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Malkin, R.A. Experimental Cardiac Tachyarrhythmias in Guinea Pigs. J. Electrocardiol. 1999, 32, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Romero, Y.; Montoya Ortiz, G.; Novoa Herrán, S.; Osorio Mendez, J.; Gomez Grosso, L.A. MiRNA Expression Profiles in Isolated Ventricular Cardiomyocytes: Insights into Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2024, 25, 5272. [Google Scholar] [CrossRef]
- Vanden Hoek, T.L.; Li, C.; Shao, Z.; Schumacker, P.T.; Becker, L.B. Significant Levels of Oxidants Are Generated by Isolated Cardiomyocytes during Ischemia Prior to Reperfusion. J. Mol. Cell. Cardiol. 1997, 29, 2571–2583. [Google Scholar] [CrossRef]
- Bassino, E.; Fornero, S.; Gallo, M.P.; Gallina, C.; Femminò, S.; Levi, R.; Tota, B.; Alloatti, G. Catestatin Exerts Direct Protective Effects on Rat Cardiomyocytes Undergoing Ischemia/Reperfusion by Stimulating PI3K-Akt-GSK3β Pathway and Preserving Mitochondrial Membrane Potential. PLoS ONE 2015, 10, e0119790. [Google Scholar] [CrossRef]
- Bernas, T.; Dobrucki, J. Mitochondrial and Nonmitochondrial Reduction of MTT: Interaction of MTT with TMRE, JC-1, and NAO Mitochondrial Fluorescent Probes. Cytometry 2002, 47, 236–242. [Google Scholar] [CrossRef]
- Hagen, B.M.; Boyman, L.; Kao, J.P.Y.; Lederer, W.J. A Comparative Assessment of Fluo Ca2+ Indicators in Rat Ventricular Myocytes. Cell Calcium 2012, 52, 170–181. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Méndez, J.J.; Gómez-Grosso, L.A.; Montoya-Ortiz, G.; Novoa-Herrán, S.; Domínguez-Romero, Y. Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity. Int. J. Mol. Sci. 2025, 26, 945. https://doi.org/10.3390/ijms26030945
Osorio-Méndez JJ, Gómez-Grosso LA, Montoya-Ortiz G, Novoa-Herrán S, Domínguez-Romero Y. Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity. International Journal of Molecular Sciences. 2025; 26(3):945. https://doi.org/10.3390/ijms26030945
Chicago/Turabian StyleOsorio-Méndez, Jhon Jairo, Luis Alberto Gómez-Grosso, Gladis Montoya-Ortiz, Susana Novoa-Herrán, and Yohana Domínguez-Romero. 2025. "Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity" International Journal of Molecular Sciences 26, no. 3: 945. https://doi.org/10.3390/ijms26030945
APA StyleOsorio-Méndez, J. J., Gómez-Grosso, L. A., Montoya-Ortiz, G., Novoa-Herrán, S., & Domínguez-Romero, Y. (2025). Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity. International Journal of Molecular Sciences, 26(3), 945. https://doi.org/10.3390/ijms26030945






