Mechanistic Wound Healing of Ficus trijuja Leaf Extract and Its Lipid Nanocapsule Supported by Metabolomic Profiling and In Vivo Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. LC-HRESIMS Metabolomic Analysis
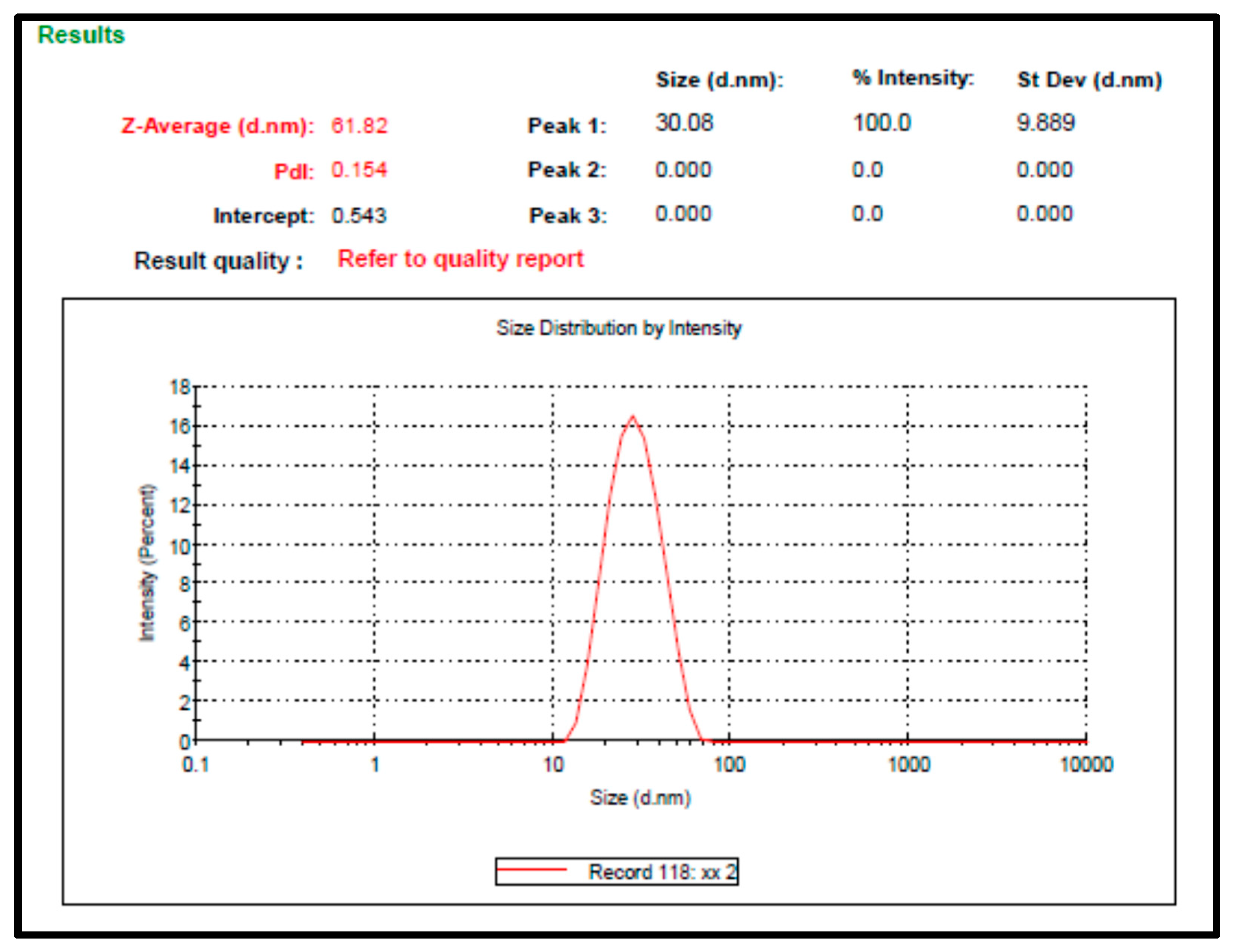
2.2. Preparation and Characterization of F. trijuja Extract LNCs
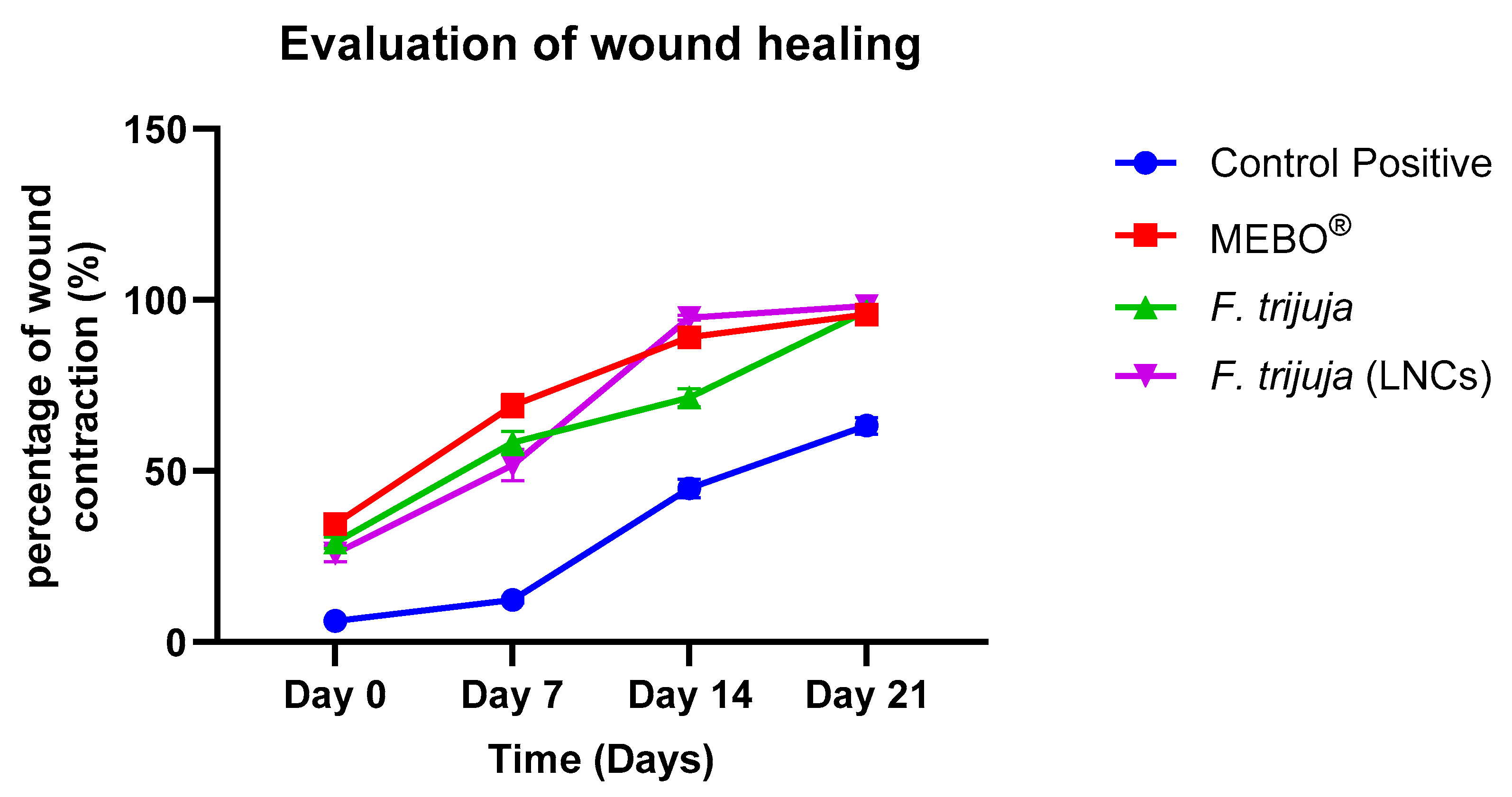
2.3. Effect of F. trijuja Extract LNCs on the Promotion of Wound Healing
2.4. Morphometric Evaluation of the Rat Skin After Treatment with F. trijuja Extract
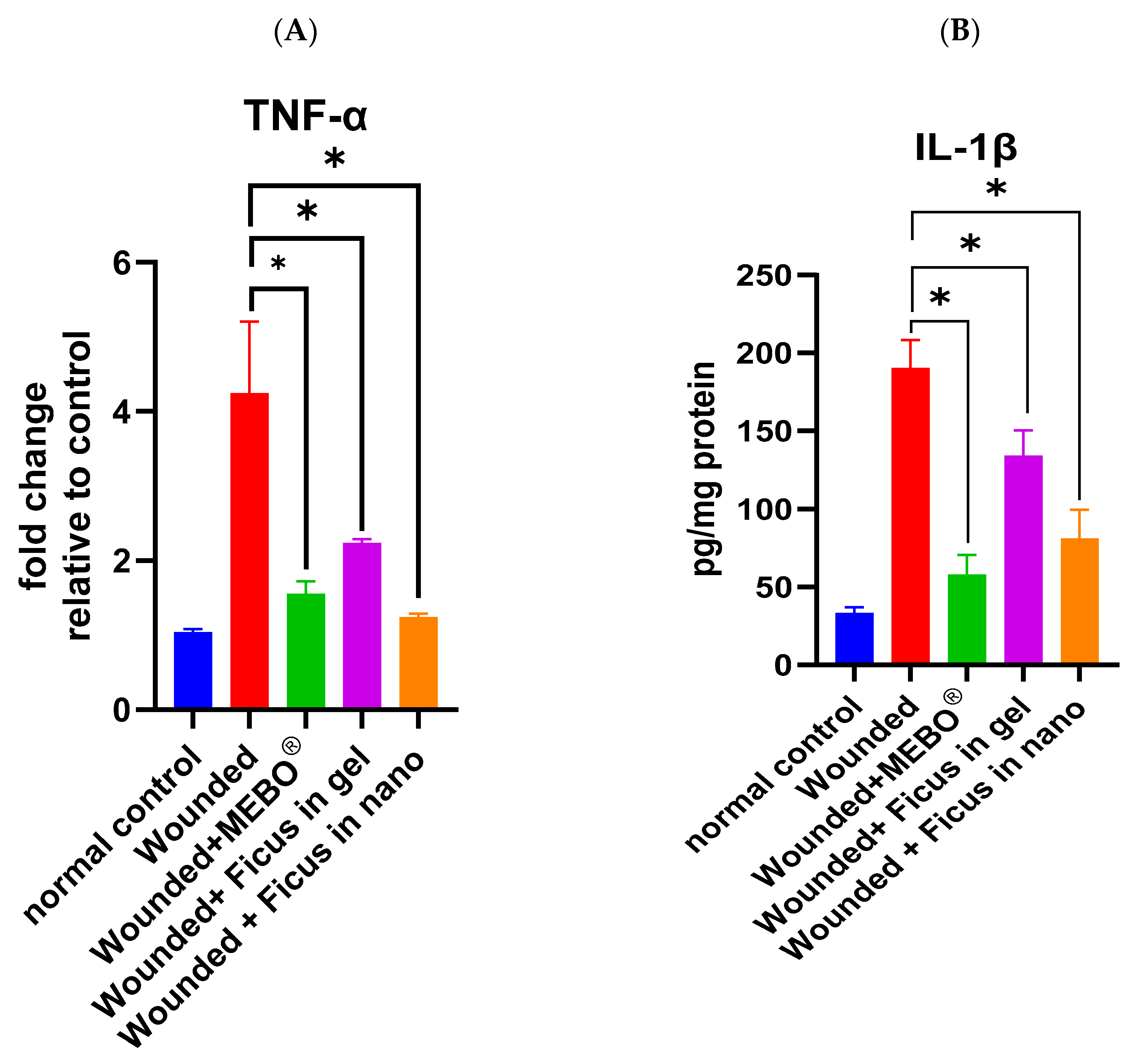
2.5. Effect of F. trijuja Extract Treatment on the mRNA Expression of TNF-α and the Protein Expression of IL-1β in Wounded Rats
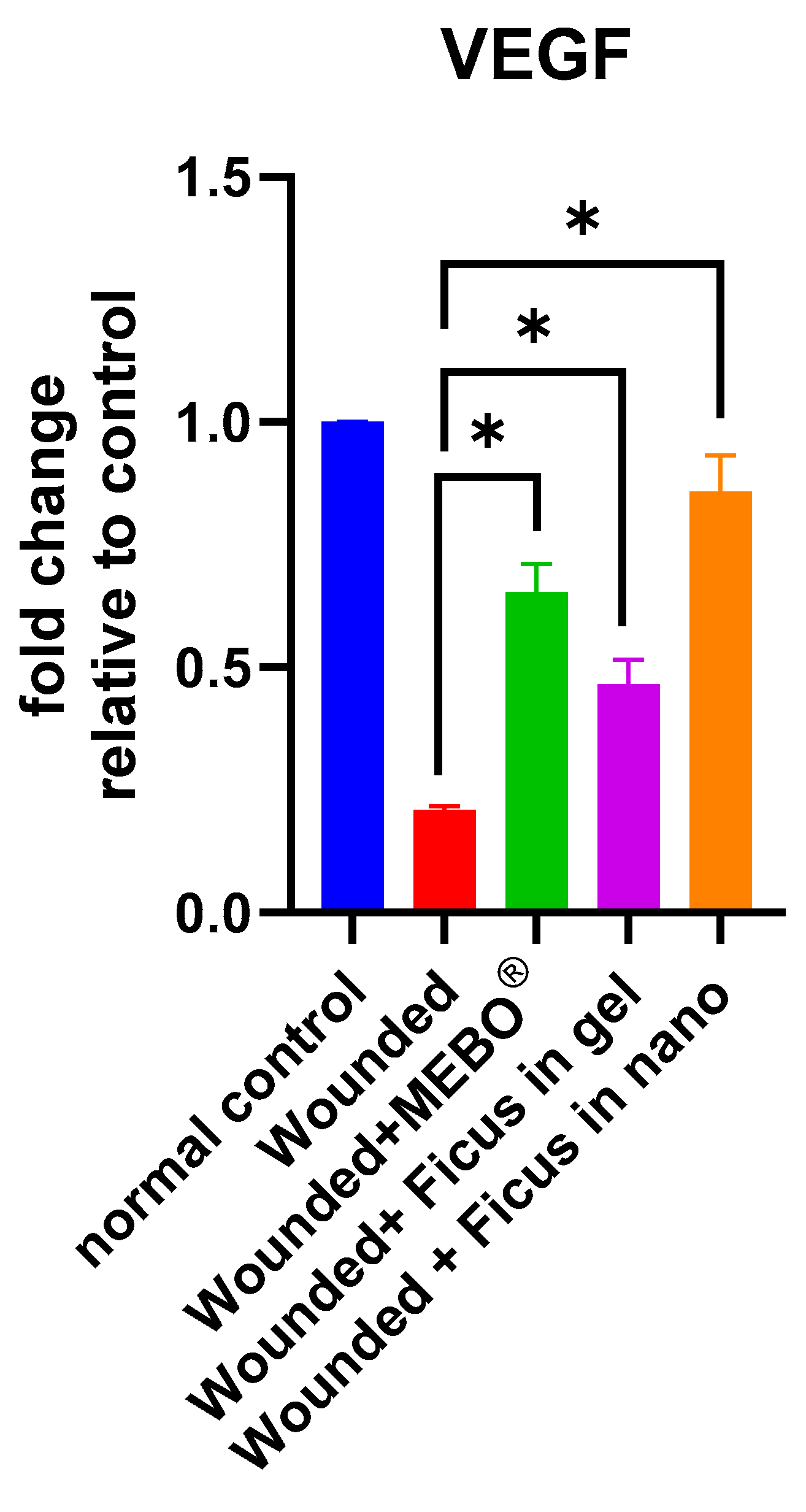
2.6. Effect of F. trijuja Extract Treatment on the mRNA Gene Expression Levels of VEGF in Wounded Rats
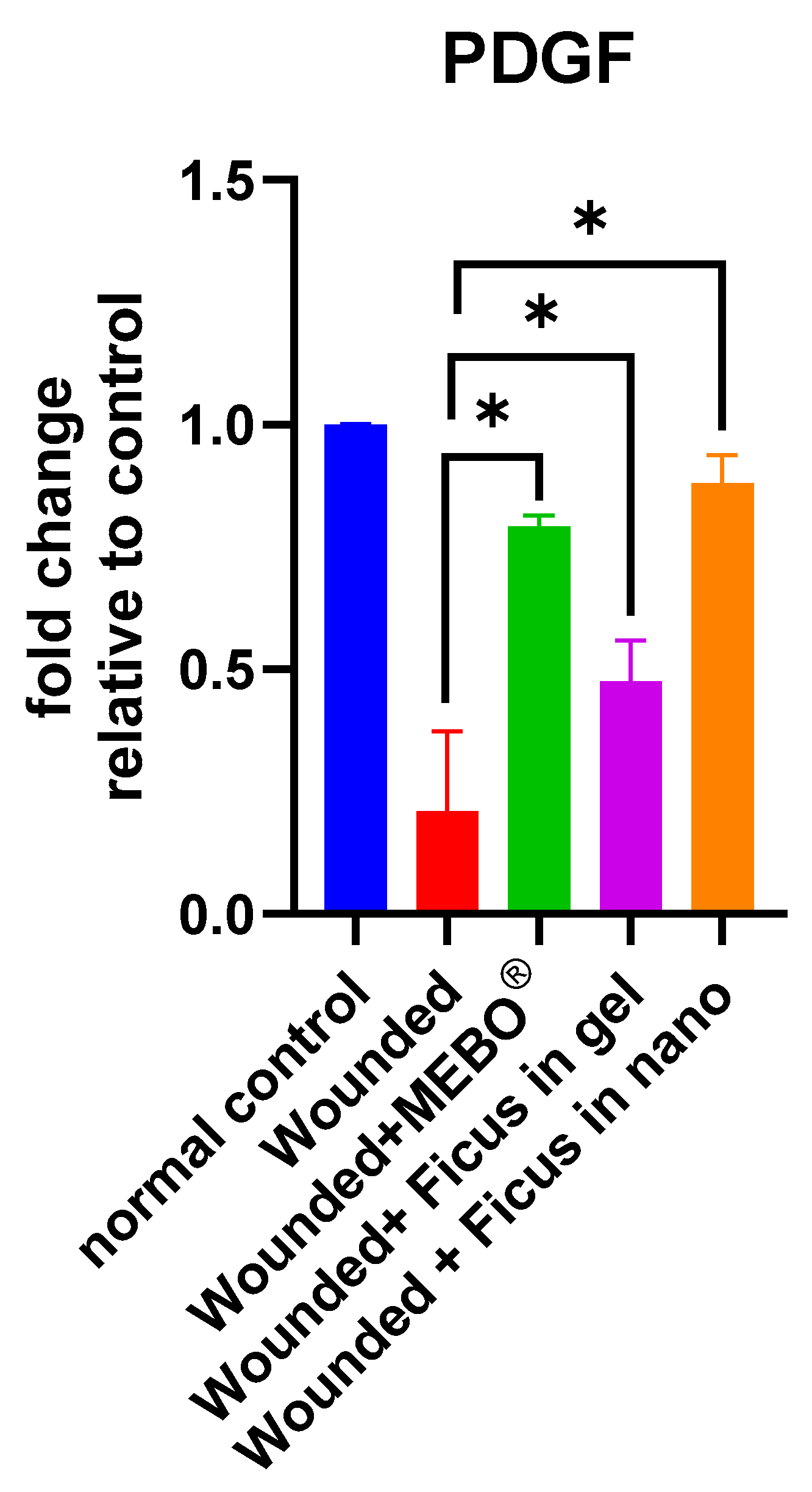
2.7. Effect of F. trijuja Extract Treatment on the mRNA Gene Expression Levels of PDGF in Wounded Rats
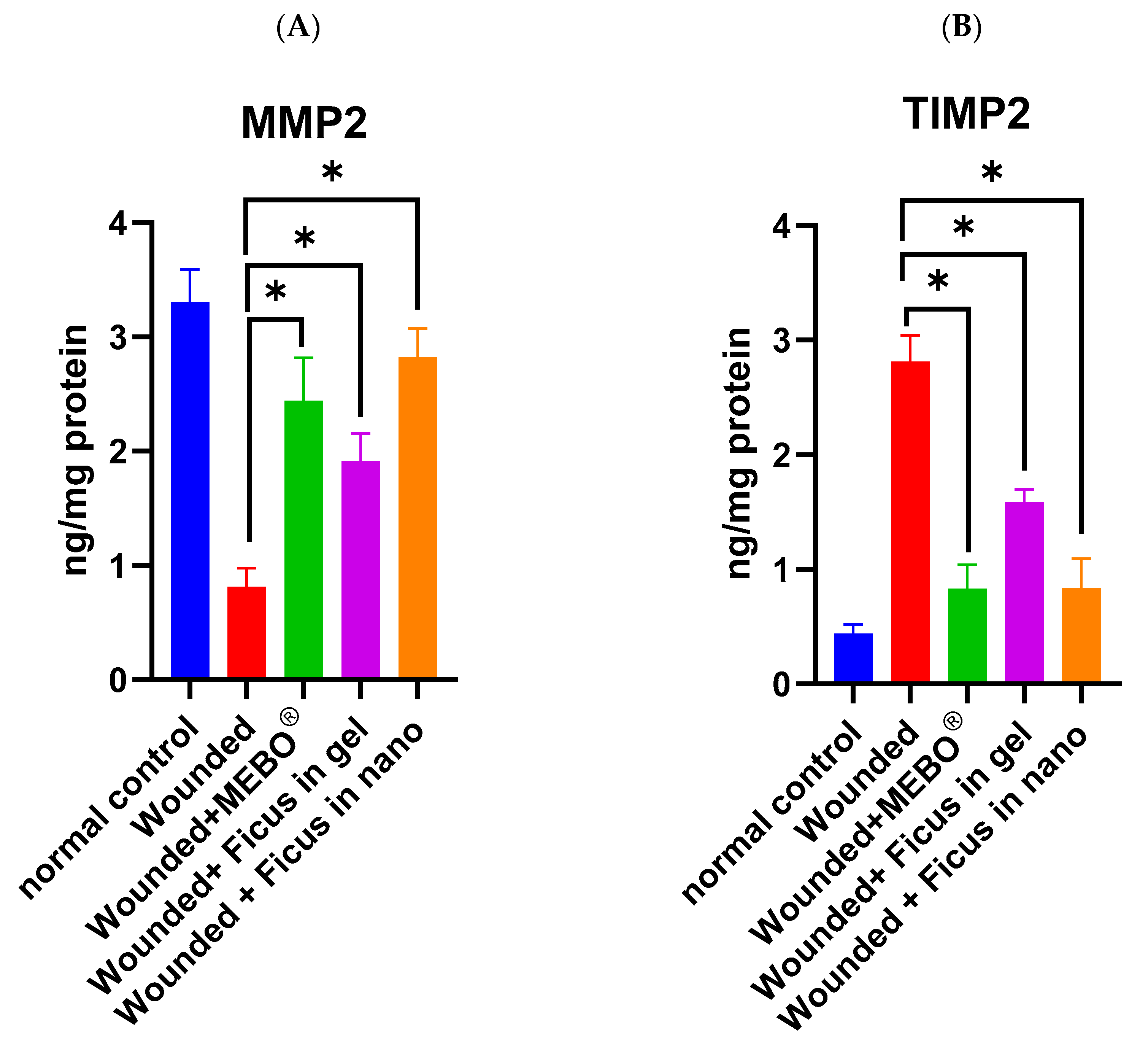
2.8. Effect of F. trijuja Extract Treatment on the Protein Expression Levels of MMP2 and TIMP2 in Wounded Rats
2.9. Immunohistochemical Staining of Wounded Tissue Using the CD86 Marker
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of the Plant Extract
3.3. LC-HRESIMS Metabolomic Profiling
3.4. Preparation of F. trijuja Extract Lipid Nanocapsules (LNCs)
3.5. Preparation of Topical F. trijuja Extract Hydrogel and F. trijuja Extract-Loaded LNC Hydrogels
3.6. Characterization of the Prepared LNCs
3.6.1. Measurement of Particle Size and the Polydispersity Index (PDI)
3.6.2. Determination of Zeta Potential
3.7. In Vivo Wound Healing Study
3.7.1. Animals
3.7.2. Surgery for the Induction of Skin Injury
3.7.3. Morphometric and Histopathological Evaluation
3.8. Preparation of Tissue Homogenate
3.9. Determination of Tissue Protein
3.10. Determination of TIMP-2 Protein Levels
3.11. Determination of MMP-2 Protein Levels
3.12. Determination of IL1β Protein Levels
3.13. RNA Extraction
3.14. Real-Time PCR
3.15. Immunohistochemical Staining
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Lipid nanocapsules | (LNCs) |
| Medium chain triglycerides | (MCT) |
| Polyethylene glycol | (PEG) |
| Tumor Necrosis Factor-alpha | (TNF-α) |
| Interleukin 1Beta | (IL1β) |
| Vascular Endothelial Growth Factor | (VEGF) |
| Platelet-Derived Endothelial Growth Factor | (PDGF) |
| Tissue Inhibitor of Metalloproteinases 2 | (TIMP2) |
| Matrix Metalloproteinase 2 | (MMP2) |
| Optical density | (OD) |
| Polydispersity index | (PDI) |
| Horseradish Peroxidase | (HRP) |
| Cycle threshold | (Ct) |
| Phosphate-buffered saline | (PBS) |
| Relative quantitation | (RQ) |
| Standard deviation | (SD) |
| Analysis of variance | (ANOVA) |
References
- Somashekhar, M.; Nayeem, N.; Sonnad, B. A review on family Moraceae (Mulberry) with a focus on Artocarpus species. World J. Pharm. Pharm. Sci. 2013, 2, 2614–2626. [Google Scholar]
- Sermboonpaisarn, T.; Sawasdee, P. Potent and selective butyrylcholinesterase inhibitors from Ficus foveolata. Fitoterapia 2012, 83, 780–784. [Google Scholar] [CrossRef]
- Cheng, J.X.; Zhang, B.D.; Zhu, W.F.; Zhang, C.F.; Qin, Y.M.; Abe, M.; Akihisa, T.; Liu, W.Y.; Feng, F.; Zhang, J. Traditional uses, phytochemistry, and pharmacology of Ficus hispida L.f.: A review. J. Ethnopharmacol. 2020, 248, 112204. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.F.; Lei, C.; Yu, B.W.; Wang, H.Y.; Hou, A.J. New Alkaloids and alpha-Glucosidase Inhibitory Flavonoids from Ficus hispida. Chem. Biodivers. 2016, 13, 445–450. [Google Scholar] [CrossRef]
- Mawa, S.; Husain, K.; Jantan, I. Ficus carica L. (Moraceae): Phytochemistry, Traditional Uses and Biological Activities. Evid. Based Complement. Alternat Med. 2013, 2013, 974256. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Li, S.Y.; Li, W.J.; Guo, J.M.; Tian, K.; Hu, Q.F.; Huang, X.Z. Phenolic glycosides from Ficus tikoua and their cytotoxic activities. Carbohydr. Res. 2013, 382, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, W.; Ji, Z. New antifungal pyranoisoflavone from Ficus tikoua Bur. Int. J. Mol. Sci. 2012, 13, 7375–7382. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.K.; Nguyen, X.C.; Nguyen, X.N.; Vu, K.T.; Ninh, K.B.; Chau, V.M.; Bui, H.T.; Truong, N.H.; Lee, S.H.; Jang, H.D.; et al. Antioxidant activity of a new C-glycosylflavone from the leaves of Ficus microcarpa. Bioorg. Med. Chem. Lett. 2011, 21, 633–637. [Google Scholar] [PubMed]
- Asem, B.D.; Laitonjam, W.S. Isolation and antimicrobial studies of the compounds isolated from the stem bark of Ficus hispida Linn. Asian J. Chem. 2008, 20, 6027–6032. [Google Scholar]
- Umeh, V.N.; Ilodigwe, E.E.; Ajaghaku, D.L.; Erhirhie, E.O.; Moke, G.E.; Akah, P.A. Wound-healing Activity of the Aqueous Leaf Extract and Fractions of Ficus exasperata (Moraceae) and its Safety Evaluation on Albino Rats. J. Tradit. Complement. Med. 2014, 4, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.K.; Paliwal, S.K. Wound-healing activity of ethanolic and aqueous extracts of Ficus benghalensis. J. Adv. Pharm. Technol. Res. 2011, 2, 110–114. [Google Scholar] [CrossRef]
- Imran, M.; Sharma, J.N.; Kamal, M.; Asif, M. Standardization and Wound-Healing Activity of Petroleum, Ethanolic and Aqueous Extracts of Ficus benghalensis Leaves. Pharm. Chem. J. 2021, 54, 1057–1062. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Anti-inflammatory, wound healing and in-vivo antioxidant properties of the leaves of Ficus amplissima Smith. J. Ethnopharmacol. 2013, 145, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dimri, A.; Negi, A.; Semwal, A.; Singh, N. Wound-healing activity of ethanolic and aqueous extracts of Ficus sarmentosa bark. Int. J. Pharm. Res. Technol. 2019, 9, 38–42. [Google Scholar]
- Feng, J.; Niu, Y.; Zhang, Y.; Zuo, H.; Wang, S.; Liu, X. Ficus carica extract impregnated amphiphilic polymer scaffold for diabetic wound tissue regenerations. Artif. Cells Nanomed. Biotechnol. 2021, 49, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Murti, K.; Kumar, U.; Panchal, M. Healing promoting potentials of roots of Ficus benghalensis L. in albino rats. Asian Pac. J. Trop. Med. 2011, 4, 921–924. [Google Scholar] [CrossRef]
- Aly, S.H.; El-Hassab, M.A.; Elhady, S.S.; Gad, H.A. Comparative Metabolic Study of Tamarindus indica L.’s Various Organs Based on GC/MS Analysis, In Silico and In Vitro Anti-Inflammatory and Wound Healing Activities. Plants 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elissawy, A.M.; Mahmoud, A.M.; El-Tokhy, F.S.E.; Mageed, S.S.A.; Almahli, H.; Al-Rashood, S.T.; Binjubair, F.A.; Hassab, M.A.E.; Eldehna, W.M. Synergistic Effect of Sophora japonica and Glycyrrhiza glabra Flavonoid-Rich Fractions on Wound Healing: In Vivo and Molecular Docking Studies. Molecules 2023, 28, 2994. [Google Scholar] [CrossRef] [PubMed]
- Shady, N.H.; Mostafa, N.M.; Fayez, S.; Abdel-Rahman, I.M.; Maher, S.A.; Zayed, A.; Saber, E.A.; Khowdiary, M.M.; Elrehany, M.A.; Alzubaidi, M.A.; et al. Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies. Antioxidants 2022, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Hosgood, G. Stages of wound healing and their clinical relevance. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 667–685. [Google Scholar] [CrossRef] [PubMed]
- El-Tokhy, F.S.; Abdel-Mottaleb, M.M.A.; Abdel Mageed, S.S.; Mahmoud, A.M.A.; El-Ghany, E.A.; Geneidi, A.S. Boosting the In Vivo Transdermal Bioavailability of Asenapine Maleate Using Novel Lavender Oil-Based Lipid Nanocapsules for Management of Schizophrenia. Pharmaceutics 2023, 15, 490. [Google Scholar] [CrossRef] [PubMed]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts-A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor. Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Bitto, A.; Minutoli, L.; Galeano, M.R.; Altavilla, D.; Polito, F.; Fiumara, T.; Calo, M.; Lo Cascio, P.; Zentilin, L.; Giacca, M.; et al. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin. Sci. 2008, 114, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Majack, R.A.; Majesky, M.W.; Goodman, L.V. Role of PDGF-A expression in the control of vascular smooth muscle cell growth by transforming growth factor-beta. J. Cell Biol. 1990, 111, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, S.Z.; Wang, Y.; Tian, Y.J.; Liu, M.H. The roles of MMP-2/TIMP-2 in extracellular matrix remodelling in the hearts of STZ-induced diabetic rats. Acta Cardiol. 2007, 62, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor. Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Saafan, H.A.; Al-Tawashi, A.S.; Kasem, M.H.; Alaa, A.M.; Eltobgy, M.M.; Moubarak, A.S.; Gharib, M.M.; Awwad, M.A.; Omar, H.M.; et al. Interleukin-17 haplotyping predicts hepatocellular carcinoma in sofosbuvir, pegylated interferon-alpha-2a & ribavirin treated chronic hepatitis C patients. Virus Res. 2021, 292, 198226. [Google Scholar]
- Doersch, K.M.; DelloStritto, D.J.; Newell-Rogers, M.K. The contribution of interleukin-2 to effective wound healing. Exp. Biol. Med. 2017, 242, 384–396. [Google Scholar] [CrossRef]
- Abdallah, S.H.; Mostafa, N.M.; Mohamed, M.; Nada, A.S.; Singab, A.N.B. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat. Prod. Res. 2022, 36, 5619–5625. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, I.; Jeong, Y.J.; Kim, Y.; Chung, J.J.; Kim, S.W. Comparative Analysis of Antibacterial and Wound Healing Activities of Chitosan and Povidone-Iodine-Based Hydrogels. Ann. Plast. Surg. 2024, 92, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Neeru, J.; Yadava, R. Flavonoids of the leaves of Ficus infectoria. Fitoterapia 1994, 65, 169–175. [Google Scholar]
- Li, C.; Yu, M.; Li, S.; Yang, X.; Qiao, B.; Shi, S.; Zhao, C.; Fu, Y. Valorization of Fig (Ficus carica L.) Waste Leaves: HPLC-QTOF-MS/MS-DPPH System for Online Screening and Identification of Antioxidant Compounds. Plants 2021, 10, 2532. [Google Scholar] [CrossRef]
- Kundap, U.; Jaiswal, Y.; Sarawade, R.; Williams, L.; Shaikh, M.F. Effect of Pelargonidin isolated from Ficus benghalensis L. on phenotypic changes in zebrafish (Danio rerio) embryos. Saudi Pharm. J. 2017, 25, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-c.; Kuo, Y.-H. A monoterpenoid and two simple phenols from heartwood of Ficus microcarpa. Phytochemistry 1998, 49, 2417–2419. [Google Scholar] [CrossRef]
- Bai, M.; Cai, Y.; Wu, S.Y.; Song, X.P.; Chen, G.Y.; Zheng, C.J.; Han, C.R. A new norisoprenoid from the leaves of Ficus pumila. Nat. Prod. Res. 2019, 33, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; Zhang, X.Q.; Wang, Y.; Zhang, Q.W.; Chen, J.X.; Ye, W.C. Two new phenolic compounds from the roots of Ficus hirta. Nat. Prod. Res. 2010, 24, 621–625. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 493–505. [Google Scholar] [CrossRef]
- Sheu, Y.-W.; Chiang, L.-C.; Chen, I.-S.; Chen, Y.-C.; Tsai, I.-L. Cytotoxic flavonoids and new chromenes from Ficus formosana f. formosana. Planta Med. 2005, 71, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Marwat, S.K.; Khan, M.A.; Khan, M.A.; Akbari, A.H.; Ahmad, M.; Zafar, M.; Ahmad, F. Medicinal and pharmacological potentiality of the plant At-Tîn-Common fig (Ficus carica L.). Asian J. Chem. 2011, 23, 1. [Google Scholar]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Somwong, P.; Suttisri, R.; Buakeaw, A. New sesquiterpenes and phenolic compound from Ficus foveolata. Fitoterapia 2013, 85, 1–7. [Google Scholar] [CrossRef]
- Van Kiem, P.; Cuong, N.X.; Nhiem, N.X.; Hang, D.T.; Nam, N.H.; Ban, N.K.; Minh, C.V.; Bing, Z.; Jang, H.D.; Kim, Y.H. Chemical constituents and antioxidant activity of Ficus callosa. Nat. Prod. Commun. 2011, 6, 159–162. [Google Scholar] [PubMed]
- Chiang, Y.M.; Kuo, Y.H. Novel triterpenoids from the aerial roots of Ficus microcarpa. J. Org. Chem. 2002, 67, 7656–7661. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, H.Y. Two novel triterpenes from the leaves of Ficus microcarpa. Helv. Chim. Acta 2004, 87, 1071–1076. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chiang, Y.M. Six new ursane- and oleanane-type triterpenes from the aerial roots of Ficus microcarpa. Chem. Pharm. Bull. 2000, 48, 593–596. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Chaiang, Y.-M. Five new taraxastane-type triterpenes from the aerial roots of Ficus microcarpa. Chem. Pharm. Bull. 1999, 47, 498–500. [Google Scholar] [CrossRef]
- Kitajima, J.; Arai, M.; Tanaka, Y. Triterpenoid constituents of Ficus thunbergii. Chem. Pharm. Bull. 1994, 42, 608–610. [Google Scholar] [CrossRef]
- Ouyang, M.A.; Kuo, Y.H. Water-soluble constituents from aerial roots of Ficus microcarpa. J. Asian Nat. Prod. Res. 2006, 8, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.P.; Luan, J.Y.; Lu, L.N.; Wu, W.J.; Ji, Z.Q. A new benzofuran glucoside from Ficus tikoua Bur. Int. J. Mol. Sci. 2011, 12, 4946–4952. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.M.; Zheng, C.J.; Han, C.R.; Chen, G.Y.; Dai, C.Y.; Song, X.P.; Zhang, J.C.; Chen, W.H. Lactones from Ficus auriculata and their effects on the proliferation function of primary mouse osteoblasts in vitro. Bioorg. Med. Chem. Lett. 2014, 24, 3952–3955. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Wathoni, N.; Suhandi, C.; Elamin, K.M.; Lesmana, R.; Hasan, N.; Mohammed, A.F.A.; El-Rayyes, A.; Wilar, G. Advancements and Challenges of Nanostructured Lipid Carriers for Wound Healing Applications. Int. J. Nanomed. 2024, 19, 8091–8113. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Sedaghat, M.; Hoseini, A.; Mohammadi, N.; Bodaghi, M. Combinational System of Lipid-Based Nanocarriers and Biodegradable Polymers for Wound Healing: An Updated Review. J. Funct. Biomater. 2023, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound Healing Study of Eucalyptus Essential Oil Containing Nanoemulsion in Rat Model. J. Oleo Sci. 2018, 67, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Woollard, A.C.; Tatham, K.C.; Barker, S. The influence of essential oils on the process of wound healing: A review of the current evidence. J. Wound Care 2007, 16, 255–257. [Google Scholar] [CrossRef] [PubMed]
- El-Tokhy, F.S.; Abdel-Mottaleb, M.M.A.; El-Ghany, E.A.; Geneidi, A.S. Design of long acting invasomal nanovesicles for improved transdermal permeation and bioavailability of asenapine maleate for the chronic treatment of schizophrenia. Int. J. Pharm. 2021, 608, 121080. [Google Scholar] [CrossRef] [PubMed]
- Sano, C.; Shimizu, T.; Tomioka, H. Effects of secretory leukocyte protease inhibitor on the tumor necrosis factor-alpha production and NF-kappaB activation of lipopolysaccharide-stimulated macrophages. Cytokine 2003, 21, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Rajkumar, V.S.; Shiwen, X.; Bostrom, M.; Leoni, P.; Muddle, J.; Ivarsson, M.; Gerdin, B.; Denton, C.P.; Bou-Gharios, G.; Black, C.M.; et al. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 2006, 169, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Sayed, A.M.; El-Sherief, H.A.M.; Rateb, M.E.; Akil, L.; Khadra, I.; Majrashi, T.A.; Al-Rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; et al. Metabolomic profile, anti-trypanosomal potential and molecular docking studies of Thunbergia grandifolia. J. Enzyme Inhib. Med. Chem. 2023, 38, 2199950. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.; Neumann, D.; Lamprecht, A. In vitro drug release mechanism from lipid nanocapsules (LNC). Int. J. Pharm. 2010, 390, 208–213. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef]
- Esimone, C.; Ibezim, E.; Chah, K. The wound healing effect of herbal ointments formulated with Napoleona imperialis. J. Pharm. Allied Sci. 2006, 3, 294–299. [Google Scholar] [CrossRef]
- Bonjoch, N.P.; Tamayo, P.R. Protein content quantification by Bradford method. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2001; pp. 283–295. [Google Scholar]



| No. | RT (min.) | m/z | Ionization Mode | Accurate Mass | Molecular Formula | Metabolite Name | Chemical Class | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.7678892 | 379.08275 | N | 380.09002 | C21H16O7 | Gancaonin F; 9-Hydroxy | Prenylcoumarin | [39] |
| 2 | 1.7993858 | 193.07054 | P | 192.06326 | C7H12O6 | Quinic acid | Cyclohexanecarboxylic acid | [40] |
| 3 | 1.9179893 | 335.09294 | N | 336.10022 | C20H16O5 | Carpachromene | Flavonoids | [41] |
| 4 | 4.674109778 | 166.08615 | P | 165.07887 | C9H11NO2 | Phenylalanine | Amino acid | [42] |
| 5 | 5.007641778 | 169.01432 | N | 170.0216 | C7H6O5 | Gallic acid | Phenolic acid | [43] |
| 6 | 8.1189884 | 327.10715 | P | 326.09988 | C15H18O8 | Foveospirolide | Sesquiterpenoid | [44] |
| 7 | 8.3530242 | 389.1439 | P | 388.13662 | C17H24O10 | Ficuscarpanoside B | Phenylpropanoid | [51] |
| 8 | 8.6699946 | 225.1484 | P | 224.14112 | C13H20O3 | 9,10-Dihydroxy-4,7- megastigmadien-3- one | Megastigmane | [38] |
| 9 | 10.403668 | 507.27947 | P | 506.2722 | C24H42O11 | Ficalloside | Megastigmane glycoside | [45] |
| 10 | 10.471478 | 539.21185 | P | 538.20458 | C26H34O12 | 3,3′,4,4′,5,5′,7-Heptahydroxyflavan; 3′,4′,5,5′,7-Penta-Me ether, 3-O-α-L-rhamnopyranoside | Flavonoid | [9] |
| 11 | 11.035368 | 595.16502 | P | 594.15774 | C27H30O15 | Sorbifolin-6-O-[α-L-arabinopyranosyl-(1→2)-β-D-glucopyranoside] | Flavonoid | [34] |
| 12 | 11.035501 | 611.15993 | P | 610.15265 | C27H30O16 | Rutin | Flavonoid | [35] |
| 13 | 11.093782 | 511.18053 | P | 510.17326 | C24H30O12 | 5, 7, 3 Trimethoxy leucodelphinidin 3-O-α-L-Rhamnoside | Leucoanthocyanidin | [36] |
| 14 | 11.38093 | 197.11712 | P | 196.10985 | C11H16O3 | Ficusic acid | Monoterpenoid | [37] |
| 15 | 12.467357 | 399.12809 | P | 398.12082 | C18H22O10 | 6-Carboxyethyl-7- methoxyl-5-hydroxy-benzofuran 5-O-β-D-glucopyranoside | Benzofuran Glucoside | [52] |
| 16 | 19.130958 | 469.33085 | P | 468.32357 | C30H44O4 | Foveolide B | Sesquiterpenoid dimer | [44] |
| 17 | 20.171168 | 473.36217 | P | 472.35489 | C30H48O4 | 29,30-Dinor-3β-acetoxy-18,19-dioxo-18,19-secolupane | Triterpenoid | [46] |
| 18 | 20.591852 | 457.36732 | P | 456.36004 | C30H48O3 | 19,20-secoursane-3,19,20-trione | Triterpenoid | [47] |
| 19 | 21.175209 | 457.36733 | P | 456.36005 | C30H48O3 | 3α-Hydroxyisohop-22(29)-en-24-oic acid | Triterpenoid | [50] |
| 20 | 23.35141 | 279.15897 | P | 278.15169 | C16H22O4 | (+)-(R)-de-O-Methyllasiodiplodin | Benzoic acid lactones | [53] |
| 21 | 25.980935 | 459.38296 | P | 458.37569 | C30H50O3 | 3β-hydroxy-11α-hydroperoxy-12-ursene | Triterpenoid | [48] |
| 22 | 26.121905 | 427.39316 | P | 426.38589 | C30H50O | Rhoiptelenol | Triterpenoid | [50] |
| 23 | 27.17658 | 443.3881 | P | 442.38083 | C30H50O2 | 20α,21α-Epoxytaraxastan-3β-ol | Triterpenoid | [49] |
| 24 | 27.364269 | 441.37248 | P | 440.3652 | C30H48O2 | 29(20→19) Abeolupane-3,20-dione | Triterpenoid | [47] |
| Control Positive | MEBO® | F. trijuja | F. trijuja (LNCs) | |
|---|---|---|---|---|
| Day 0 | 6.18 ± 0.42 | 34.58 ± 1.66 * | 29.12 ± 1.70 * | 25.89 ± 2.29 * |
| Day 7 | 12.35 ± 0.84 | 69.16 ± 3.31 * | 58.25 ± 3.41 * | 51.78 ± 4.58 * |
| Day 14 | 44.90 ± 2.68 | 89.11 ± 1.23 * | 71.44 ± 2.59 * | 94.77 ± 0.65 * |
| Day 21 | 63.22 ± 2.39 | 95.77 ± 0.46 * | 96.73 ± 0.59 * | 98.28 ± 0.06 * |
| Constituents | Percentage (%w/w) |
|---|---|
| Herbal extract | 2% |
| Propylene glycol | 5% |
| Eucalyptus oil | 15% |
| Kolliphor® HS15 | 15% |
| Phospholipon®90G | 1% |
| Sodium Chloride | 1% |
| Deionized water | 61% |
| Gene Symbol | Forward | Reverse | Gene Bank Accession |
|---|---|---|---|
| PDGF | TGAGTGGCGAGCACTGTC | TCTGGATTTCCCAGCTGT | AB003156.1 |
| VEGF | GGCTCTGAAACCATGAACTTTCT | GCAGTAGCTGCGCTGGTAGAC | NM_001287114.1 |
| TNFα | TGCCTCAGCCTCTTCTCATT | GAGCCCATTTGGGAACTTCT | NM_012675.3 |
| GAPDH | GTGCCAACCCCAAACGTATC | CTGCTTTCACAGCCTCCTTGA | NM_023964.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashad, I.M.; Aly, S.H.; Saleh, D.O.; Abo El-Nasr, N.M.E.; Shabana, M.E.; El-Tokhy, F.S.; El-Nashar, H.A.S.; Abdelmohsen, U.R.; Mostafa, N.M.; Mostafa, A.M. Mechanistic Wound Healing of Ficus trijuja Leaf Extract and Its Lipid Nanocapsule Supported by Metabolomic Profiling and In Vivo Studies. Int. J. Mol. Sci. 2025, 26, 928. https://doi.org/10.3390/ijms26030928
Hashad IM, Aly SH, Saleh DO, Abo El-Nasr NME, Shabana ME, El-Tokhy FS, El-Nashar HAS, Abdelmohsen UR, Mostafa NM, Mostafa AM. Mechanistic Wound Healing of Ficus trijuja Leaf Extract and Its Lipid Nanocapsule Supported by Metabolomic Profiling and In Vivo Studies. International Journal of Molecular Sciences. 2025; 26(3):928. https://doi.org/10.3390/ijms26030928
Chicago/Turabian StyleHashad, Ingy M., Shaza H. Aly, Dalia O. Saleh, Nesma M. E. Abo El-Nasr, Marwa E. Shabana, Fatma Sa’eed El-Tokhy, Heba A. S. El-Nashar, Usama R. Abdelmohsen, Nada M. Mostafa, and Ahmed M. Mostafa. 2025. "Mechanistic Wound Healing of Ficus trijuja Leaf Extract and Its Lipid Nanocapsule Supported by Metabolomic Profiling and In Vivo Studies" International Journal of Molecular Sciences 26, no. 3: 928. https://doi.org/10.3390/ijms26030928
APA StyleHashad, I. M., Aly, S. H., Saleh, D. O., Abo El-Nasr, N. M. E., Shabana, M. E., El-Tokhy, F. S., El-Nashar, H. A. S., Abdelmohsen, U. R., Mostafa, N. M., & Mostafa, A. M. (2025). Mechanistic Wound Healing of Ficus trijuja Leaf Extract and Its Lipid Nanocapsule Supported by Metabolomic Profiling and In Vivo Studies. International Journal of Molecular Sciences, 26(3), 928. https://doi.org/10.3390/ijms26030928









