Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery
Abstract
1. Introduction
2. Results
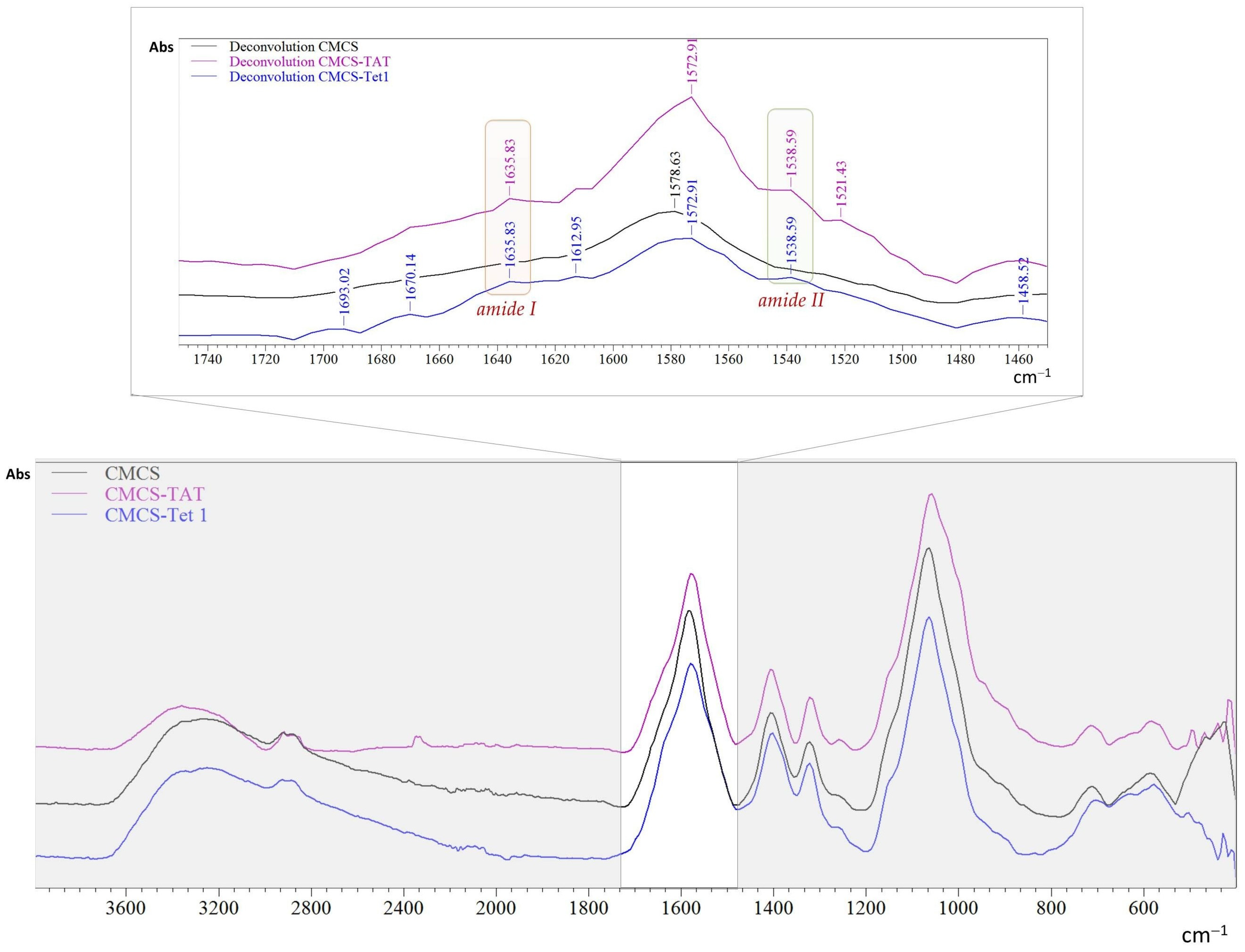
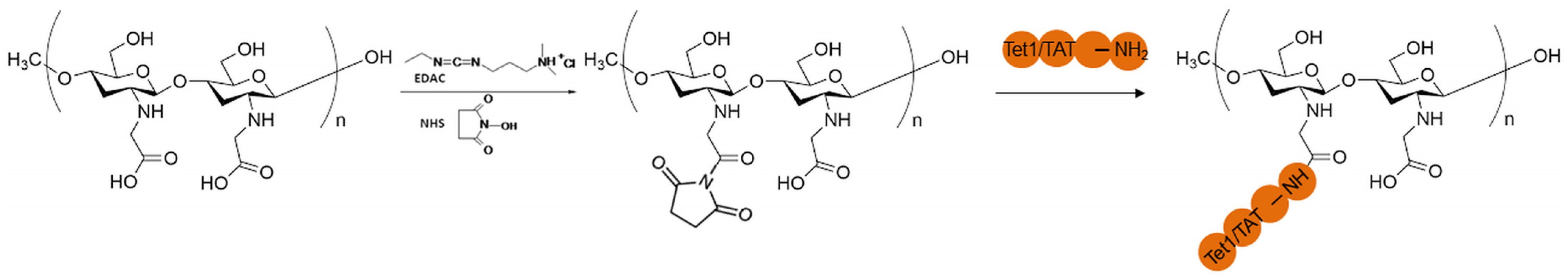
2.1. Structural Characteristics
2.2. Characterization of Peptide-Functionalized Liposomes
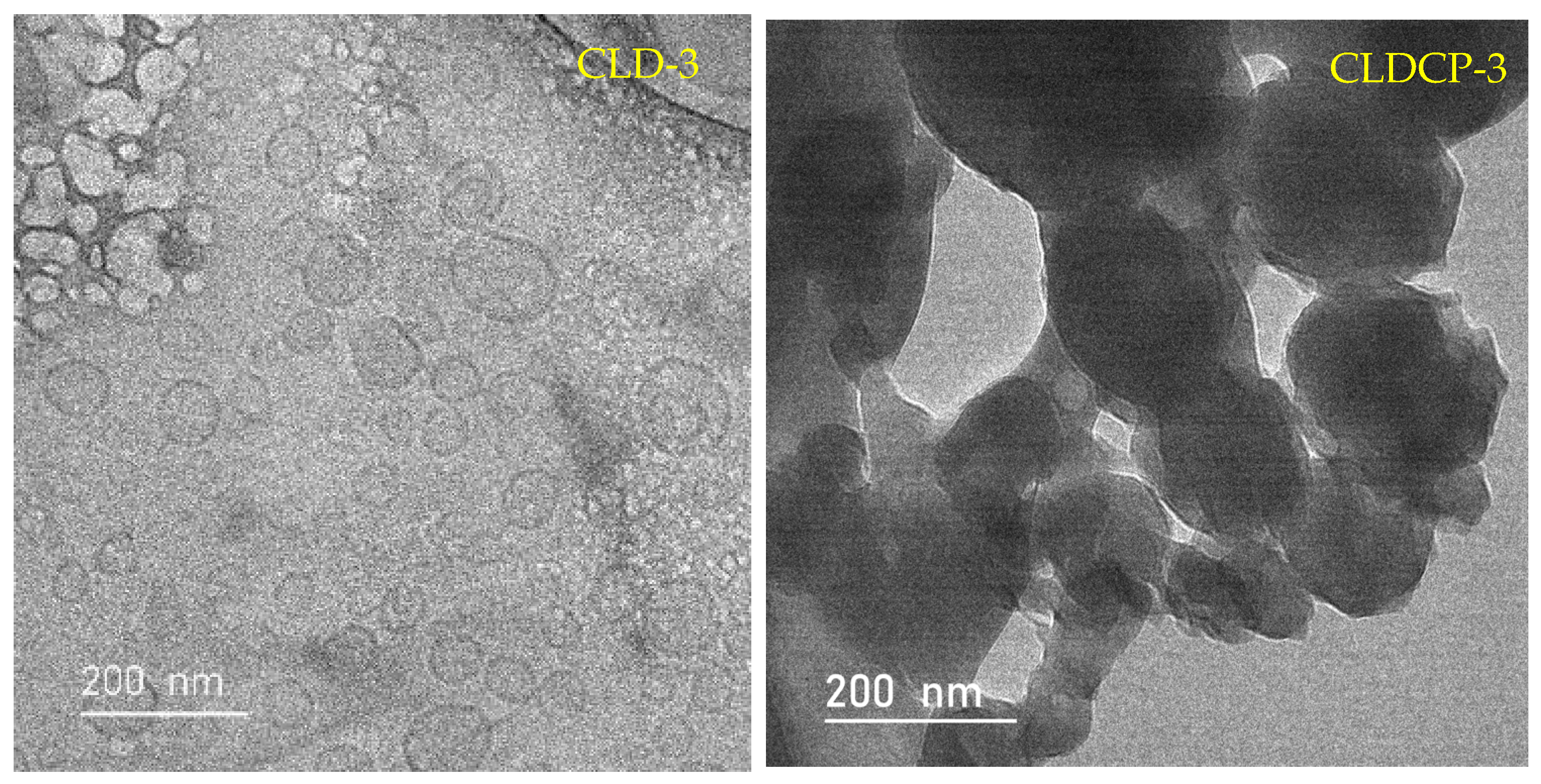
2.2.1. The Morphology of Liposomes
2.2.2. The Encapsulation Efficiency
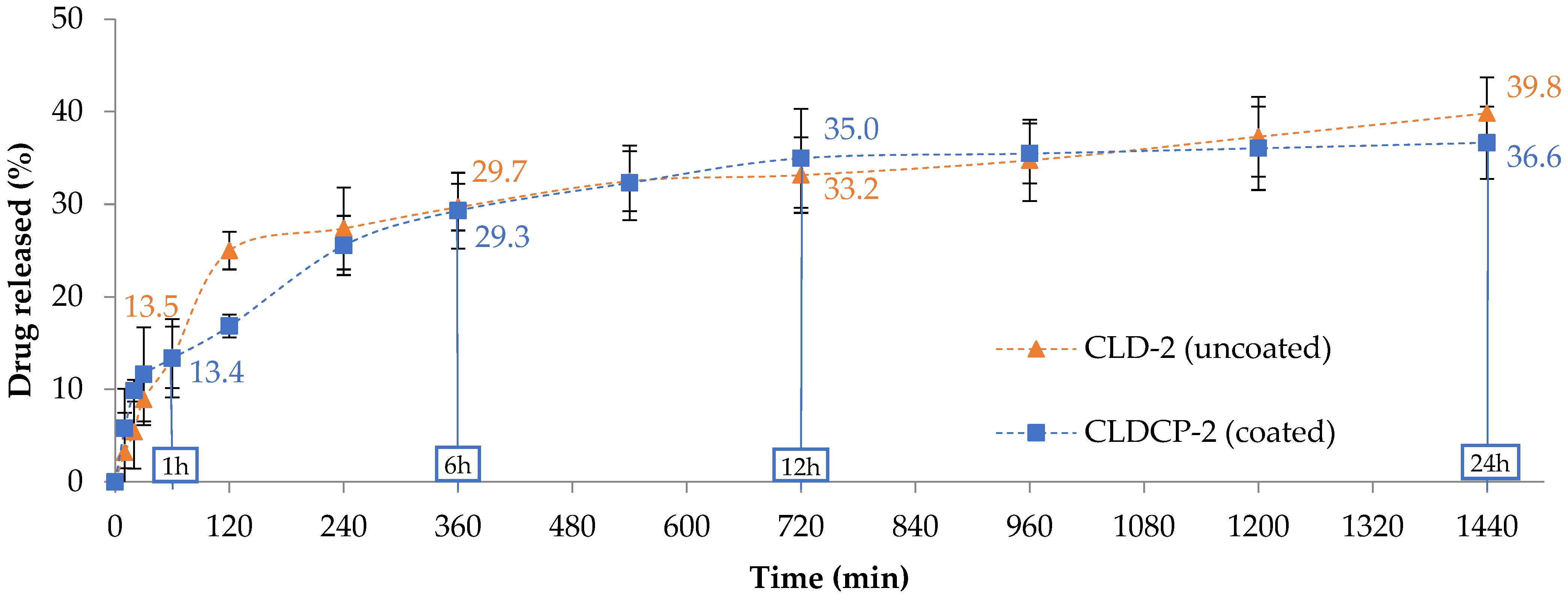
2.2.3. The Drug Release Tests
2.2.4. The Stability Tests
2.2.5. The Hemolytic Potential
2.3. Assessment of the In Vitro Cytotoxicity
2.4. The Half-Maximal Inhibitory Concentration (IC50) Values
2.5. Morphology Assay
3. Materials and Methods
3.1. Materials
3.2. Preparation Methods
3.2.1. Functionalization of CMCS with the Peptides
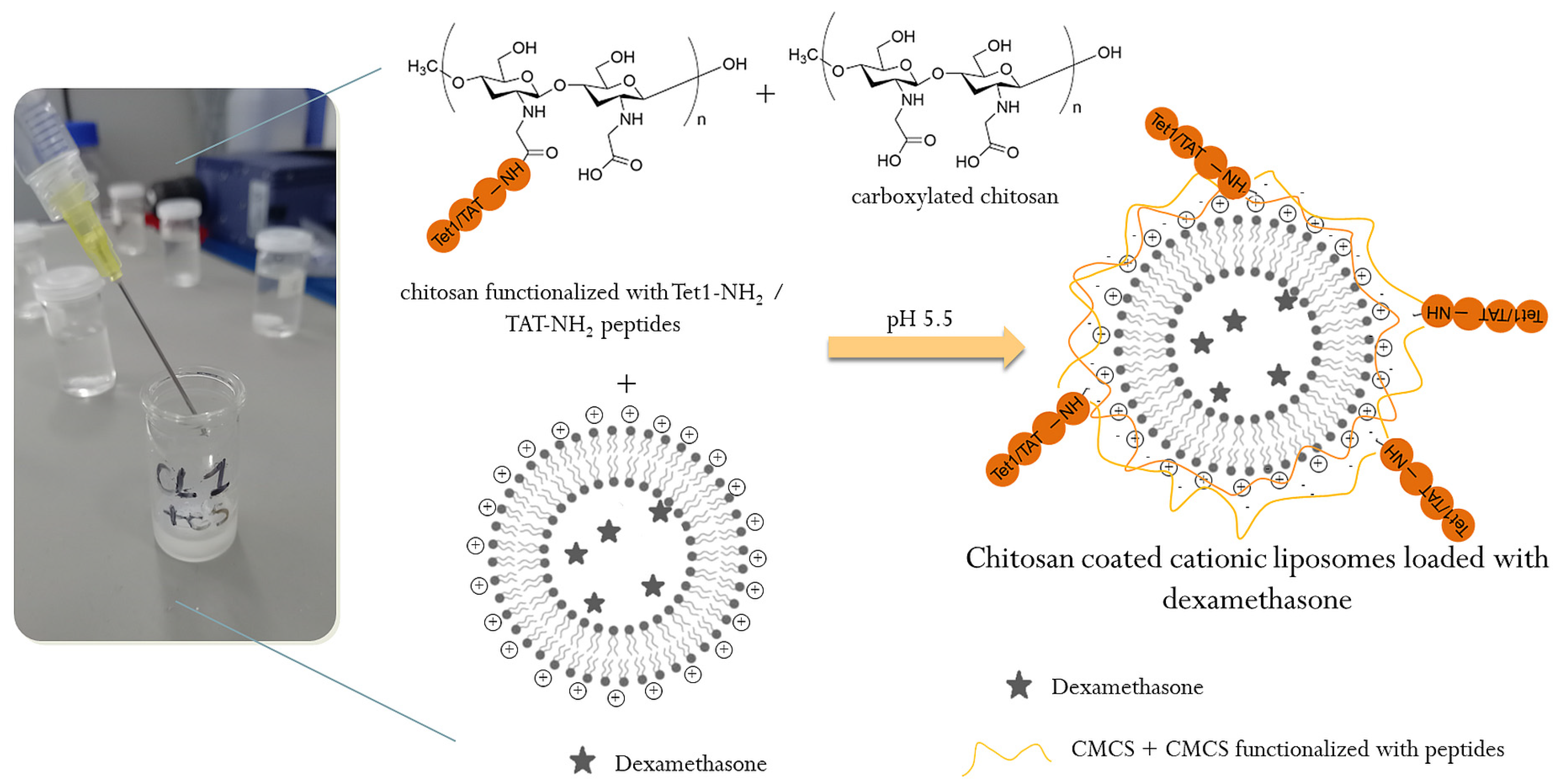
3.2.2. Preparation of Cationic Liposomes
3.3. Characterization of Peptide-Functionalized CMCS and Peptide-Functionalized Liposomes
3.3.1. Characterization of Peptide-Functionalized CMCS
3.3.2. Characterization of Peptide-Functionalized Liposomes
3.3.3. Assessment of the in Vitro Cytotoxicity
3.3.4. The Half-Maximal Inhibitory Concentration (IC50) Values
3.3.5. Cell Morphology Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barone, A.; Zimbo, A.M.; d’Avanzo, N.; Tolomeo, A.M.; Ruga, S.; Cardamone, A.; Celia, C.; Scalise, M.; Torella, D.; La Deda, M.; et al. Thermoresponsive M1 macrophage-derived hybrid nanovesicles for improved in vivo tumor targeting. Drug Deliv. Transl. Res. 2023, 13, 3154–3168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, X.; Li, B.; Yan, C.; Wu, C.; He, L.; Cao, J.; Lu, F.; Chen, H.; Li, W. Potent cancer therapy by liposome microstructure tailoring with active-to-passive targeting and shell-to-core thermosensitive features. Mater. Today Bio 2024, 26, 101035. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- d’Avanzo, N.; Paolino, D.; Barone, A.; Ciriolo, L.; Mancuso, A.; Christiano, M.C.; Tolomeo, A.M.; Celia, C.; Deng, X.; Fresta, M. OX26-cojugated gangliosilated liposomes to improve the post-ischemic therapeutic effect of CDP-choline. Drug. Deliv. Transl. Res. 2024, 14, 2771–2787. [Google Scholar] [CrossRef]
- Greenlee, J.D.; Zhang, Z.; Subramanian, T.; Liu, K.; King, M.R. TRAIL-conjugated liposomes that bind natural killer cells to induce colorectal cancer cell apoptosis. J. Biomed. Mater. Res. A 2024, 112, 110–120. [Google Scholar] [CrossRef]
- Lin, Q.; Guo, Q.; Zhu, M.; Zhang, J.; Chen, B.; Wu, T.; Jiang, W.; Tang, W. Application of Nanomedicine in Inner Ear Diseases. Front. Bioeng. Biotechnol. 2022, 9, 809443. [Google Scholar] [CrossRef] [PubMed]
- d’Avanzo, N.; Sidorenko, V.; Simón-Gracia, L.; Rocchi, A.; Ottonelli, I.; Ruozi, B.; Longo, F.; Celia, C.; Teesalu, T. C-end rule peptide-guided niosomes for prostate cancer cell targeting. J. Drug Deliv. Sci. Technol. 2023, 91, 105162. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168. [Google Scholar] [CrossRef]
- Mahon, E.; Salvati, A.; Bombelli, F.B.; Lynch, I.; Dawson, K.A. Designing the nanoparticle-biomolecule interface for “targeting and therapeutic delivery”. J. Control. Release 2012, 161, 164–174. [Google Scholar] [CrossRef]
- Guan, J.; Shen, Q.; Zhang, Z.; Jiang, Z.; Yang, Y.; Lou, M.; Qian, J.; Lu, W.; Zhan, C. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat. Commun. 2018, 9, 2982. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.H.; Cohen, Y.; Andrew Emili, A.; Chan, W.C.W. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef]
- Bigdeli, A.; Palchetti, S.; Pozzi, D.; Hormozi-Nezhad, M.R.; Bombelli, F.B.; Caracciolo, G.; Mahmoudi, M. Exploring Cellular Interactions of Liposomes Using Protein Corona Fingerprints and Physicochemical Properties. ACS Nano 2016, 10, 3723–3737. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Jois, S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control. Release 2021, 329, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2022, 12, 803304. [Google Scholar] [CrossRef]
- Nel, J.; Elkhoury, K.; Velot, É.; Bianchi, A.; Acherar, S.; Francius, G.; Tamayol, A.; Grandemange, S.; Arab-Tehrany, E. Functionalized liposomes for targeted breast cancer drug delivery. Bioact. Mater. 2023, 24, 401–437. [Google Scholar] [CrossRef]
- Wang, C.; Lan, X.; Zhu, L.; Wang, Y.; Gao, X.; Li, J.; Tian, H.; Liang, Z.; Xu, W. Construction Strategy of Functionalized Liposomes and Multidimensional Application. Small 2024, 20, e2309031. [Google Scholar] [CrossRef]
- Majzoub, R.N.; Ewert, K.K.; Safinya, C.R. Cationic liposome–nucleic acid nanoparticle assemblies with applications in gene delivery and gene silencing. Phil. Trans. R. Soc. A 2016, 374, 20150129. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Ewert, K.K.; Scodeller, P.; Simón-Gracia, L.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Safinya, C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics 2021, 13, 1365. [Google Scholar] [CrossRef]
- Bianco, A.; Bonadies, F.; Napolitano, R.; Ortaggi, G.; Esposito, C.; Mossa, G.; Cametti, C. Synthesis of novel cationic lipids for preparation of liposomes to be used in gene therapy of glioma. Comptes Rendus Chim. 2003, 6, 589–595. [Google Scholar] [CrossRef]
- Gao, J.; Ochyl, L.J.; Yang, E.; Moon, J.J. Cationic liposomes promote antigen cross-presentation in dendritic cells by alkalizing the lysosomal pH and limiting the degradation of antigens. Int. J. Nanomed. 2017, 12, 1251–1264. [Google Scholar] [CrossRef]
- Hattori, Y.; Nakamura, M.; Takeuchi, N.; Tamaki, K.; Shimizu, S.; Yoshiike, Y.; Taguchi, M.; Ohno, H.; Ozaki, K.-I.; Onishi, H. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J. Drug Target. 2019, 27, 217–227. [Google Scholar] [CrossRef]
- Hattori, Y.; Tang, M.; Torii, S.; Tomita, K.; Sagawa, A.; Inoue, N.; Yamagishi, R.; Ozaki, K.I. Optimal combination of cationic lipid and phospholipid in cationic liposomes for gene knockdown in breast cancer cells and mouse lung using siRNA lipoplexes. Mol. Med. Rep. 2022, 2, 253. [Google Scholar] [CrossRef] [PubMed]
- Filion, M.C.; Phillips, N.C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta 1997, 1329, 345. [Google Scholar] [CrossRef]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Induction of apoptosis in macrophages by cationic liposomes. FEBS Lett. 1999, 460, 472. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.I.; El-Refaie, W.M. Bioadhesive Chitosan-Coated Cationic Nanoliposomes with Improved Insulin Encapsulation and Prolonged Oral Hypoglycemic Effect in Diabetic Mice. J. Pharm. Sci. 2018, 107, 2136–2143. [Google Scholar] [CrossRef]
- Yao, Y.; Su, Z.; Liang, Y.; Zhang, N. pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery. Int. J. Nanomed. 2015, 10, 6185–6197. [Google Scholar] [CrossRef] [PubMed]
- Alomrani, A.; Badran, M.; Harisa, G.I.; ALshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dong, X.; Chen, X. Polymer-Modified Liposomes for Drug Delivery: From Fundamentals to Applications. Pharmaceutics 2022, 14, 778. [Google Scholar] [CrossRef]
- Basinska, T.; Gadzinowski, M.; Mickiewicz, D.; Slomkowski, S. Functionalized Particles Designed for Targeted Delivery. Polymers 2021, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Vo, T. Theory and simulation of ligand functionalized nanoparticles—A pedagogical overview. Soft Matter 2024, 20, 3554–3576. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef]
- Aronson, M.R.; Medina, S.H.; Mitchell, M.J. Peptide functionalized liposomes for receptor targeted cancer therapy. APL Bioeng. 2021, 5, 011501. [Google Scholar] [CrossRef] [PubMed]
- d’Avanzo, N.; Torrieri, G.; Figueiredo, P.; Celia, C.; Paolino, D.; Correia, A.; Moslova, K.; Teesalu, T.; Fresta, M.; Santos, H.A. LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy. Int. J. Pharm. 2021, 597, 120346. [Google Scholar] [CrossRef]
- Giofrè, S.; Renda, A.; Sesana, S.; Formicola, B.; Vergani, B.; Leone, B.E.; Denti, V.; Paglia, G.; Groppuso, S.; Romeo, V.; et al. Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions. Pharmaceutics 2022, 14, 2402. [Google Scholar] [CrossRef]
- Nanjaiah, H.; Moudgil, K.D. The Utility of Peptide Ligand-Functionalized Liposomes for Subcutaneous Drug Delivery for Arthritis Therapy. Int. J. Mol. Sci. 2023, 24, 6883. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Zhang, Y.; Wang, L.; Zhang, Z. Comparison of two methods for tumour-targeting peptide modification of liposomes. Acta Pharmacol. Sin. 2023, 44, 832–840. [Google Scholar] [CrossRef]
- Jourdain, M.A.; Dupont, A.; Lautram, N.; Eyer, J. Investigating the functionalization of liposomes with NFL-TBS. 40-63 peptide as a promising drug delivery system. Int. J. Pharm. 2024, 652, 123805. [Google Scholar] [CrossRef]
- Bartomeu Garcia, C.; Shi, D.; Webster, T.J. Tat-functionalized liposomes for the treatment of meningitis: An in vitro study. Int. J. Nanomed. 2017, 12, 3009–3021. [Google Scholar] [CrossRef] [PubMed]
- Zarazvand, F.; Karimi, M.; Moosavian, S.A.; Arabi, L.; Badiee, A.; Jaafari, M.R.; Masreghi, M. Eddicacy comparison of TAT peptide-functionalized PEGylated liposomal Doxorubicin in C26 and B16F0 tumor micel models. Int. J. Pept. Res. Ther. 2021, 27, 2099–2109. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Schwieger, J.; Matin-Mann, F.; Behrens, P.; Lenarz, T.; Scheper, V. Dexamethasone for Inner Ear Therapy: Biocompatibility and Bio-Efficacy of Different Dexamethasone Formulations In Vitro. Biomolecules 2021, 11, 1896. [Google Scholar] [CrossRef]
- McCall, A.A.; Swan, E.E.; Borenstein, J.T.; Sewell, W.F.; Kujawa, S.G.; McKenna, M.J. Drug delivery for treatment of inner ear disease: Current state of knowledge. Ear Hear. 2010, 31, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, D.; Wang, H.; Chai, R.; Zhao, Y. Cutting-Edge Achievements of Inner Ear Drug Delivery Systems. Adv. NanoBiomed Res. 2024, 4, 2400004. [Google Scholar] [CrossRef]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2019, 5, 122–131. [Google Scholar] [CrossRef]
- Mfoafo, K.A.; Mittal, R.; Eshraghi, A.A.; Omidi, Y.; Omidian, H. Improved inner ear drug delivery using hydrogel carriers. J. Drug Deliv. Sci. Technol. 2023, 79, 104086. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, J.; He, Y.; Lin, K.; Li, S.; Zhang, Y.; Song, P.; Zhou, Y.; Chen, X. Nanocarriers for Inner Ear Disease Therapy. Front. Cell. Neurosci. 2021, 15, 791573. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, Q.; Li, S.; Zhou, Y.; He, Z.; Lin, K.; Yang, M.; Song, P.; Chen, X. Promising Applications of Nanoparticles in the Treatment of Hearing Loss. Front. Cell Dev. Biol. 2021, 9, 750185. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Park, J.S. Intratympanic drug delivery systems to treat inner ear impairments. J. Pharm. Investig. 2023, 53, 93–118. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Rabbani, G.; Ahmad, S.; Hasan, Q.; Khan, R.H.; Alam, K.; Choi, I. Glycation of H1 Histone by 3-Deoxyglucosone: Effects on Protein Structure and Generation of Different Advanced Glycation End Products. PLoS ONE 2015, 10, e0130630. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Suchý, T.; Bartoš, M.; Sedláček, R.; Šupová, M.; Žaloudková, M.; Martynková, G.S.; Foltán, R. Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds. Materials 2021, 14, 4388. [Google Scholar] [CrossRef]
- Sainaga Jyothi, V.G.S.; Bulusu, R.; Venkata Krishna Rao, B.; Pranothi, M.; Banda, S.; Kumar Bolla, P.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Designing liposomes as a drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Vochita, G. Peptide-functionalized chitosan-based microcapsules for dual active targeted treatment of lung infections. Int. J. Biol. Macromol. 2024, 265 Pt 2, 131027. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Kondratowicz, A.; Weiss, M.; Juzwa, W.; Majchrzycki, Ł.; Lewandowicz, G. Characteristics of liposomes derived from egg yolk. Open Chem. 2019, 17, 763–778. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Dong, M.; Han, X. Effect of cholesterol on the fluidity of supported lipid bilayers. Colloids Surf. B Biointerfaces 2020, 196, 111353. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://lipoid.com/en/products/natural-phospholipids/unsaturated-phospholipids/ (accessed on 8 January 2025).
- Available online: https://lipoid.com/en/products/oils-fatty-acids-lipids/cationic-synthetic-lipids/ (accessed on 8 January 2025).
- Zhuxin, L.; Biao, Y.; Badamkhand, D.; Yifan, C.; Honghong, S.; Xiao, X.; Mingqian, T.; Zhixiang, W.; Chongjiang, C. Carboxylated chitosan improved the stability of phycocyanin under acidified conditions. Int. J. Biol. Macromol. 2023, 233, 123474. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, V.G.; Freshney, R.I. Culture of Cells for Tissue Engineering; John and Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Cann, A.J. Maths from Scratch for Biologists; John and Wiley and Sons: Hoboken, NJ, USA, 2002; pp. 83–146. [Google Scholar]
- Collins, T.J. ImageJ for icroscopy. BioTechniques 2007, 43 (Suppl. S1), 25–30. [Google Scholar] [CrossRef]
- Cerrutti, B.M.; Lamas, J.C.; Campana-Filho, S.P.; Frollini, E. Carboxymethyl Chitosan: Preparation and Use in Colloidal Ceramic Processing. J. Polym. Environ. 2012, 21, 816–825. [Google Scholar] [CrossRef]
- Vaghani, S.S.; Patel, M.M.; Satish, C.S.; Patel, K.M.; Jivani, N.P. Synthesis and characterization of carboxymethyl chitosan hydrogel: Application as site specific delivery for lercanidipine hydrochloride. Bull. Mater. Sci. 2012, 35, 1133–1142. [Google Scholar] [CrossRef]
- Du, J.; Hsieh, Y.L. Nanofibrous membranes from aqueous electrospinning of carboxymethyl chitosan. Nanotechnology 2008, 19, 125707. [Google Scholar] [CrossRef] [PubMed]
- Malta, L.F.; Senra, J.D.; Tinoco, L.W.; Medeiros, M.E.; Antunes, O.A. Chiral Recognition of 2-Hydroxypropyl-alpha-cyclodextrin Towards DLTryptophan. Lett. Org. Chem. 2009, 6, 258–263. [Google Scholar] [CrossRef]
- Tchipilov, T.; Meyer, K.; Weller, M.G. Quantitative 1H Nuclear Magnetic Resonance (qNMR) of Aromatic Amino Acids for Protein Quantification. Methods Protoc. 2023, 6, 11. [Google Scholar] [CrossRef]
- Aslan, H.K.; Kuşçulu, N.G. Investigation of chemical activity, SCHIFF base reactions and staining effects of some amino acids by spectrophotometric and theorical methods. J. Indian Chem. Soc. 2021, 99, 100315. [Google Scholar] [CrossRef]
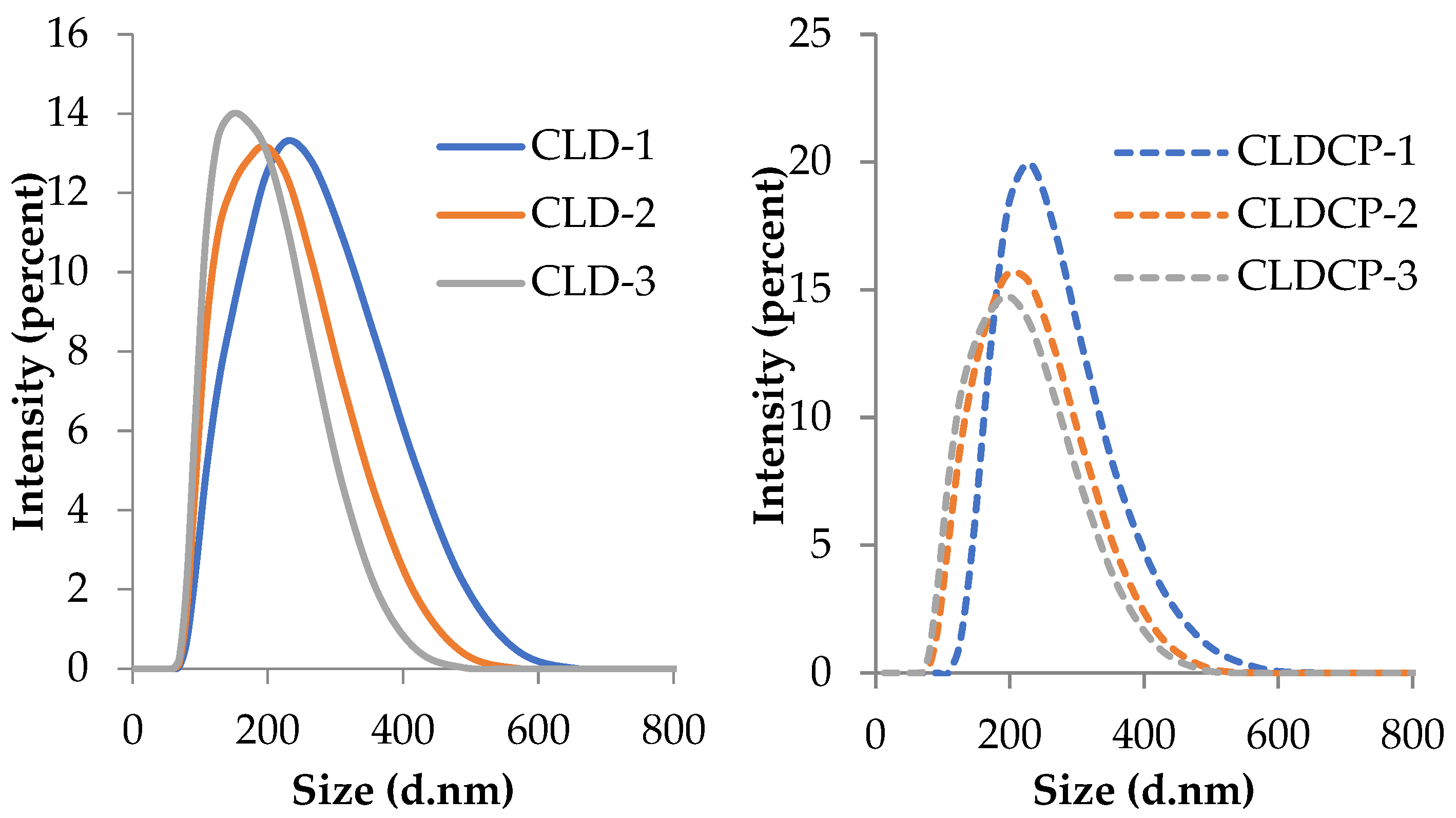
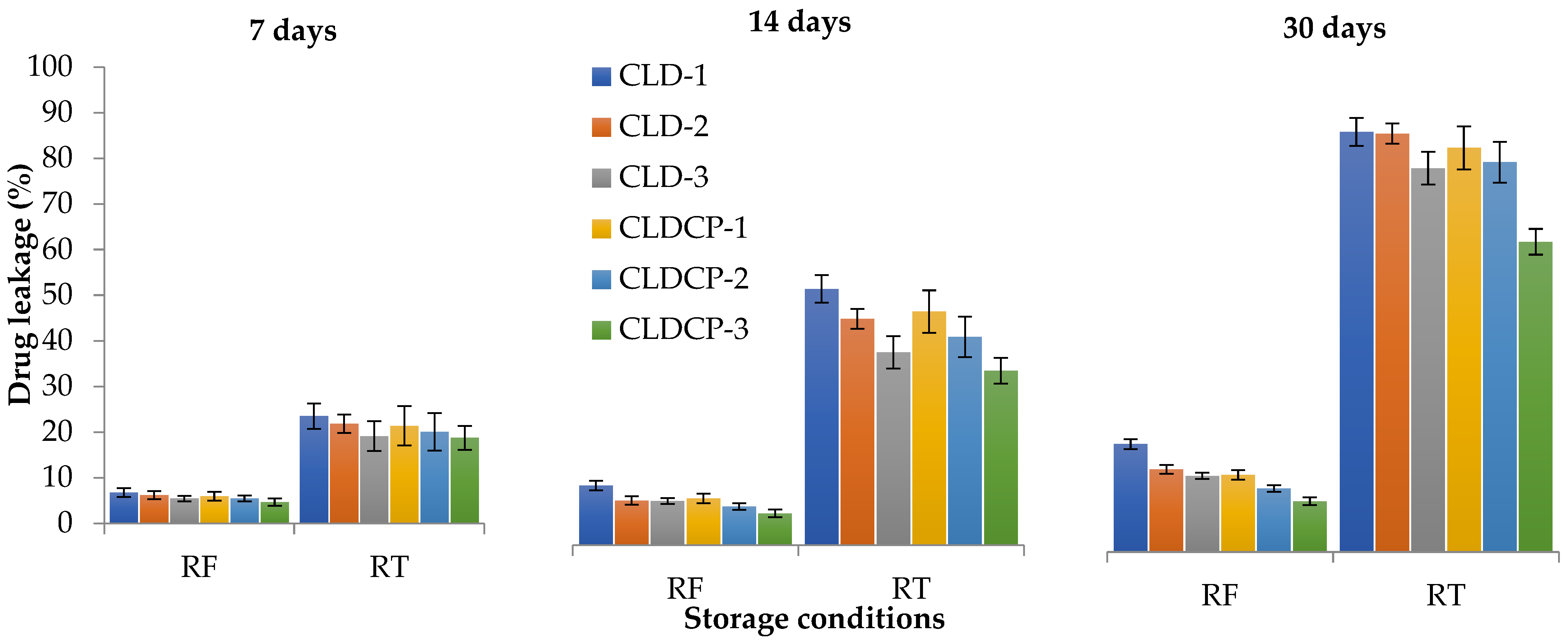
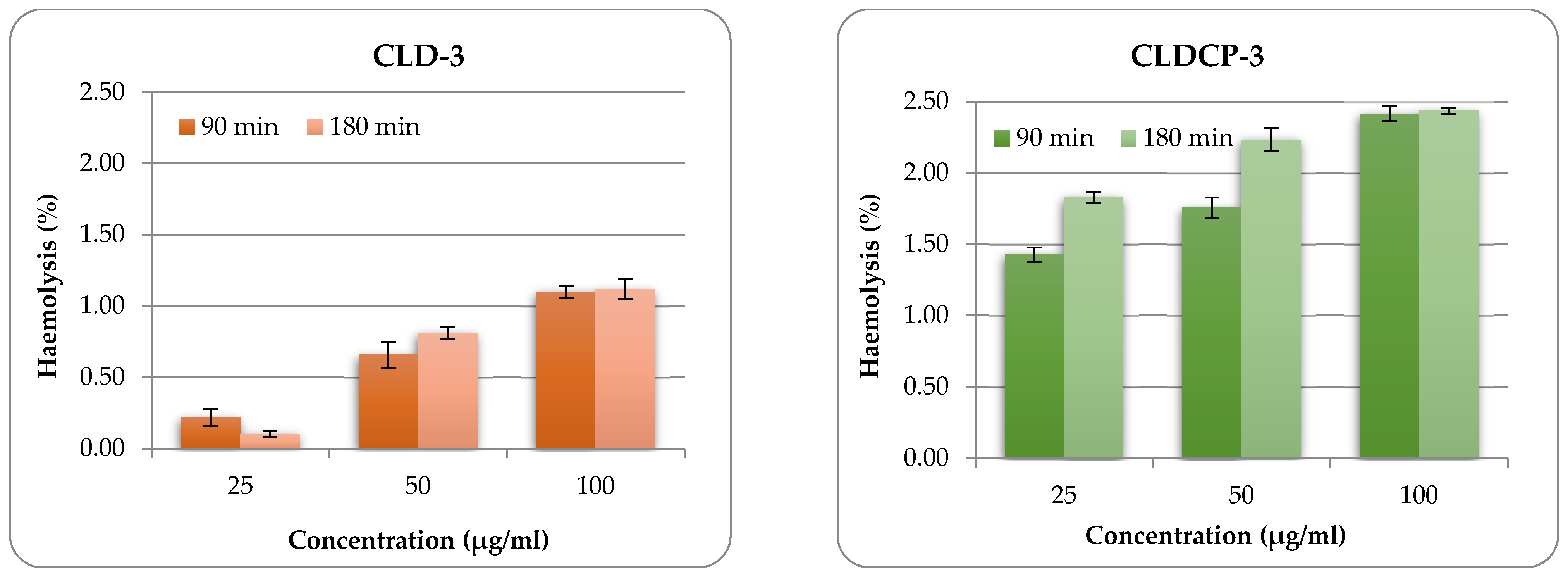

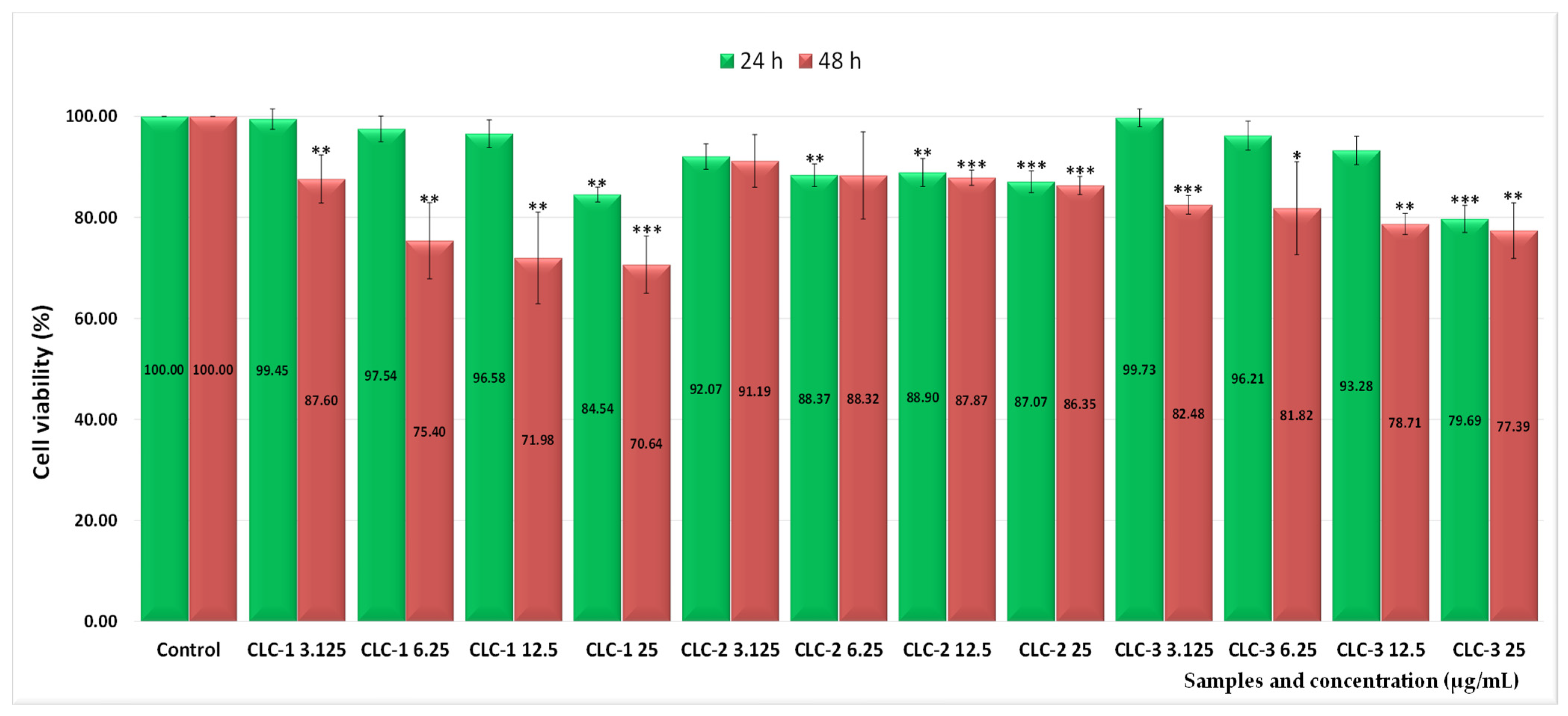
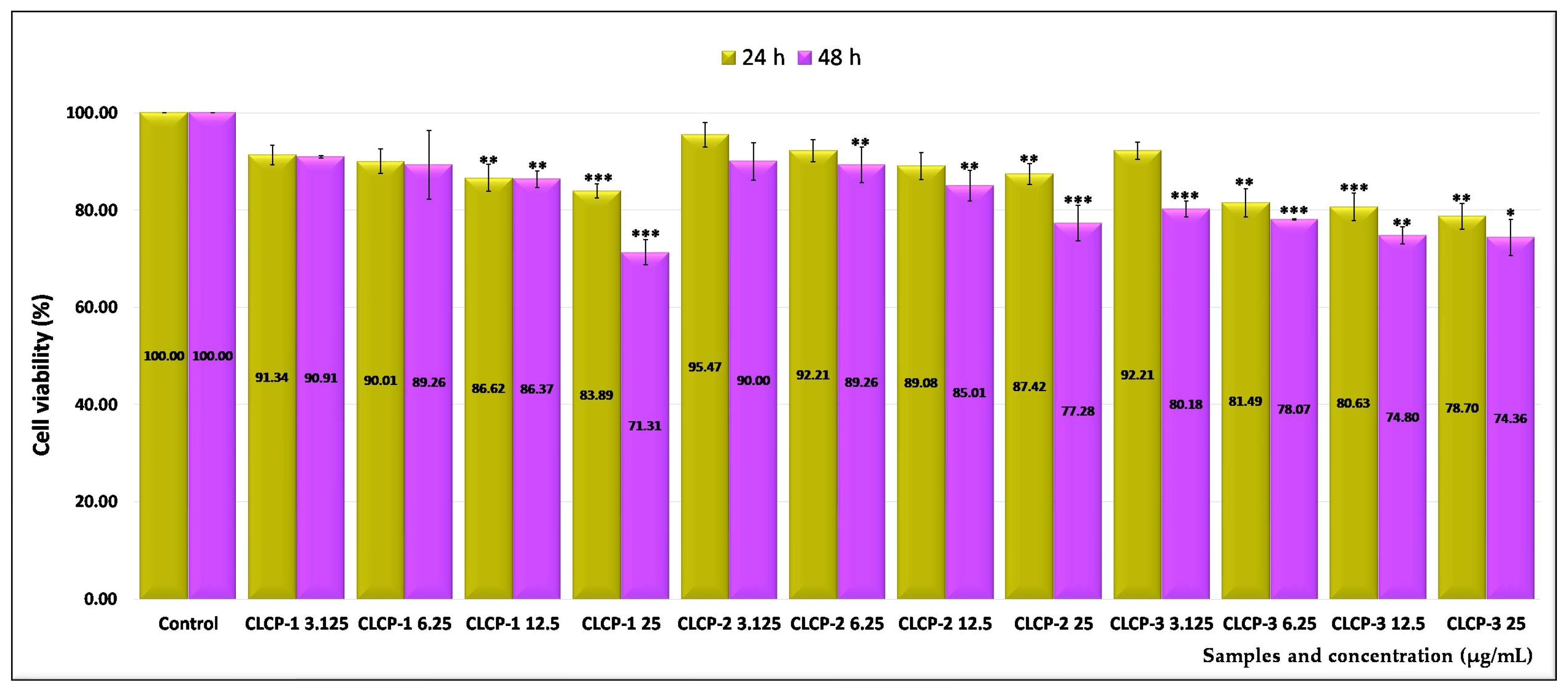
| Sample Code | Lipid Mixture (Weight Ratio) | Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|---|
| Uncovered liposomes | ||||
| CLD-1 | EPC/Chol/DOTAP 22/2/1 | 197 ± 3 | 0.12 ± 0.02 | +11 ± 0.7 |
| CLD-2 | EPC/Chol/DOTAP 21/2/2 | 179 ± 1 | 0.13 ± 0.01 | +27 ± 0.9 |
| CLD-3 | EPC/Chol/DOTAP 19/4/2 | 167 ± 2 | 0.14 ± 0.02 | +25 ± 2.1 |
| Liposomes coated with CMCS and CMCS functionalized with peptide mixture | ||||
| CLDCP-1 | EPC/Chol/DOTAP 22/2/1 | 202 ± 1 | 0.06 ± 0.01 | −14 ± 0.4 |
| CLDCP-2 | EPC/Chol/DOTAP 21/2/2 | 187 ± 1 | 0.15 ± 0.03 | −17 ± 0.1 |
| CLDCP-3 | EPC/Chol/DOTAP 19/4/2 | 179 ± 2 | 0.07 ± 0.01 | −18 ± 1.1 |
| Sample Code | Loading Efficiency (%) | Release Efficiency (%) |
|---|---|---|
| CLD-1 | 25 ± 4 | 37 ± 2 |
| CLD-2 | 23 ± 3 | 40 ± 4 |
| CLD-3 | 22 ± 3 | 38 ± 4 |
| CLDCP-1 | 24 ± 2 | 34 ± 4 |
| CLDCP-2 | 21 ± 2 | 37 ± 4 |
| CLDCP-3 | 19 ± 2 | 34 ± 4 |
| Samples | IC50 24 h (µg/mL) | IC50 48 h (µg/mL) |
|---|---|---|
| CL-1 | 106.13 | 61.61 |
| CL-2 | 84.59 | 31.22 |
| CL-3 | 190.39 | 37.03 |
| CLC-1 | 77.61 | 28.91 |
| CLC-2 | 66.75 | 63.90 |
| CLC-3 | 60.40 | 35.19 |
| CLCP-1 | 65.46 | 44.02 |
| CLCP-2 | 84.07 | 52.29 |
| CLCP-3 | 40.70 | 29.38 |
| Sample Code | EPC (mg) | Chol (mg) | DOTAP (mg) | EPC/Chol/DOTAP (Molar Ratio) | Dexamethasone Phosphate (mg) | CMCS + CMCS-P1 + CMCS-P2 * (1%) /Liposomes Suspension (v/v) |
|---|---|---|---|---|---|---|
| Uncoated liposomes | ||||||
| CLD-1 | 22 | 2 | 1 | 1.00/0.18/0.05 | 5 | - |
| CLD-2 | 21 | 2 | 2 | 0.95/0.18/0.10 | ||
| CLD-3 | 19 | 4 | 2 | 0.86/0.36/0.10 | ||
| Liposomes coated with CMCS + CMCS-P1 + CMCS-P2 | ||||||
| CLDCP-1 | 22 | 2 | 1 | 1.00/0.18/0.05 | 5 | 1/1 |
| CLDCP-2 | 21 | 2 | 2 | 0.95/0.18/0.10 | ||
| CLDCP-3 | 19 | 4 | 2 | 0.86/0.36/0.10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftode, L.; Cadinoiu, A.N.; Raţă, D.M.; Atanase, L.I.; Vochiţa, G.; Rădulescu, L.; Popa, M.; Gherghel, D. Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery. Int. J. Mol. Sci. 2025, 26, 922. https://doi.org/10.3390/ijms26030922
Iftode L, Cadinoiu AN, Raţă DM, Atanase LI, Vochiţa G, Rădulescu L, Popa M, Gherghel D. Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery. International Journal of Molecular Sciences. 2025; 26(3):922. https://doi.org/10.3390/ijms26030922
Chicago/Turabian StyleIftode, Loredana, Anca Niculina Cadinoiu, Delia Mihaela Raţă, Leonard Ionuț Atanase, Gabriela Vochiţa, Luminița Rădulescu, Marcel Popa, and Daniela Gherghel. 2025. "Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery" International Journal of Molecular Sciences 26, no. 3: 922. https://doi.org/10.3390/ijms26030922
APA StyleIftode, L., Cadinoiu, A. N., Raţă, D. M., Atanase, L. I., Vochiţa, G., Rădulescu, L., Popa, M., & Gherghel, D. (2025). Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery. International Journal of Molecular Sciences, 26(3), 922. https://doi.org/10.3390/ijms26030922










